Duration of Protection and Humoral Immune Response in Nile Tilapia (Oreochromis niloticus L.) Vaccinated against Streptococcus agalactiae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Fish
2.3. Vaccination Trial
2.4. Blood Sampling
2.5. Experimental S. agalactiae Challenge
2.6. Evaluation of Humoral Immune Response by ELISA
2.6.1. Coating Antigen Preparation
2.6.2. Development of Indirect ELISA Assay
2.7. Statistical Analysis
3. Results
3.1. Duration of Vaccine Protection against S. agalactiae
3.2. Detection of Antibody Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Towards Blue Transformation. State World Fish. Aquacult; Food and Agriculture Organization: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- IBGE. Produção da Pecuária Municipal 2022. Rio de Janeiro. 2023. Available online: https://biblioteca.ibge.gov.br/index.php/bibliotecacatalogo?view=detalhes&id=784 (accessed on 6 November 2023).
- Mian, G.F.; Godoy, D.T.; Leal, C.A.G.; Yuhara, T.Y.; Costa, G.M.; Figueiredo, H.C.P. Aspects of the natural history and virulence of S. agalactiae infection in Nile tilapia. Vet. Microbiol. 2009, 136, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Delphino, M.K.V.C.; Leal, C.A.G.; Gardner, I.A.; Assis, G.B.N.; Roriz, G.D.; Ferreira, F.; Figueiredo, H.C.P.; Gonçalves, V.S.P. Seasonal dynamics of bacterial pathogens of Nile tilapia farmed in a Brazilian reservoir. Aquaculture 2019, 498, 100–108. [Google Scholar] [CrossRef]
- Oliveira, T.F.; Queiroz, G.A.; Teixeira, J.P.; Figueiredo, H.C.P.; Leal, C.A.G. Recurrent Streptoccoccus agalactiae infection in Nile tilapia (Oreochromis niloticus) treated with florfenicol. Aquaculture 2018, 493, 51–60. [Google Scholar] [CrossRef]
- Caputo, A.; Bondad-Reantaso, M.G.; Karunasagar, I.; Hao, B.; Gaunt, P.; Verner-Jeffreys, D.; Fridman, S.; Dorado-Garcia, A. Antimicrobial resistance in aquaculture: A global analysis of literature and national action plans. Rev. Aquac. 2023, 15, 568–578. [Google Scholar] [CrossRef]
- Delphino, M.K.V.C.; Barone, R.S.C.; Leal, C.A.G.; Figueiredo, H.C.P.; Gardner, I.A.; Gonçalves, V.S.P. Economic appraisal of vaccination against Streptoccocus agalactiae in Nile tilapia farms in Brazil. Prev. Vet. Med. 2019, 162, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Maulu, S.; Hasimuna, O.J.; Mphande, J.; Munang’andu, H.M. Prevention and control of streptococcosis in tilapia culture: A systematic review. J. Aquat. Anim. Health 2021, 33, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Quagrainie, K.; Hishamunda, N. Social and Economic Performance of Tilapia Farming in Africa; FAO Fisheries and Aquaculture Circular no 1130; Food and Agriculture Organization: Rome, Italy, 2017; Available online: https://www.fao.org/3/i7258en/I7258EN.pdf (accessed on 20 July 2023).
- Liu, J.; Li, Z.; Li, X.; Wang, Y. On-Farm Feed Management Practices for Nile Tilapia in Southern China; FAO Fisheries and Aquaculture Technical Paper; Food and Agriculture Organization: Rome, Italy, 2013; Volume 583, pp. 71–99. [Google Scholar]
- Barroso, R.; Muñoz, A.E.; Cai, J. Social and economic performance of tilapia farming in Brazil. FAO Fisheries and Aquaculture Circular no 1181; Food and Agriculture Organization: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- PeixeBR. Anuário Brasileiro da Piscicultura PEIXE. Br. São Paulo, Brazil. 2023. Available online: https://www.peixebr.com.br/anuario/ (accessed on 20 July 2023).
- Liu, G.; Zhu, J.; Chen, K.; Gao, T.; Yao, H.; Liu, Y.; Zhang, W.; Lu, C. Development of Streptococcus agalactiae vaccines for tilapia. Dis. Aquat. Organ. 2016, 122, 163–170. [Google Scholar] [CrossRef]
- Eldar, A.; Horovitcz, A.; Bercovier, H. Development and efficacy of a vaccine against Streptococcus iniae infection in farmed rainbow trout. Vet. Immunol. Immunopathol. 1997, 56, 175–183. [Google Scholar] [CrossRef]
- Pasnik, D.J.; Evans, J.J.; Klesius, P.H. Duration of protective antibodies and correlation with survival in Nile tilapia Oreochromis niloticus following Streptococcus agalactiae vaccination. Dis. Aquat. Organ. 2005, 66, 129–134. [Google Scholar] [CrossRef]
- Brasil, S. Tilapicultura Cresce com Sanidade e Adoção de Novas Tecnologias. Revista Seafood Brasil. São Paulo, Brazil. 2020. Available online: http://seafoodbrasil.com.br/tilapicultura-cresce-com-sanidade-e-adocao-de-novas-784tecnologias (accessed on 15 January 2023).
- Wendover, N.; Aguirre, M.; Zanolo, R.; Cericato, L.; Wardle, R. Streptococcus in tilapia: Implications for vaccine development and field experiences from Asia. In Bacterial Disease in Warmwater Fish: New Strategies for Sustainable Control; MSD Animal Health Global Aquaculture Advocate (Ed.), New Hampshire, USA. 2011, pp. 1–44. Available online: https://thefishsite.com/articles/streptococcus-in-tilapia-implications-for-vaccine-development-field-experiences-from-asia (accessed on 20 July 2023).
- Rocha, I.S.F.; Freitas, P.V.D.; Oliveira, R.P.C.; Teodoro, A.G.; Iskandar, G.R.; Neto, C.M.S. Análise de ângulos e pontos para vacinação de tilápias-do-Nilo. Vet. Zootec. 2023, 30, 1–8. [Google Scholar] [CrossRef]
- Gallage, S.; Katagiri, T.; Endo, M.; Maita, M. Comprehensive evaluation of immunomodulation by moderate hypoxia in S. agalactiae vaccinated Nile tilapia. Fish Shellfish Immunol. 2017, 66, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, D.Q.; Jiang, B.; Mo, X.B.; Du, J.J.; Li, A.X. Influence of temperature on the vaccine efficacy against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 521, 734943. [Google Scholar] [CrossRef]
- Arguello, H.; Carvajal, A.; Naharro, G.; Rubio, P. Evaluation of protection conferred by a Salmonella Typhimurium inactivated vaccine in Salmonella-infected finishing pig farms. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.A.; Amin, Z.; Rodrigues, K.F.; Saallah, S.; Shaarani, S.M.; Sarker, S.; Siddiquee, S. Infectious bronchitis virus (Gammacoronavirus) in poultry farming: Vaccination, immune response and measures for mitigation. Vet. Sci. 2021, 8, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, A.; Garvey, M. A review of diagnostic tests recommended by the World Organisation for Animal Health Manual of Diagnostic Tests and vaccines for Terrestrial Animals. OIE Rev. Sci. Tech. 2021, 40, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, D.; Peeler, E.J.; Laurin, E.; Gardner, I.A.; Whittington, R.J. Serology in finfish for diagnosis, surveillance, and research: A systematic review. J. Aquat. Anim. Health. 2017, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kahieshesfandiari, M.; Sabri, M.Y.; Ina-Salwany, M.Y.; Hassan, M.D.; Noraini, O.; Ajadi, A.A.; Isiaku, A.I. Streptococcosis in Oreochromis sp.: Is feed-based biofilm vaccine of Streptococcus agalactiae effective? Aquac. Int. 2019, 27, 817–832. [Google Scholar] [CrossRef]
- Linh, N.V.; Dien, L.T.; Sangpo, P.; Senapin, S.; Thapinta, A.; Panphut, W.; St-Hilaire, S.; Rodkhum, C.; Dong, H.T. Pre-treatment of Nile tilapia (Oreochromis niloticus) with ozone nanobubbles improve efficacy of heat-killed Streptococcus agalactiae immersion vaccine. Fish Shellfish Immunol. 2022, 123, 229–237. [Google Scholar] [CrossRef]
- Ma, Y.; Hao, L.; Liang, Z.; Ma, J.; Ke, H.; Kang, H.; Yang, H.; Wu, J.; Feng, G.; Liu, Z. Characterization of novel antigenic vaccine candidates for Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae infection. Fish Shellfish Immunol. 2020, 105, 405–414. [Google Scholar] [CrossRef]
- Nurani, F.S.; Sukenda, S.; Nuryati, S. Maternal immunity of tilapia broodstock vaccinated with polyvalent vaccine and resistance of their offspring against Streptococcus agalactiae. Aquac Res. 2020, 51, 1513–1522. [Google Scholar] [CrossRef]
- Ramos-Espinoza, F.C.; Cueva-Quiroz, V.A.; Yunis-Aguinaga, J.; de Moraes, J.R.E. A comparison of novel inactivation methods for production of a vaccine against Streptococcus agalactiae in Nile tilapia Oreochromis niloticus. Aquaculture 2020, 528, 735484. [Google Scholar] [CrossRef]
- Ross, L.G.; Ross, B. Anaesthetic and Sedative Techniques for Aquatic Animals, 3rd ed.; Blackwell Publishing: Oxford, UK, 2008; pp. 1–222. [Google Scholar] [CrossRef]
- Poyart, C.; Tazi, A.; Réglier-Poupet, H.; Billoët, A.; Tavares, N.; Raymond, J.; Trieu-Cuot, P. Multiplex PCR assay for rapid and accurate capsular typing of group B streptococci. J. Clin. Microbiol. 2007, 45, 1985–1988. [Google Scholar] [CrossRef] [PubMed]
- Reed; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Amend, D.F. Potency testing of fish vaccines. Dev. Biol. Stand. 1982, 49, 447–452. [Google Scholar]
- Leal, C.A.G.; Carvalho-Castro, G.A.; Sacchetin, P.S.C.; Lopes, C.O.; Moraes, A.M.; Figueiredo, H.C.P. Oral and parenteral vaccines against Flavobacterium columnare: Evaluation of humoral immune response by ELISA and in vivo efficiency in Nile tilapia (Oreochromis niloticus). Aquacult. Int. 2010, 18, 657–666. [Google Scholar] [CrossRef]
- Romstad, A.B.; Reitan, L.J.; Midtlyng, P.; Gravningen, K.; Emilsen, V.; Evensen, Ø. Comparison of a serological potency assay for furunculosis vaccines (Aeromonas salmonicida subsp. salmonicida) to intraperitoneal challenge in Atlantic salmon (Salmo salar L.). Biologicals 2014, 42, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, R.; Li, L.P.; Liang, W.W.; Li, J.; Huang, Y.; Lei, A.Y.; Huang, W.Y.; Gan, X. Screening vaccine candidate strains against Streptococcus agalactiae of tilapia based on PFGE genotype. Vaccine 2012, 30, 6088–6092. [Google Scholar] [CrossRef]
- Li, L.P.; Wang, R.; Liang, W.W.; Huang, T.; Huang, Y.; Luo, F.G.; Lei, A.Y.; Chen, M.; Gan, X. Development of live attenuated Streptococcus agalactiae vaccine for tilapia via continuous passage in vitro. Fish Shellfish Immunol. 2015, 45, 955–963. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef]
- Wangkaghart, E.; Deville, S.; Wang, B.; Srisapoome, P.; Wang, T.; Secombes, C.J. Immune response and protective efficacy of two new adjuvants, MontanideTM ISA 763B VG and MontanideTM GEL02, administered with a Streptococcus agalactiae ghost vaccine in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 116, 19–29. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Evensen, Ø. Correlates of protective immunity for fish vaccines. Fish Shellfish Immunol. 2019, 85, 132–140. [Google Scholar] [CrossRef]
- Bricknell, I.R.; King, J.A.; Bowden, T.J.; Ellis, A.E. Duration of protective antibodies, and the correlation with protection in Atlantic salmon (Salmo salar L.), following vaccination with an Aeromonas salmonicida vaccine containing iron-regulated outer membrane proteins and secretory polysaccharide. Fish Shellfish Immunol. 1999, 9, 139–151. [Google Scholar] [CrossRef]
- Villumsen, K.R.; Dalsgaard, I.; Holten-Andersen, L.; Raida, M.K. Potential Role of Specific Antibodies as Important Vaccine Induced Protective Mechanism against Aeromonas salmonicida in Rainbow Trout. PLoS ONE. 2012, 7, e46733. [Google Scholar] [CrossRef] [PubMed]
- Raida, M.K.; Nylén, J.; Holten-Andersen, L.; Buchmann, K. Association between plasma antibody response and protection in rainbow trout Oncorhynchus mykiss immersion vaccinated against Yersinia ruckeri. PLoS ONE. 2011, 6, e18832. [Google Scholar] [CrossRef]
- Pasnik, D.J.; Evans, J.J.; Klesius, P.H. Passive immunization of Nile tilapia (Oreochromis niloticus) provides significant protection against Streptococcus agalactiae. Fish Shellfish Immunol. 2006, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, Ø. Acquired immunity and vaccination against infectious pancreatic necrosis virus of salmon. Dev. Comp. Immunol. 2014, 43, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Mu, L.; Fu, S.; Wu, L.; Han, K.; Wu, H.; Bian, X.; Wei, X.; Guo, Z.; Wang, A.; et al. Expression and characterization of Nile tilapia (Oreochromis niloticus) secretory and membrane-bound IgM in response to bacterial infection. Aquaculture 2019, 508, 214–222. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Kong, W.; Yin, Y.X.; Dong, F.; Huang, Z.Y.; Yin, G.M.; Dong, S.; Salinas, I.; Zhang, Y.A.; Xu, Z. Mucosal immunoglobulins protect the olfactory organ of teleost fish against parasitic infection. PLOS Pathog. 2018, 14, e1007251. [Google Scholar] [CrossRef]
- Bilal, S.; Etayo, A.; Hordvik, I. Immunoglobulins in teleosts. Immunogenetics 2021, 73, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I.; Parra, D. Fish mucosal immunity: Intestine. In Mucosal Health in Aquaculture; Beck, B.H., Peatman, E., Eds.; Elsevier Inc.: New York, NY, USA, 2015; pp. 135–170. [Google Scholar] [CrossRef]
- Velázquez, J.; Acosta, J.; Lugo, J.M.; Reyes, E.; Herrera, F.; González, O.; Morales, A.; Carpio, Y.; Estrada, M.P. Discovery of immunoglobulin T in Nile tilapia (Oreochromis niloticus): A potential molecular marker to understand mucosal immunity in this species. Dev. Comp. Immunol. 2018, 88, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, P.; Wu, Z.H.; Lu, Y.S.; Wang, Z.L.; Jian, J.C. Molecular cloning and expression analysis of IgD in Nile tilapia (Oreochromis niloticus) in response to Streptococcus agalactiae stimulus. Int. J. Mol. Sci. 2016, 17, 348. [Google Scholar] [CrossRef]
- Midtlyng, P.J. Methods for measuring efficacy, safety and potency of fish vaccines. In Fish Vaccines; Adams, A., Ed.; Springer: Basel, Switzerland, 2016; pp. 119–141. [Google Scholar] [CrossRef]
- Mai, T.T.; Kayansamruaj, P.; Taengphu, S.; Senapin, S.; Costa, J.Z.; del-Pozo, J.; Thompson, K.D.; Rodkhum, C.; Dong, H.T. Efficacy of heat-killed and formalin-killed vaccines against Tilapia tilapinevirus in juvenile Nile tilapia (Oreochromis niloticus). J. Fish Dis. 2021, 44, 2097–2109. [Google Scholar] [CrossRef] [PubMed]

| Experimental Group | Days Post-Vaccination (dpv) | No. Fish Challenged | Cumulative Mortality (%) | RPS (%) | p-Value * |
|---|---|---|---|---|---|
| NonVac | 15 | 20 | 85 a | 71 | 0.0003 |
| Vac | 15 | 20 | 25 b | ||
| NonVac | 30 | 20 | 70 a | 93 | ˂0.0001 |
| Vac | 30 | 20 | 5 b | ||
| NonVac | 150 | 20 | 80 a | 94 | ˂0.0001 |
| Vac | 150 | 20 | 5 b | ||
| NonVac | 180 | 20 | 65 a | 70 | 0.0095 |
| Vac | 180 | 20 | 20 b | ||
| NonVac | 210 | 20 | 70 a | 86 | 0.0002 |
| Vac | 210 | 20 | 10 b | ||
| NonVac | 300 | 20 | 90 a | 67 | 0.0002 |
| Vac | 300 | 20 | 30 b |
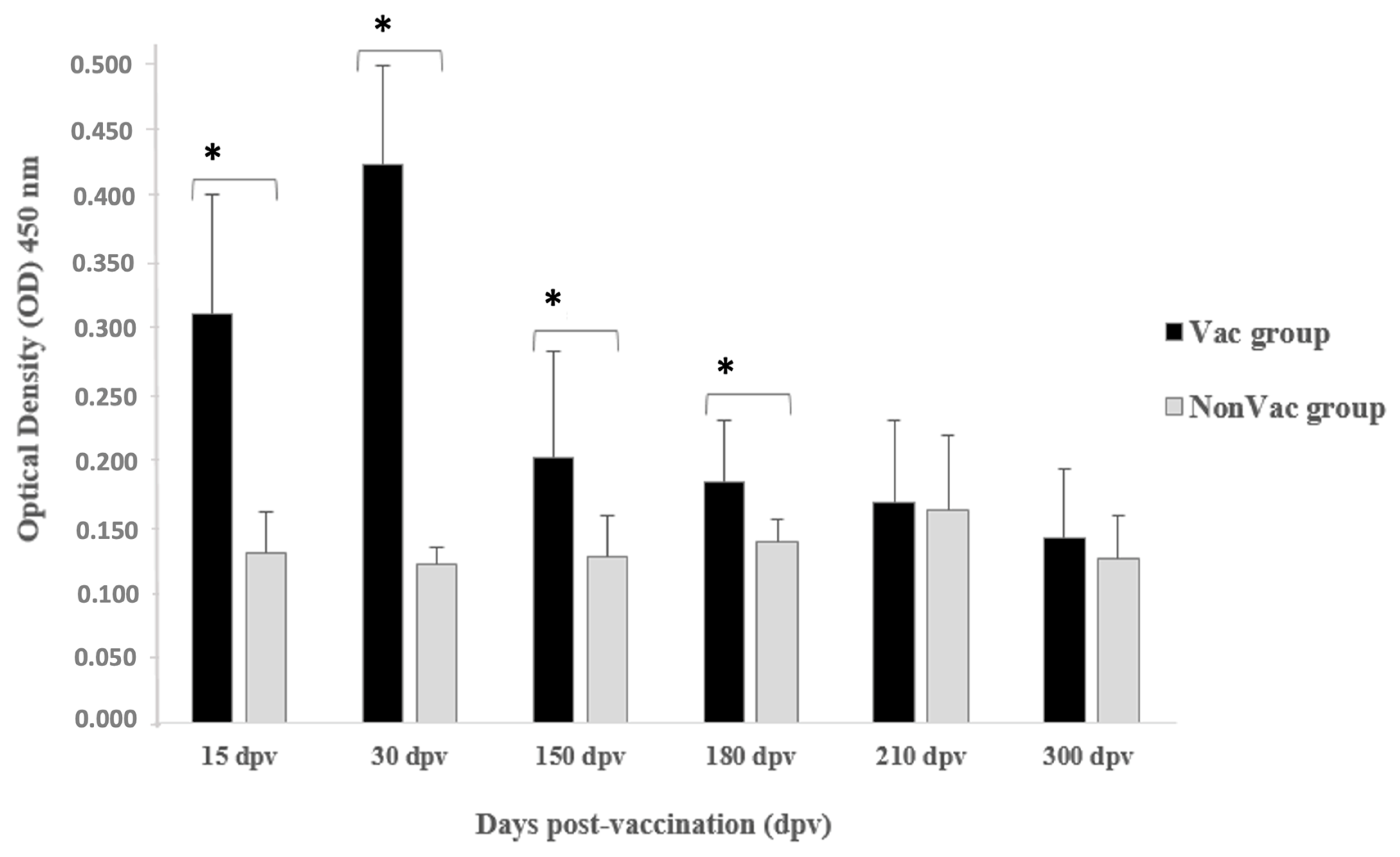
| Experimental Group | Days Post-Vaccination (dpv) | OD450 Pre-Challenge (Mean ± SD) | IC95% * | p-Value ** |
|---|---|---|---|---|
| NonVac | 15 | 0.129 ± 0.031 | 0.106–0.151 | <0.0001 |
| Vac | 15 | 0.311 ± 0.090 | 0.246–0.375 | |
| NonVac | 30 | 0.120 ± 0.013 | 0.110–0.129 | <0.0001 |
| Vac | 30 | 0.423 ± 0.075 | 0.369–0.477 | |
| NonVac | 150 | 0.126 ± 0.031 | 0.103–0.149 | 0.0142 |
| Vac | 150 | 0.201 ± 0.081 | 0.143–0.260 | |
| NonVac | 180 | 0.138 ± 0.017 | 0.125–0.150 | 0.0119 |
| Vac | 180 | 0.183 ± 0.047 | 0.149–0.217 | |
| NonVac | 210 | 0.162 ± 0.056 | 0.122–0.202 | 0.8501 |
| Vac | 210 | 0.167 ± 0.062 | 0.122–0.212 | |
| NonVac | 300 | 0.124 ± 0.033 | 0.100–0.148 | 0.4223 |
| Vac | 300 | 0.140 ± 0.052 | 0.103–0.177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiróz, G.A.d.; Silva, T.M.F.e.; Leal, C.A.G. Duration of Protection and Humoral Immune Response in Nile Tilapia (Oreochromis niloticus L.) Vaccinated against Streptococcus agalactiae. Animals 2024, 14, 1744. https://doi.org/10.3390/ani14121744
Queiróz GAd, Silva TMFe, Leal CAG. Duration of Protection and Humoral Immune Response in Nile Tilapia (Oreochromis niloticus L.) Vaccinated against Streptococcus agalactiae. Animals. 2024; 14(12):1744. https://doi.org/10.3390/ani14121744
Chicago/Turabian StyleQueiróz, Guilherme Alves de, Tarcísio Martins França e Silva, and Carlos Augusto Gomes Leal. 2024. "Duration of Protection and Humoral Immune Response in Nile Tilapia (Oreochromis niloticus L.) Vaccinated against Streptococcus agalactiae" Animals 14, no. 12: 1744. https://doi.org/10.3390/ani14121744






