Phenotypic Identification, Genetic Characterization, and Selective Signal Detection of Huitang Duck
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Traits Measurements
2.2.2. Whole-Genome Resequencing
2.2.3. Data Pre-Processing
2.2.4. Population Structure Analysis
2.2.5. Selective-Sweep Analysis
2.2.6. Functional Enrichment Analysis
3. Results
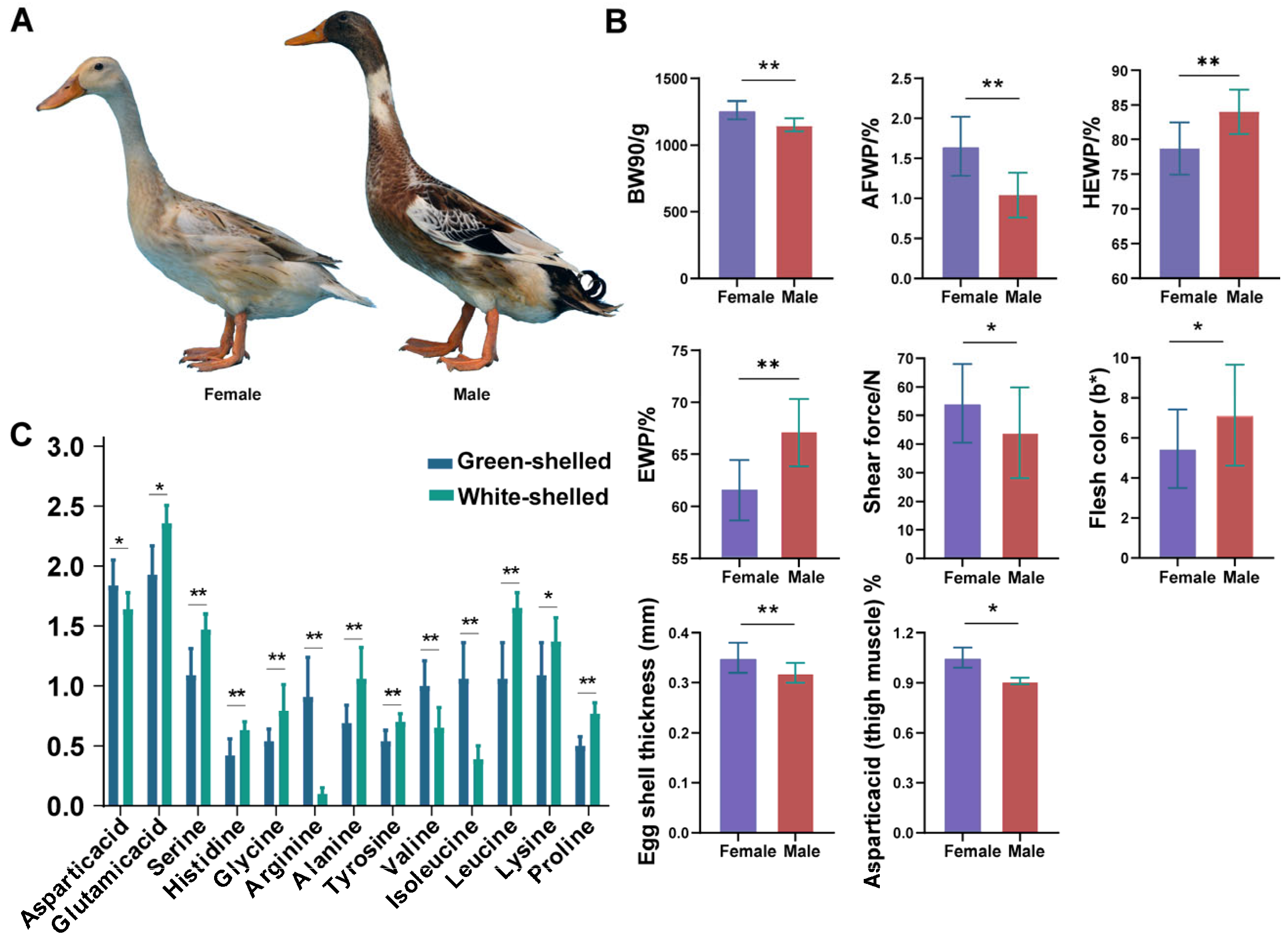
3.1. Summary of HT Traits
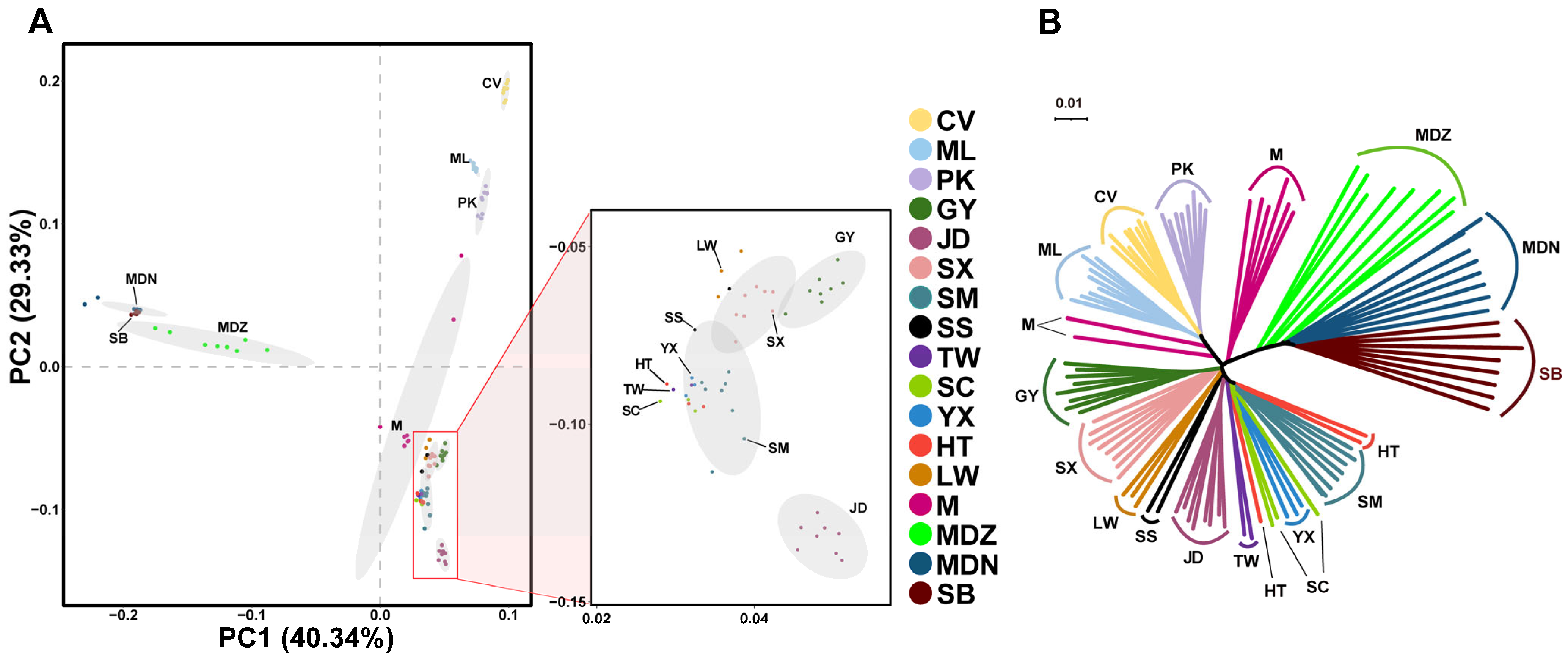
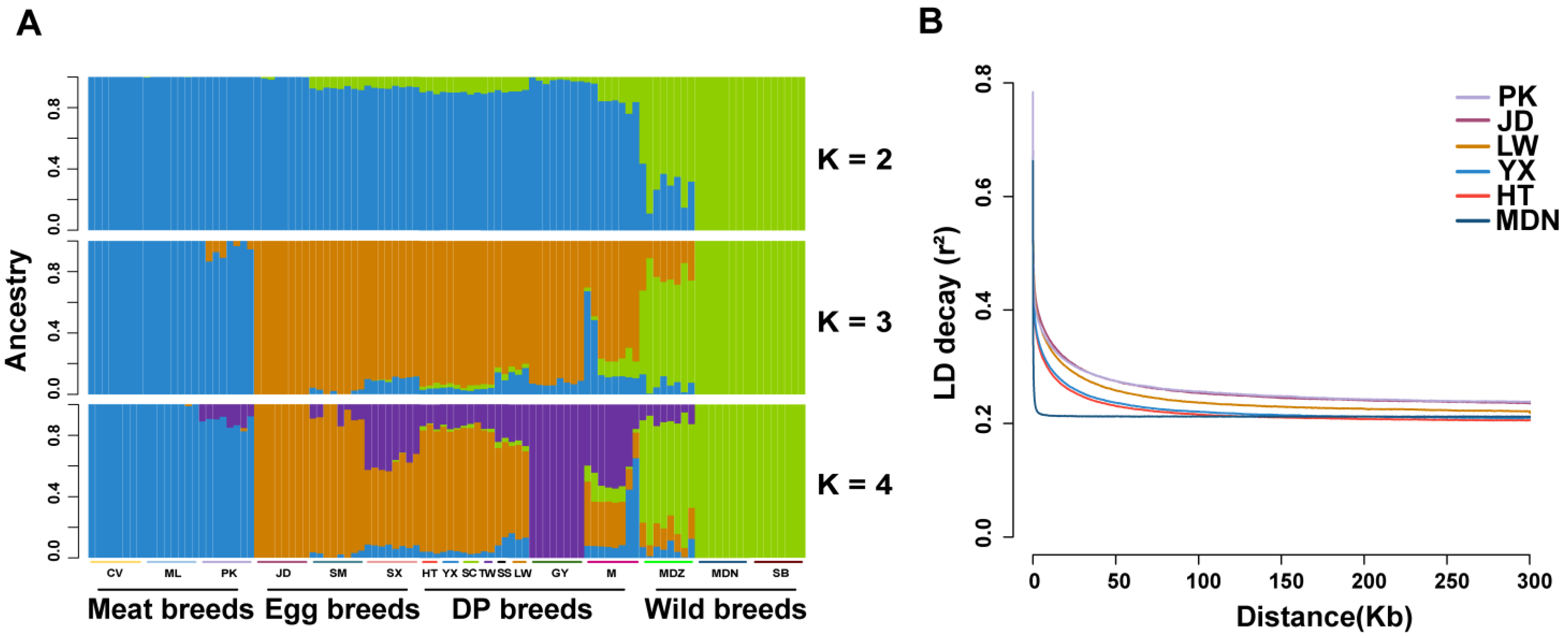
3.2. Population Structure Analysis
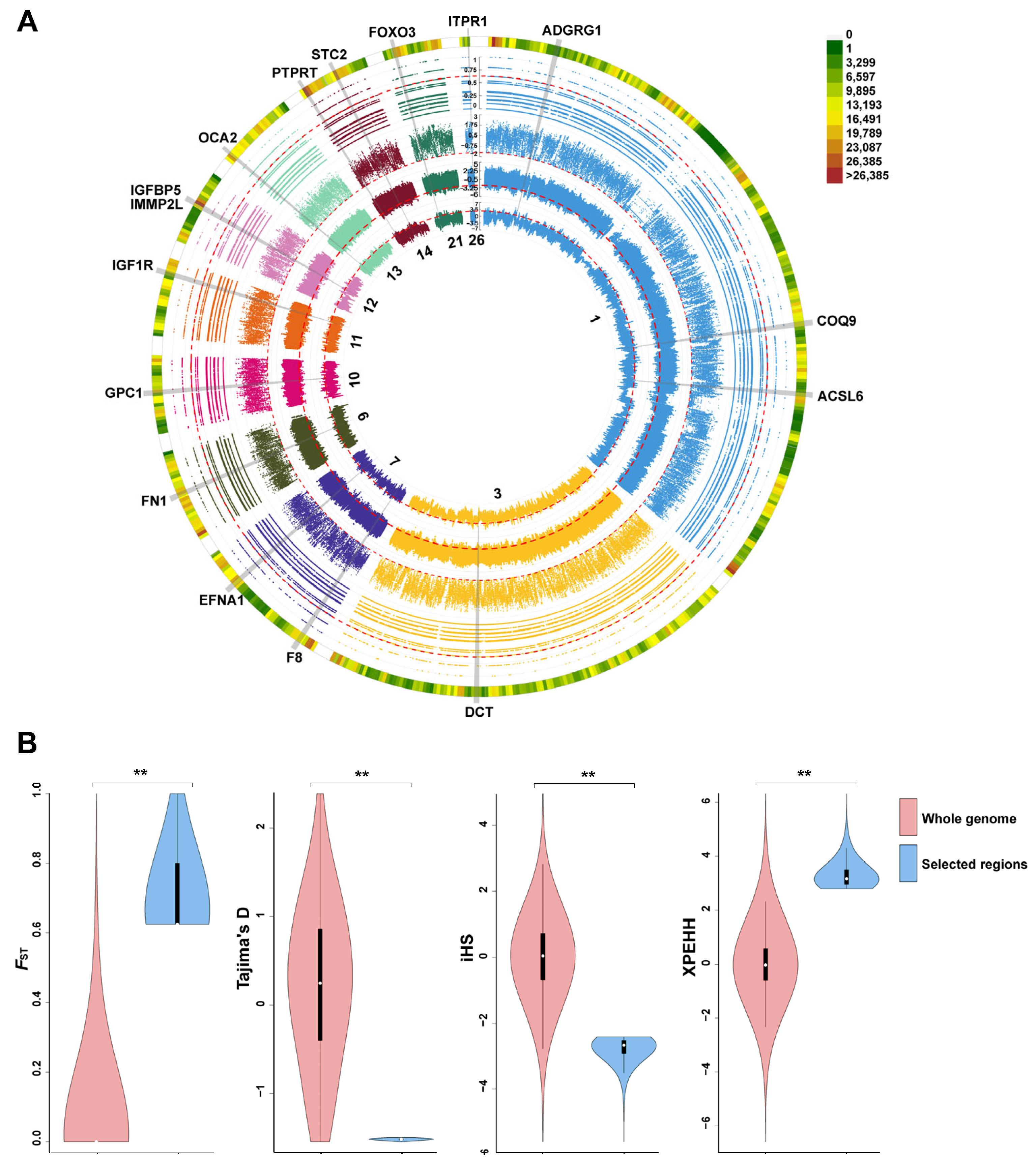
3.3. Genome-Wide Selective Sweep Analysis
3.4. Detection of Candidate Divergent Regions between HT and YX
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fanatico, A.C.; Cavitt, L.C.; Pillai, P.B.; Emmert, J.L.; Owens, C.M. Evaluation of Slower-Growing Broiler Genotypes Grown with and without Outdoor Access: Meat Quality. Poult. Sci. 2005, 84, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.H.; Shi, S.R.; Dou, T.C.; Sun, H.J. Effect of a Free-Range Raising System on Growth Performance, Carcass Yield, and Meat Quality of Slow-Growing Chicken. Poult. Sci. 2009, 88, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, W.; Tan, H.Z.; Xu, G.F.; Zhang, X.B.; Wei, S.; Wang, X.Q. Effects of Outdoor Access on Growth Performance, Carcass Composition, and Meat Characteristics of Broiler Chickens. Poult. Sci. 2013, 92, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Weng, K.; Gu, T.; Luo, X.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G. Effects of Integrated Rice-Duck Farming System on Duck Carcass Traits, Meat Quality, Amino Acid, and Fatty Acid Composition. Poult. Sci. 2021, 100, 101107. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, G.; Kebreab, E.; Li, J.; Feng, X.; Li, C.; Zhang, X.; Huang, X.; Fang, C.; Fang, R.; et al. 1H NMR-Based Metabolomics Study of Breast Meat from Pekin and Linwu Duck of Different Ages and Relation to Meat Quality. Food Res. Int. 2020, 133, 109126. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, C.; He, J.; Dai, Q.; Fang, R. Comparison of the Meat Metabolite Composition of Linwu and Pekin Ducks Using 600 MHz 1H Nuclear Magnetic Resonance Spectroscopy. Poult. Sci. 2017, 96, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.-P.; Hu, Y.-Q.; Li, Y.-B.; Huang, Z.-B.; Li, A. Polymorphisms of the Prolactin Gene and Their Association with Egg Production Traits in Two Chinese Domestic Ducks. Br. Poult. Sci. 2019, 60, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.Q.; Li, A.; Lan, J.J.; Wang, Y.M.; Yan, M.J.; Lian, S.Y.; Wu, X. Study of Formation of Green Eggshell Color in Ducks through Global Gene Expression. PLoS ONE 2018, 13, e0191564. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xiong, X.; Jiang, X.; Du, H.; Li, Q.; Liu, H.; Gan, W.; Yu, C.; Peng, H.; Xia, B.; et al. Novel miRNA Identification and Comparative Profiling of miRNA Regulations Revealed Important Pathways in Jinding Duck Ovaries by Small RNA Sequencing. 3 Biotech 2020, 10, 38. [Google Scholar] [CrossRef]
- Xin, Q.; Li, L.; Zhao, B.; Shi, W.; Hao, X.; Zhang, L.; Miao, Z.; Zhu, Z.; Huang, Q.; Zheng, N. The Network Regulation Mechanism of the Effects of Heat Stress on the Production Performance and Egg Quality of Jinding Duck Was Analyzed by miRNA–mRNA. Poult. Sci. 2024, 103, 103255. [Google Scholar] [CrossRef]
- Gu, H.; Zhu, T.; Li, X.; Chen, Y.; Wang, L.; Lv, X.; Yang, W.; Jia, Y.; Jiang, Z.; Qu, L. A Joint Analysis Strategy Reveals Genetic Changes Associated with Artificial Selection between Egg-Type and Meat-Type Ducks. Anim. Genet. 2020, 51, 890–898. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.; Heo, K.-N.; Kwon, K.; Moon, Y.; Lim, D.; Lee, K.-T.; Kim, J. Population Analysis of the Korean Native Duck Using Whole-Genome Sequencing Data. BMC Genom. 2020, 21, 554. [Google Scholar] [CrossRef]
- Jiang, F.; Lin, R.; Xiao, C.; Xie, T.; Jiang, Y.; Chen, J.; Ni, P.; Sung, W.-K.; Han, J.; Du, X.; et al. Analysis of Whole-Genome Re-Sequencing Data of Ducks Reveals a Diverse Demographic History and Extensive Gene Flow between Southeast/South Asian and Chinese Populations. Genet. Sel. Evol. 2021, 53, 35. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luo, K.; Wang, C.; Xuan, R.; Zheng, S.; Tang, H.; Li, Y.; Xiong, Y.; Wu, Y.; Wang, L.; et al. Genomic Characteristics and Selection Signals of Zhongshan Ducks. Animal 2023, 17, 100797. [Google Scholar] [CrossRef]
- Li, L.; Quan, J.; Gao, C.; Liu, H.; Yu, H.; Chen, H.; Xia, C.; Zhao, S. Whole-Genome Resequencing to Unveil Genetic Characteristics and Selection Signatures of Specific Pathogen-Free Ducks. Poult. Sci. 2023, 102, 102748. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Zhu, F.; Yin, Z.-T.; Wang, Z.; Smith, J.; Zhang, F.; Martin, F.; Ogeh, D.; Hincke, M.; Lin, F.-B.; Burt, D.W.; et al. Three Chromosome-Level Duck Genome Assemblies Provide Insights into Genomic Variation during Domestication. Nat. Commun. 2021, 12, 5932. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing next-Generation DNA Sequencing Data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The Variant Call Format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Browning, B.L.; Zhou, Y.; Browning, S.R. A One-Penny Imputed Genome from Next-Generation Reference Panels. Am. J. Hum. Genet. 2018, 103, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff: SNPs in the Genome of Drosophila Melanogaster Strain w 1118; Iso-2; Iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast Model-Based Estimation of Ancestry in Unrelated Individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, S.-S.; Xu, J.-Y.; He, W.-M.; Yang, T.-L. PopLDdecay: A Fast and Effective Tool for Linkage Disequilibrium Decay Analysis Based on Variant Call Format Files. Bioinformatics 2019, 35, 1786–1788. [Google Scholar] [CrossRef]
- Szpiech, Z.A. Selscan 2.0: Scanning for Sweeps in Unphased Data. Bioinformatics 2024, 40, btae006. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. rMVP: A Memory-Efficient, Visualization-Enhanced, and Parallel-Accelerated Tool for Genome-Wide Association Study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent Prioritization and Exploratory Visualization of Biological Functions for Gene Enrichment Analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Lyu, S.; Arends, D.; Nassar, M.K.; Weigend, A.; Weigend, S.; Wang, E.; Brockmann, G.A. High-Density Genotyping Reveals Candidate Genomic Regions for Chicken Body Size in Breeds of Asian Origin. Poult. Sci. 2023, 102, 102303. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, Z.; Hu, J.; Guo, Z.; Xu, Y.; Li, Y.; Wang, L.; Fan, W.; Liang, S.; Liu, D.; et al. Genetic Variations for Egg Internal Quality of Ducks Revealed by Genome-Wide Association Study. Anim. Genet. 2021, 52, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Xin, Q.; Zhu, Z.; Miao, Z.; Zheng, N. Metabolomic Characterization of Liancheng White and Cherry Valley Duck Breast Meat and Their Relation to Meat Quality. Poult. Sci. 2023, 102, 103020. [Google Scholar] [CrossRef]
- Weng, K.; Song, L.; Bao, Q.; Cao, Z.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Comparative Characterization of Key Volatile Compounds in Slow- and Fast-Growing Duck Raw Meat Based on Widely Targeted Metabolomics. Foods 2022, 11, 3975. [Google Scholar] [CrossRef] [PubMed]
- Halpern, B.P. Glutamate and the Flavor of Foods. J. Nutr. 2000, 130, 910S–914S. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Chen, K.; Chang, H.; Tang, Q.; Zhang, J. Genetic Diversity and Systematic Evolution of Chinese Domestic Ducks Along the Yangtze-Huai River. Biochem. Genet. 2007, 45, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liu, Y.; Peng, S.; Liu, C.; Lv, T.; Liao, L.; Li, Y.; Wang, Y.; Fan, Z.; Wu, W.; et al. Magnolol Additive Improves Growth Performance of Linwu Ducklings by Modulating Antioxidative Status. PLoS ONE 2021, 16, e0259896. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Cai, Y.; Li, Y.; Li, T.; Zhang, J.; Hu, Z.; Zhang, J. Characterization and Comparative Transcriptomic Analysis of Skeletal Muscle in Female Pekin Duck and Hanzhong Ma Duck during Different Growth Stages Using RNA-Seq. Poult. Sci. 2023, 102, 103122. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X. Integration of Transcriptomics and Non-Targeted Metabolomics Reveals the Underlying Mechanism of Skeletal Muscle Development in Duck during Embryonic Stage. Int. J. Mol. Sci. 2023, 24, 5214. [Google Scholar] [CrossRef]
- Hu, Z.; Cao, J.; Liu, G.; Zhang, H.; Liu, X. Comparative Transcriptome Profiling of Skeletal Muscle from Black Muscovy Duck at Different Growth Stages Using RNA-Seq. Genes 2020, 11, 1228. [Google Scholar] [CrossRef]
- Hu, Z.; Cao, J.; Ge, L.; Zhang, J.; Zhang, H.; Liu, X. Characterization and Comparative Transcriptomic Analysis of Skeletal Muscle in Pekin Duck at Different Growth Stages Using RNA-Seq. Animals 2021, 11, 834. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, G.; Li, T.; Ling, J.; Zhang, X.; Wang, J. Transcriptomic Profile of Leg Muscle during Early Growth in Chicken. PLoS ONE 2017, 12, e0173824. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Ge, K.; Li, M.; Yang, L.; Jin, S.; Zhang, C.; Chen, X.; Geng, Z. Egg-Laying and Brooding Stage-Specific Hormonal Response and Transcriptional Regulation in Pituitary of Muscovy Duck (Cairina moschata). Poult. Sci. 2019, 98, 5287–5296. [Google Scholar] [CrossRef] [PubMed]
- Estermann, M.A.; Hirst, C.E.; Major, A.T.; Smith, C.A. The Homeobox Gene TGIF1 Is Required for Chicken Ovarian Cortical Development and Generation of the Juxtacortical Medulla. Development 2021, 148, dev199646. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ouyang, Q.; Chen, Q.; Song, Y.; Hu, J.; Hu, S.; He, H.; Li, L.; Liu, H.; Wang, J. Molecular Mechanisms of Hypothalamic-Pituitary-Ovarian/Thyroid Axis Regulating Age at First Egg in Geese. Poult. Sci. 2024, 103, 103478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, J.; Sun, G.; Ji, R.; Li, X.; Zhang, X.; Wang, J. Identification of Differentially Expressed Genes and Signaling Pathways in Gaoyou Duck Ovary at Different Physiological Stages. Front. Vet. Sci. 2023, 10, 1190998. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Z.; Qu, Y.; Li, Q.; Tian, Y.; Chen, L.; Tang, J.; Li, C.; Li, G.; Shen, J.; et al. Genome-Wide Association Studies and Haplotype-Sharing Analysis Targeting the Egg Production Traits in Shaoxing Duck. Front. Genet. 2022, 13, 828884. [Google Scholar] [CrossRef] [PubMed]
- Bello, S.F.; Xu, H.; Guo, L.; Li, K.; Zheng, M.; Xu, Y.; Zhang, S.; Bekele, E.J.; Bahareldin, A.A.; Zhu, W.; et al. Hypothalamic and Ovarian Transcriptome Profiling Reveals Potential Candidate Genes in Low and High Egg Production of White Muscovy Ducks (Cairina moschata). Poult. Sci. 2021, 100, 101310. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Huang, H.; Ma, S.; Gan, X.; Zhu, M.; Liu, H.; Li, L.; Wang, J. Akirin2 Could Promote the Proliferation but Not the Differentiation of Duck Myoblasts via the Activation of the mTOR/p70S6K Signaling Pathway. Int. J. Biochem. Cell Biol. 2016, 79, 298–307. [Google Scholar] [CrossRef]
- Chen, R.; Wen, C.; Cheng, Y.; Chen, Y.; Zhuang, S.; Zhou, Y. Effects of Dietary Supplementation with Betaine on Muscle Growth, Muscle Amino Acid Contents and Meat Quality in Cherry Valley Ducks. Anim. Physiol. Nutr. 2019, 103, 1050–1059. [Google Scholar] [CrossRef]
- Ran, M.; Ouyang, Q.; Li, X.; Hu, S.; Hu, B.; Hu, J.; Dong, D.; Li, L.; He, H.; Liu, H.; et al. Exploring Right Ovary Degeneration in Duck and Goose Embryos by Histology and Transcriptome Dynamics Analysis. BMC Genom. 2023, 24, 389. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Deng, X.; Li, L.; Feng, J.; Du, X.; Amevor, F.K.; Tian, Y.; Li, L.; Rao, Y.; Yi, Z.; et al. miR-128-3p Regulates Chicken Granulosa Cell Function via 14-3-3β/FoxO and PPAR-γ/LPL Signaling Pathways. Int. J. Biol. Macromol. 2023, 241, 124654. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Guan, L.; Ge, L.; Liu, G.; Bai, Y.; Liu, X. Nanopore-Based Full-Length Transcriptome Sequencing of Muscovy Duck (Cairina moschata) Ovary. Poult. Sci. 2021, 100, 101246. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.G.; Xia, W.G.; Chen, W.; Abouelezz, K.F.M.; Ruan, D.; Wang, S.; Zhang, Y.N.; Huang, X.B.; Li, K.C.; Zheng, C.T.; et al. Effects of Capsaicin on Laying Performance, Follicle Development, and Ovarian Antioxidant Capacity in Aged Laying Ducks. Poult. Sci. 2021, 100, 100901. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Lin, B.; Yan, Z.; Tong, Y.; Liu, H.; He, X.; Zhang, H. Phenotypic Identification, Genetic Characterization, and Selective Signal Detection of Huitang Duck. Animals 2024, 14, 1747. https://doi.org/10.3390/ani14121747
Ma H, Lin B, Yan Z, Tong Y, Liu H, He X, Zhang H. Phenotypic Identification, Genetic Characterization, and Selective Signal Detection of Huitang Duck. Animals. 2024; 14(12):1747. https://doi.org/10.3390/ani14121747
Chicago/Turabian StyleMa, Haojie, Bingjin Lin, Zhiyao Yan, Yueyue Tong, Huichao Liu, Xi He, and Haihan Zhang. 2024. "Phenotypic Identification, Genetic Characterization, and Selective Signal Detection of Huitang Duck" Animals 14, no. 12: 1747. https://doi.org/10.3390/ani14121747
APA StyleMa, H., Lin, B., Yan, Z., Tong, Y., Liu, H., He, X., & Zhang, H. (2024). Phenotypic Identification, Genetic Characterization, and Selective Signal Detection of Huitang Duck. Animals, 14(12), 1747. https://doi.org/10.3390/ani14121747



