Description and Evaluation of Dye and Contrast Media Distribution of Ultrasound-Guided Rectus Sheath Block in Cat Cadavers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Phase 1: Anatomical Study
2.2. Phase 2
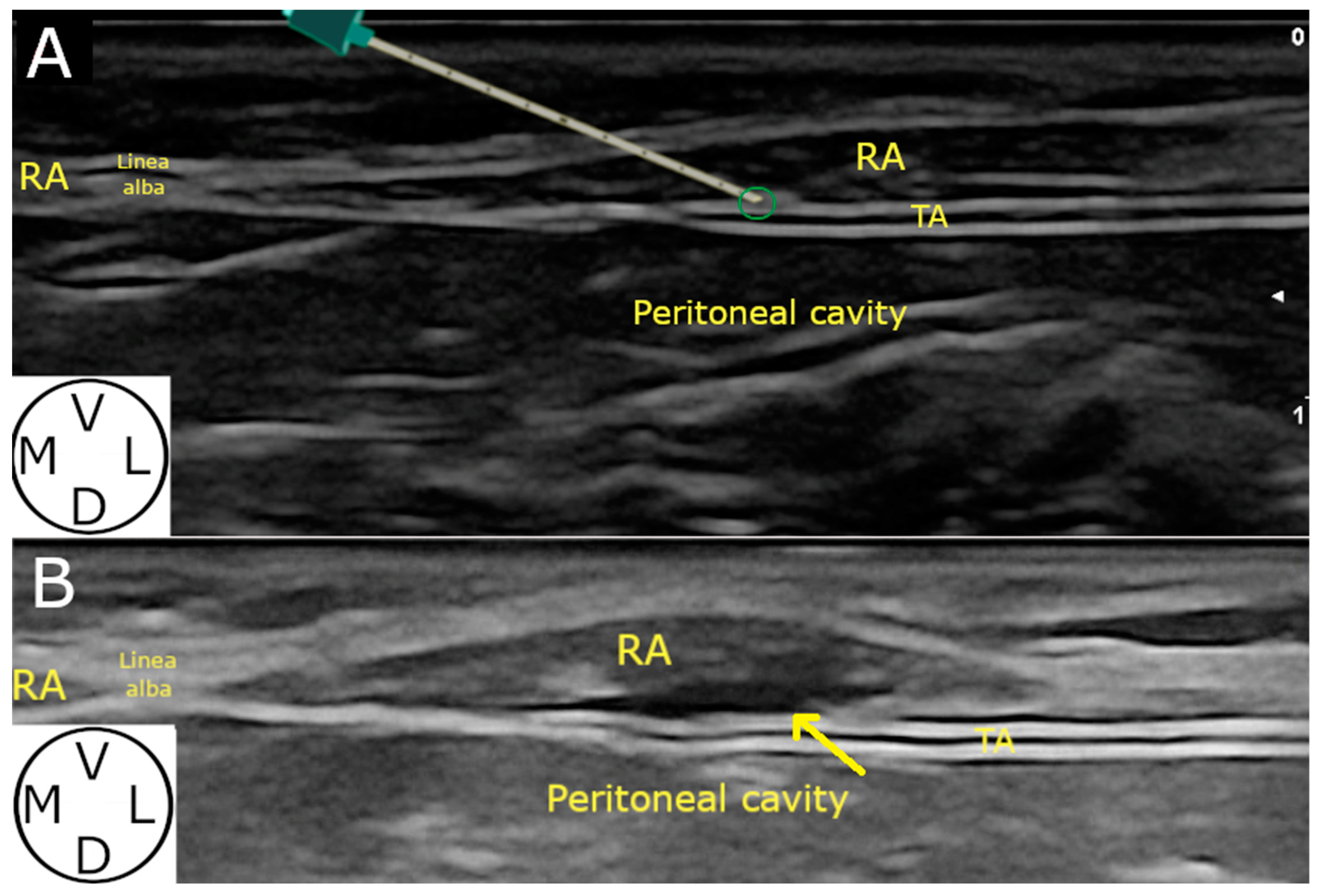
2.2.1. Ultrasound-Guided Technique
2.2.2. Computed Tomography (CT) Study
2.2.3. Dyeing Study
2.3. Statistical Analysis
3. Results
3.1. Phase 1: Anatomical Study
3.2. Phase 2
3.2.1. Demographic Distribution
3.2.2. Ultrasound-Guided Technique
3.2.3. Computed Tomography Study
3.2.4. Spread Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, B.L. Introduction to Fascial Plane Blocks. In Small Animal Regional Anesthesia and Analgesia; Read, M., Campoy, L., Fischer, B., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 117–125. ISBN 978-1-119-51415-2. [Google Scholar]
- Elsharkawy, H.; Pawa, A.; Mariano, E.R. Interfascial Plane Blocks: Back to Basics. Reg. Anesth. Pain Med. 2018, 43, 341–346. [Google Scholar] [CrossRef]
- Chin, K.J.; Versyck, B.; Elsharkawy, H.; Rojas Gomez, M.F.; Sala-Blanch, X.; Reina, M.A. Anatomical basis of fascial plane blocks. Reg. Anesth. Pain Med. 2021, 46, 581–599. [Google Scholar] [CrossRef]
- Campoy, L. Development of Enhanced Recovery After Surgery (ERAS) protocols in veterinary medicine through a one-health approach: The role of anesthesia and locoregional techniques. J. Am. Vet. Med. Assoc. 2022, 260, 1751–1759. [Google Scholar] [CrossRef]
- Otero, P.E.; Romano, M.; Zaccagnini, A.S.; Fuensalida, S.E.; Verdier, N.; Sanchez, F.; Portela, D.A. Transversus abdominis plane block in cat cadavers: Anatomical description and comparison of injectate spread using two- and three-point approaches. Vet. Anaesth. Analg. 2021, 48, 432–441. [Google Scholar] [CrossRef]
- Garbin, M.; Marangoni, S.; Finck, C.; Steagall, P.V. An Anatomical, Sonographic, and Computed Tomography Study of the Transversus Abdominis Plane Block in Cat Cadavers. Animals 2022, 12, 2674. [Google Scholar] [CrossRef]
- dos-Santos, J.D.; Ginja, M.; Alves-Pimenta, S.; Otero, P.E.; Ribeiro, L.; Colaço, B. A description of an ultrasound-guided technique for a quadratus lumborum block in the cat: A cadaver study. Vet. Anaesth. Analg. 2021, 48, 804–808. [Google Scholar] [CrossRef] [PubMed]
- dos-Santos, J.D.; Ginja, M.; Alves-Pimenta, S.; Otero, P.E.; Ribeiro, L.; Colaço, B. Comparison of dorsoventral and ventrodorsal approaches for ultrasound-guided quadratus lumborum block in cats: A cadaver study. Vet. Anaesth. Analg. 2022, 49, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Polo-Paredes, G.; Laredo, F.G.; Gil, F.; Soler, M.; Agut, A.; Belda, E. Modified Ultrasound-Guided Dorsal Quadratus Lumborum Block in Cat Cadavers. Animals 2023, 13, 3798. [Google Scholar] [CrossRef] [PubMed]
- Garbin, M.; Ruel, H.L.; Watanabe, R.; Malo, A.; Monteiro, B.P.; Steagall, P.V. Analgesic efficacy of an ultrasound-guided transversus abdominis plane block with bupivacaine in cats: A randomised, prospective, masked, placebo-controlled clinical trial. J. Feline Med. Surg. 2023, 25, 1098612X2311544. [Google Scholar] [CrossRef]
- dos-Santos, J.D.; Ginja, M.; Martins, J.; Cabral, P.; Alves-Pimenta, S.; Ribeiro, L.; Otero, P.E.; Colaço, B. Comparison between Bilateral Ultrasound-Guided Quadratus Lumborum Block and Sacrococcygeal Epidural in Cats Undergoing Ovariectomy. Vet. Sci. 2024, 11, 25. [Google Scholar] [CrossRef]
- Ferreira, T.H. Ultrasound-Guided Rectus Sheath Block. In Small Animal Regional Anesthesia and Analgesia; Read, M., Campoy, L., Fischer, B., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 203–212. ISBN 978-1-119-51415-2. [Google Scholar]
- Hamill, J.K.; Liley, A.; Hill, A.G. Rectus sheath block for laparoscopic appendicectomy: A randomized clinical trial. ANZ J. Surg. 2015, 85, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Hamill, J.K.; Rahiri, J.-L.; Liley, A.; Hill, A.G. Rectus sheath and transversus abdominis plane blocks in children: A systematic review and meta-analysis of randomized trials. Pediatr. Anesth. 2016, 26, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Manassero, A.; Bossolasco, M.; Meineri, M.; Ugues, S.; Liarou, C.; Bertolaccini, L. Spread patterns and effectiveness for surgery after ultrasound-guided rectus sheath block in adult day-case patients scheduled for umbilical hernia repair. J. Anaesthesiol. Clin. Pharmacol. 2015, 31, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Yassin, H.M.; Abd Elmoneim, A.T.; El Moutaz, H. The Analgesic Efficiency of Ultrasound-Guided Rectus Sheath Analgesia Compared with Low Thoracic Epidural Analgesia After Elective Abdominal Surgery with a Midline Incision: A Prospective Randomized Controlled Trial. Anesthesiol. Pain Med. 2017, 7, e14244. [Google Scholar] [CrossRef] [PubMed]
- Sarbay, İ.; Akın Uzan, H.S.; Yüceyar, L.; Turna, A. Rectus Sheath Block for Pain Management in Subxiphoid Single-Incision Thoracoscopic Surgery. Indian J. Surg. 2023, 85, 566–569. [Google Scholar] [CrossRef]
- Getty, R. Sisson and Grossman’s-The Anatomy of the Domestic Animals; Aufl, W.B., Ed.; Saunders Company: Philadelphia, PA, USA, 1975; Volume 2, ISBN 978-0-7216-4107-2. [Google Scholar]
- Chin, K.J.; McDonnell, J.G.; Carvalho, B.; Sharkey, A.; Pawa, A.; Gadsden, J. Essentials of Our Current Understanding: Abdominal Wall Blocks. Reg. Anesth. Pain Med. 2017, 42, 133–183. [Google Scholar] [CrossRef] [PubMed]
- El-Boghdadly, K.; Wolmarans, M.; Stengel, A.D.; Albrecht, E.; Chin, K.J.; Elsharkawy, H.; Kopp, S.; Mariano, E.R.; Xu, J.L.; Adhikary, S.; et al. Standardizing nomenclature in regional anesthesia: An ASRA-ESRA Delphi consensus study of abdominal wall, paraspinal, and chest wall blocks. Reg. Anesth. Pain Med. 2021, 46, 571–580. [Google Scholar] [CrossRef]
- Sviggum, H.P.; Niesen, A.D.; Sites, B.D.; Dilger, J.A. Trunk Blocks 101: Transversus Abdominis Plane, Ilioinguinal-Iliohypogastric, and Rectus Sheath Blocks. Int. Anesthesiol. Clin. 2012, 50, 74–92. [Google Scholar] [CrossRef]
- St James, M.; Ferreira, T.H.; Schroeder, C.A.; Hershberger-Braker, K.L.; Schroeder, K.M. Ultrasound-guided rectus sheath block: An anatomic study in dog cadavers. Vet. Anaesth. Analg. 2020, 47, 95–102. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Sakai, D.M.; Im, J.S.; Zhang, S.; Reed, R.A.; Quandt, J.E.; Baldo, C.F.; Walters, B.; Barletta, M. Antinociceptive effects of bupivacaine injected within the internal abdominis rectus sheath in standing healthy horses. Vet. Anaesth. Analg. 2023, 50, 294–301. [Google Scholar] [CrossRef]
- Gutiérrez Bautista, Á.J.; Söbbeler, F.J.; Koch, R.; Viscasillas, J.; Kästner, S. Assessment of an Ultrasound-Guided Rectus Sheath Block in Foals: A Cadaveric Study. Animals 2023, 13, 3600. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.H.; Schroeder, C.A.; St James, M.; Hershberger-Braker, K.L. Description of an ultrasound-guided rectus sheath block injection technique and the spread of dye in calf cadavers. Vet. Anaesth. Analg. 2022, 49, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ienello, L.; Kennedy, M.; Wendt-Hornickle, E.; Baldo, C.; Moshnikova, V.; Guedes, A. Ultrasound-guided rectus sheath block injections in miniature swine cadavers: Technique description and distribution of two injectate volumes. Vet. Anaesth. Analg. 2022, 49, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Schaller, O.; Constantinescu, G.M. (Eds.) Illustrated Veterinary Anatomical Nomenclature, 4th ed.; Georg Thieme: Stuttgart, NY, USA, 2018; ISBN 978-3-13-242517-0. [Google Scholar]
- Kamyabnia, M.; Rastabi, H.I.; Ghadiri, A.; Jalali, M.R.; Givi, M.E. Comparison of incisional, transverse abdominis plane, and rectus sheath blocks in dogs undergoing ovariohysterectomy. Am. J. Vet. Res. 2023, 84, ajvr.23.02.0040. [Google Scholar] [CrossRef] [PubMed]
- Alterisio, M.C.; Micieli, F.; Valle, G.D.; Chiavaccini, L.; Vesce, G.; Ciaramella, P.; Guccione, J. Cardiovascular changes, laboratory findings and pain scores in calves undergoing ultrasonography-guided bilateral rectus sheath block before herniorrhaphy: A prospective randomized clinical trial. BMC Vet. Res. 2023, 19, 191. [Google Scholar] [CrossRef] [PubMed]
- Haley, A.; Kennedy, M.; Hickey, C.; Gordon-Evans, W.; Guedes, A. Evaluation of pre-operative rectus sheath block with bupivacaine for analgesia in cats undergoing ovariohysterectomy. Vet. Anaesth. Analg. 2023, 50, e109–e110. [Google Scholar] [CrossRef]
- Touzot-Jourde, G. Ultrasound-Guided Rectus Abdominis Sheath Block in Cats Undergoing Ovariectomy: A Prospective, Randomized, Investigator-Blinded, Placebo-Controlled Clinical Trial. Open Access J. Vet. Sci. Res. 2022, 7, 1–8. [Google Scholar] [CrossRef]
- WSAVA Nutritional Assessment Guidelines Task Force Members; Freeman, L.; Becvarova, I.; Cave, N.; MacKay, C.; Nguyen, P.; Rama, B.; Takashima, G.; Tiffin, R.; Tsjimoto, H.; et al. WSAVA Nutritional Assessment Guidelines. J. Small Anim. Pract. 2011, 52, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.; Finnerty, O.; Rauf, J.; Bergin, D.; Laffey, J.G.; Mc Donnell, J.G. Studies on the spread of local anaesthetic solution in transversus abdominis plane blocks*. Anaesthesia 2011, 66, 1023–1030. [Google Scholar] [CrossRef]
- Støving, K.; Rothe, C.; Rosenstock, C.V.; Aasvang, E.K.; Lundstrøm, L.H.; Lange, K.H.W. Cutaneous Sensory Block Area, Muscle-Relaxing Effect, and Block Duration of the Transversus Abdominis Plane Block: A Randomized, Blinded, and Placebo-Controlled Study in Healthy Volunteers. Reg. Anesth. Pain Med. 2015, 40, 355–362. [Google Scholar] [CrossRef]
- Teixeira, L.G.; Pujol, D.M.; Pazzim, A.F.; Souza, R.P.; Fadel, L. Combination of Transversus abdominis plane block and Serratus plane block anesthesia in dogs submitted to masctetomy. Pesqui. Vet. Bras. 2018, 38, 315–319. [Google Scholar] [CrossRef]
- Marchina-Gonçalves, A.; Gil, F.; Laredo, F.G.; Soler, M.; Agut, A.; Belda, E. Evaluation of High-Volume Injections Using a Modified Dorsal Quadratus Lumborum Block Approach in Canine Cadavers. Animals 2021, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Viscasillas, J.; Terrado, J.; Marti-Scharfhausen, R.; Castiñeiras, D.; Esteve, V.; Clancy, N.; Redondo, J.I. A Modified Approach for the Ultrasound-Guided Quadratus Lumborum Block in Dogs: A Cadaveric Study. Animals 2021, 11, 2945. [Google Scholar] [CrossRef] [PubMed]
- Marchina-Gonçalves, A.; Laredo, F.G.; Gil, F.; Soler, M.; Agut, A.; Redondo, J.I.; Belda, E. An Ultrasound-Guided Latero-Ventral Approach to Perform the Quadratus Lumborum Block in Dog Cadavers. Animals 2023, 13, 2214. [Google Scholar] [CrossRef] [PubMed]
- Zoff, A.; Laborda-Vidal, P.; Mortier, J.; Amengual, M.; Rioja, E. Comparison of the spread of two different volumes of contrast medium when performing ultrasound-guided transversus abdominis plane injection in dog cadavers. J. Small Anim. Pract. 2017, 58, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Grubb, T.; Lobprise, H. Local and regional anaesthesia in dogs and cats: Overview of concepts and drugs (Part 1). Vet. Med. Sci. 2020, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.P.; Lascelles, B.D.X.; Murrell, J.; Robertson, S.; Steagall, P.V.M.; Wright, B. 2022 WSAVA guidelines for the recognition, assessment and treatment of pain. J. Small Anim. Pract. 2023, 64, 177–254. [Google Scholar] [CrossRef]
- Rioja Garcia, E. Local Anesthetics. In Veterinary Anesthesia and Analgesia; Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A., Robertson, S.A., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 332–354. ISBN 978-1-118-52623-1. [Google Scholar]
- Garbin, M.; Benito, J.; Ruel, H.L.M.; Watanabe, R.; Monteiro, B.P.; Cagnardi, P.; Steagall, P.V. Pharmacokinetics of Bupivacaine Following Administration by an Ultrasound-Guided Transversus Abdominis Plane Block in Cats Undergoing Ovariohysterectomy. Pharmaceutics 2022, 14, 1548. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.E.; Portela, D.A. Needles for neuraxial and peripheral nerve blocks. In Manual of Small Animal Regional Anesthesia: Illustrated Anatomy for Nerve Stimulation and Ultrasound-Guided Nerve Blocks; Otero, P.E., Portela, D.A., Eds.; Editorial Inter-Medica: Buenos Aires, Argentina, 2019; Volume 1, pp. 37–46. ISBN 978-950-555-465-2. [Google Scholar]
- Portela, D.A.; Verdier, N.; Otero, P.E. Regional anesthetic techniques for the pelvic limb and abdominal wall in small animals: A review of the literature and technique description. Vet. J. 2018, 238, 27–40. [Google Scholar] [CrossRef]
- Blanco, R. 271. Tap block under ultrasound guidance: The description of a “no pops” technique. Reg. Anesth. Pain Med. 2007, 32, 130. [Google Scholar] [CrossRef]
- Schroeder, C.A.; Snyder, L.B.C.; Tearney, C.C.; Baker-Herman, T.L.; Schroeder, K.M. Ultrasound-guided transversus abdominis plane block in the dog: An anatomical evaluation. Vet. Anaesth. Analg. 2011, 38, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, H.; El-Boghdadly, K.; Barrington, M. Quadratus Lumborum Block. Anesthesiology 2019, 130, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Garbin, M.; Portela, D.A.; Bertolizio, G.; Garcia-Pereira, F.; Gallastegui, A.; Otero, P.E. Description of ultrasound-guided quadratus lumborum block technique and evaluation of injectate spread in canine cadavers. Vet. Anaesth. Analg. 2020, 47, 249–258. [Google Scholar] [CrossRef]
- Raymond, S.A.; Steffensen, S.C.; Gugino, L.D.; Strichartz, G.R. The role of length of nerve exposed to local anesthetics in impulse blocking action. Anesth. Analg. 1989, 68, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.E.; Portela, D.A. Manual of Small Animal Regional Anesthesia: Illustrated Anatomy for Nerve Stimulation and Ultrasound-Guided Nerve Blocks, 2nd ed.; Fuensalida, S.E., Romano, M., Eds.; Editorial Inter-Medica: Buenos Aires, Argentina, 2019; ISBN 978-950-555-465-2. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polo-Paredes, G.; Soler, M.; Gil, F.; Laredo, F.G.; Agut, A.; Carrillo-Flores, S.; Belda, E. Description and Evaluation of Dye and Contrast Media Distribution of Ultrasound-Guided Rectus Sheath Block in Cat Cadavers. Animals 2024, 14, 1743. https://doi.org/10.3390/ani14121743
Polo-Paredes G, Soler M, Gil F, Laredo FG, Agut A, Carrillo-Flores S, Belda E. Description and Evaluation of Dye and Contrast Media Distribution of Ultrasound-Guided Rectus Sheath Block in Cat Cadavers. Animals. 2024; 14(12):1743. https://doi.org/10.3390/ani14121743
Chicago/Turabian StylePolo-Paredes, Gonzalo, Marta Soler, Francisco Gil, Francisco G. Laredo, Amalia Agut, Sara Carrillo-Flores, and Eliseo Belda. 2024. "Description and Evaluation of Dye and Contrast Media Distribution of Ultrasound-Guided Rectus Sheath Block in Cat Cadavers" Animals 14, no. 12: 1743. https://doi.org/10.3390/ani14121743
APA StylePolo-Paredes, G., Soler, M., Gil, F., Laredo, F. G., Agut, A., Carrillo-Flores, S., & Belda, E. (2024). Description and Evaluation of Dye and Contrast Media Distribution of Ultrasound-Guided Rectus Sheath Block in Cat Cadavers. Animals, 14(12), 1743. https://doi.org/10.3390/ani14121743








