Emergence of a Novel G4P[6] Porcine Rotavirus with Unique Sequence Duplication in NSP5 Gene in China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Viral Genomic RNA Extraction from Faecal Samples
2.2. Amplification and Sequencing of Rotavirus Genes
2.3. Phylogenetic Recombination and Mutation Analyses
2.4. Assignment of Genotypes and Accession Numbers
3. Results
3.1. RT-PCR and Agarose Gel Electrophoresis Analyses
3.2. Whole Genome Acquirement and Similarity Analyses
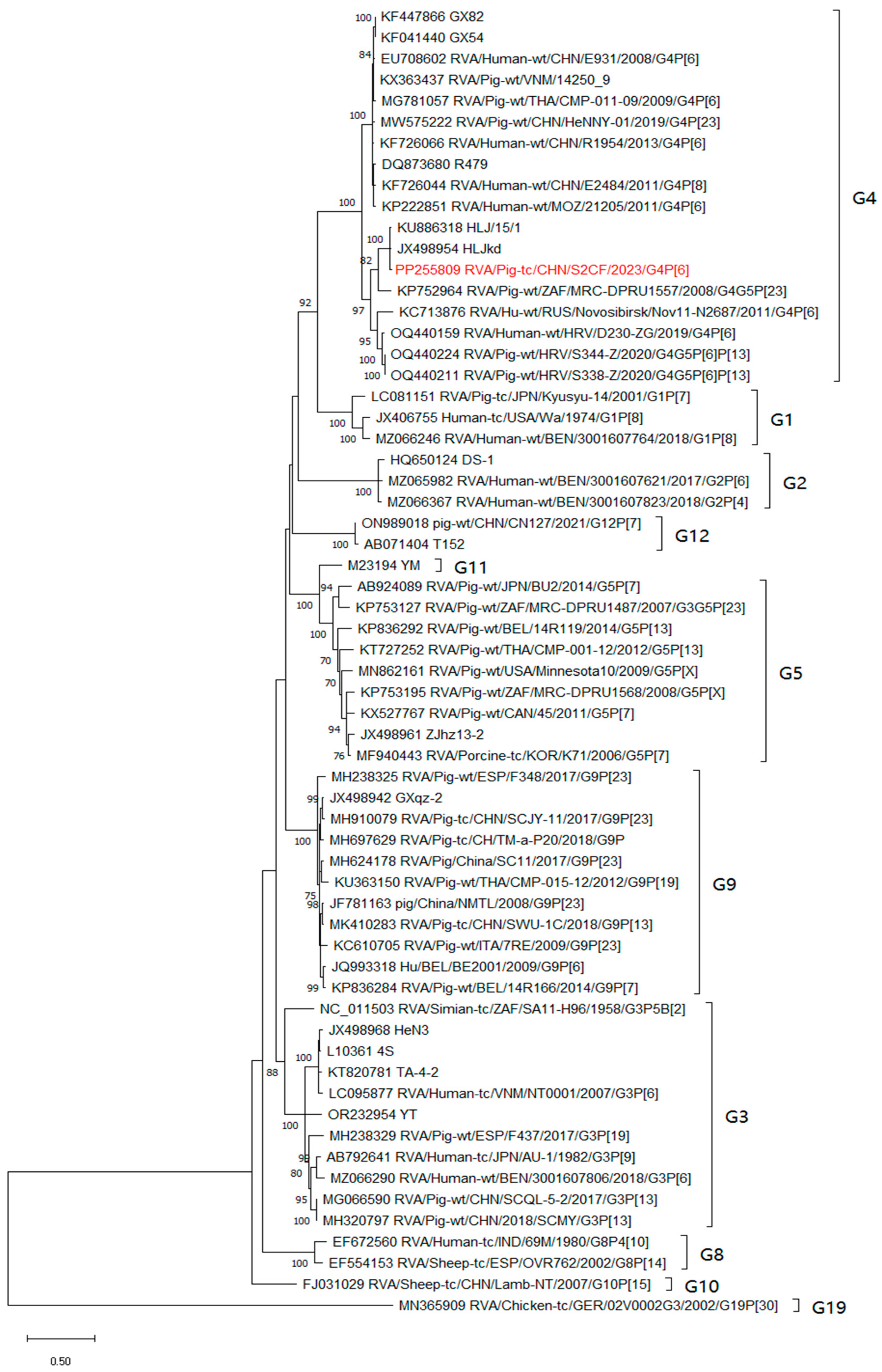
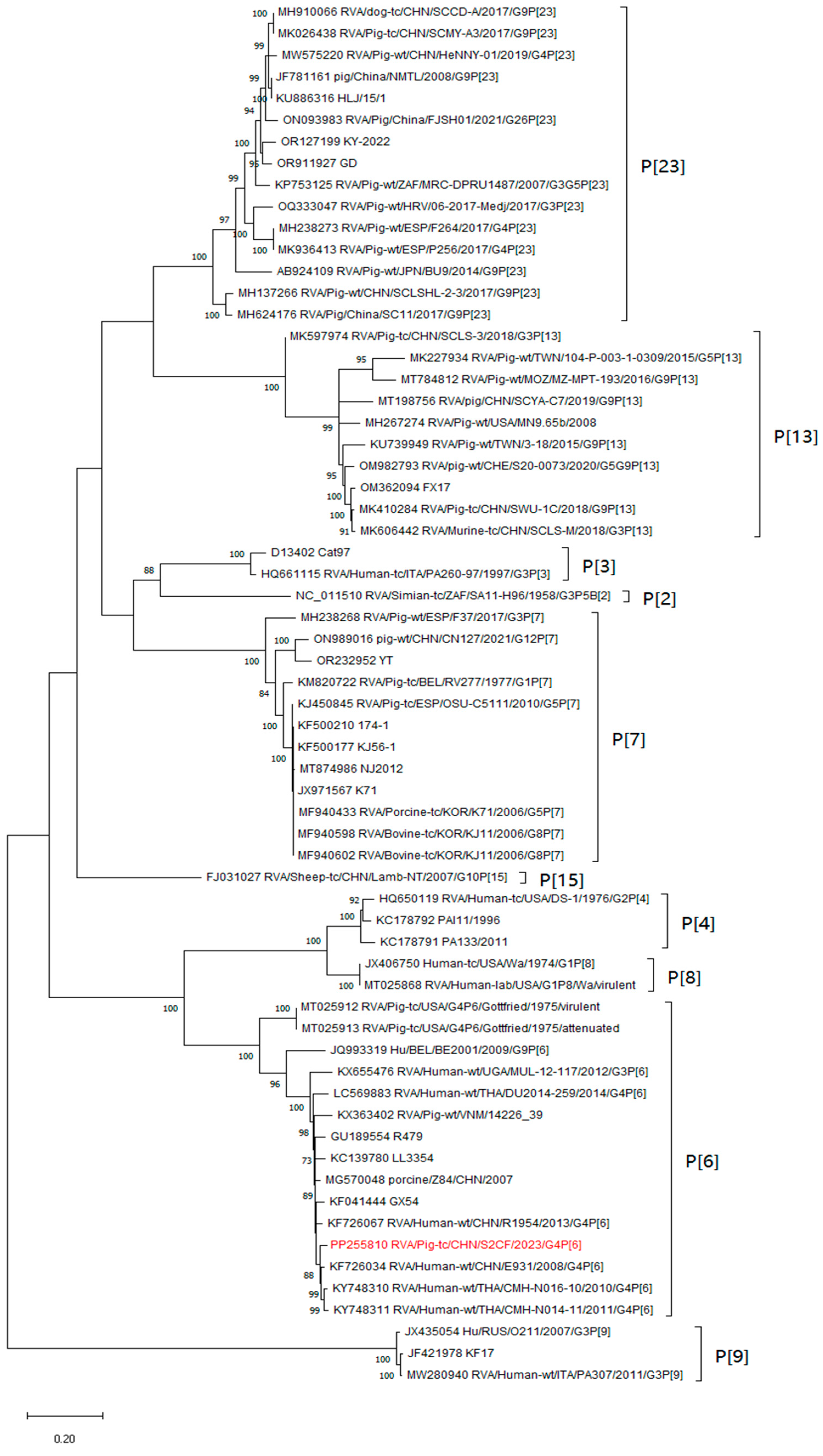
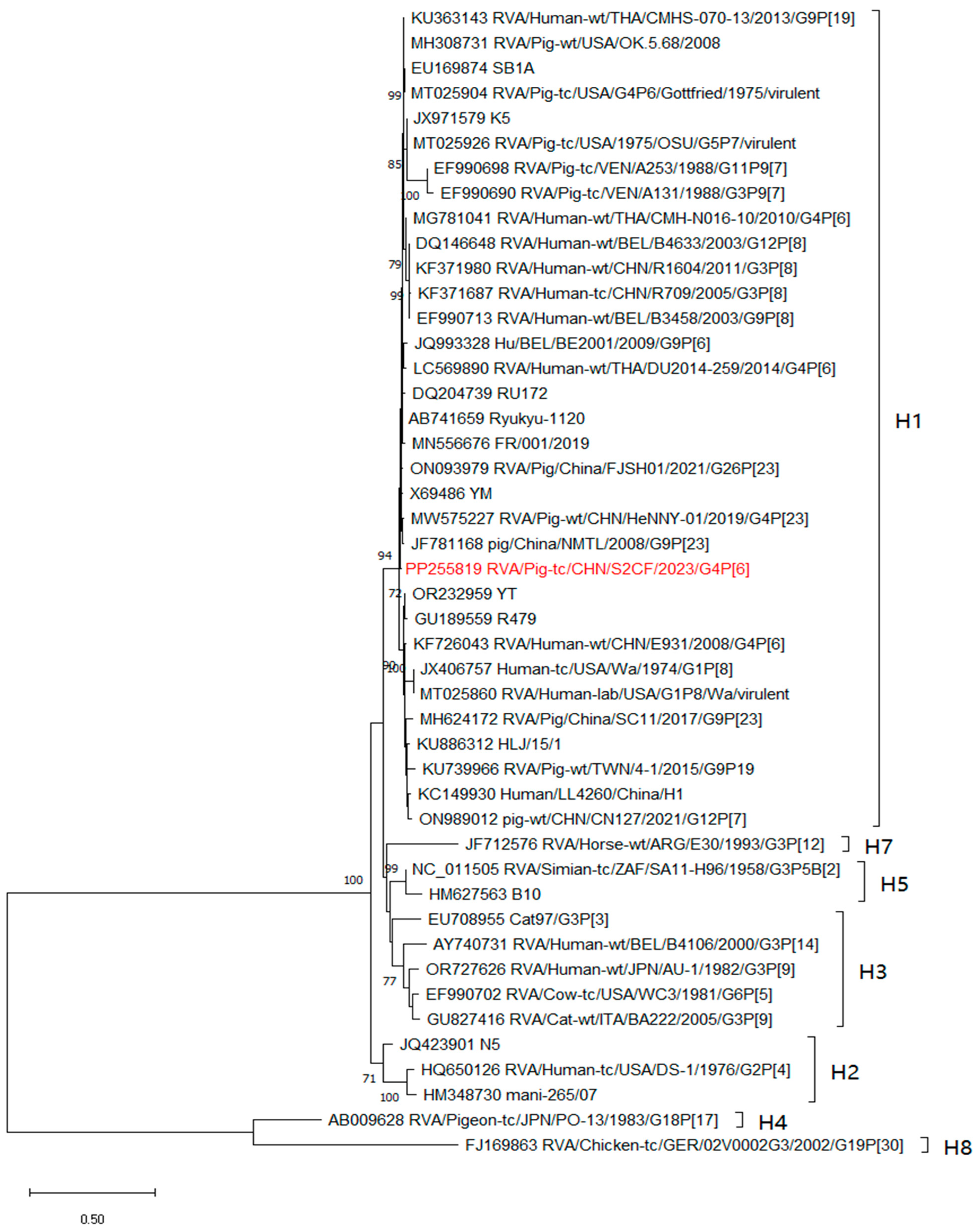
3.3. Phylogenetic and Recombination Relationships of All Genes
3.4. The VP7 and VP4 Antigenic Region Analyses
3.5. Genotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woode, G.N.; Bridger, J.; Hall, G.A.; Jones, J.M.; Jackson, G. The isolation of reovirus-like agents (rota-viruses) from acute gastroenteritis of piglets. J. Med. Microbiol. 1976, 9, 203–209. [Google Scholar] [CrossRef]
- Kishimoto, M.; Kajihara, M.; Tabata, K.; Itakura, Y.; Toba, S.; Ozono, S.; Sato, Y.; Suzuki, T.; Ito, N.; Changula, K.; et al. Isolation and Characterization of Distinct Rotavirus A in Bat and Rodent Hosts. J. Virol. 2023, 97, e0145522. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.H.; Chen, N.; Pang, B.B.; Liu, M.Q.; Cai, K.; Kobayashi, N. Surveillance of Human Rotaviruses in Wuhan, China (2019–2022): Whole-Genome Analysis of Emerging DS-1-like G8P[8] Rotavirus. Int. J. Mol. Sci. 2023, 24, 12189. [Google Scholar] [CrossRef]
- Cao, M.; Yuan, F.; Ma, X.P.; Ma, J.T.; Ma, X.M.; Chen, H.; Zhang, W.; Zhao, J.H.; Kuai, W.H. Surveillance of human Group A rotavirus in Ningxia, China (2015–2021): Emergence and prevalence of G9P[8]-E2 and G3P[8]-E2 genotypes. Infect. Genet. Evol. 2023, 113, 105469. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M.; Palombo, E.A.; Iturriza-Gómara, M.; Maes, P.; Patton, J.T.; et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008, 82, 3204–3219. [Google Scholar] [CrossRef]
- Bonura, F.; Bányai, K.; Mangiaracina, L.; Bonura, C.; Martella, V.; Giammanco, G.M.; De Grazia, S. Emergence in 2017–2019 of novel reassortant equine-like G3 rotavirus strains in Palermo, Sicily. Transbound. Emerg. Dis. 2022, 69, 813–835. [Google Scholar] [CrossRef]
- Luo, S.C.; Chen, X.Q.; Yan, G.Z.; Chen, S.N.; Pan, J.H.; Zeng, M.Y.; Han, H.; Guo, Y.J.; Zhang, H.Q.; Li, J.M.; et al. Emergence of human-porcine reassortment G9P[19] porcine rotavirus A strain in Guangdong Province, China. Front. Vet. Sci. 2023, 9, 1111919. [Google Scholar] [CrossRef]
- Miao, Q.; Pan, Y.D.; Gong, L.; Guo, L.J.; Wu, L.; Jing, Z.Y.; Zhang, G.H.; Tian, J.; Feng, L. Full genome characterization of a human-porcine reassortment G12P[7] rotavirus and its pathogenicity in piglets. Transbound. Emerg. Dis. 2022, 69, 3506–3517. [Google Scholar] [CrossRef]
- Akari, Y.; Hatazawa, R.; Kuroki, H.; Ito, H.; Negoro, M.; Tanaka, T.; Miwa, H.; Sugiura, K.; Umemoto, M.; Tanaka, S.; et al. Rotavirus Epidemiology Study Group. Full genome-based characterization of an Asian G3P[6] human rotavirus strain found in a diarrheic child in Japan: Evidence for porcine-to-human zoonotic transmission. Infect. Genet. Evol. 2023, 115, 105507. [Google Scholar] [CrossRef]
- Wandera, E.A.; Hatazawa, R.; Tsutsui, N.; Kurokawa, N.; Kathiiko, C.; Mumo, M.; Waithira, E.; Wachira, M.; Mwaura, B.; Nyangao, J.; et al. Genomic characterization of an African G4P[6] human rotavirus strain identified in a diarrheic child in Kenya: Evidence for porcine-to-human interspecies transmission and reassortment. Infect. Genet. Evol. 2021, 96, 105133. [Google Scholar] [CrossRef]
- Do, L.P.; Nakagomi, T.; Nakagomi, O. A rare G1P[6] super-short human rotavirus strain carrying an H2 genotype on the genetic background of a porcine rotavirus. Infect. Genet. Evol. 2014, 21, 334–350. [Google Scholar] [CrossRef]
- Zeller, M.; Heylen, E.; De Coster, S.; Van Ranst, M.; Matthijnssens, J. Full genome characterization of a porcine-like human G9P[6] rotavirus strain isolated from an infant in Belgium. Infect. Genet. Evol. 2012, 12, 1492–1500. [Google Scholar] [CrossRef]
- Hasegawa, A.; Inouye, S.; Matsuno, S.; Yamaoka, K.; Eko, R.; Suharyono, W. Isolation of human rotaviruses with a distinct RNA electrophoretic pattern from Indonesia. Microbiol. Immunol. 1984, 28, 719–722. [Google Scholar] [CrossRef]
- Guo, D.H.; Liu, J.S.; Lu, Y.; Sun, Y.; Yuan, D.W.; Jiang, Q.; Lin, H.; Li, C.W.; Si, C.D.; Qu, L.D. Full genomic analysis of rabbit rotavirus G3P[14] strain N5 in China: Identification of a novel VP6 genotype. Infect. Genet. Evol. 2012, 12, 1567–1576. [Google Scholar] [CrossRef]
- Vetter, J.; Papa, G.; Tobler, K.; Rodriguez, J.M.; Kley, M.; Myers, M.; Wiesendanger, M.; Schraner, E.M.; Luque, D.; Burrone, O.R.; et al. The recruitment of TRiC chaperonin in rotavirus viroplasms correlates with virus replication. mBio 2024, 15, e0049924. [Google Scholar] [CrossRef]
- Buttafuoco, A.; Michaelsen, K.; Tobler, K.; Ackermann, M.; Fraefel, C.; Eichwald, C. Conserved rotavirus NSP5 and VP2 domains interact and affect viroplasm. J. Virol. 2020, 94, e01965-19. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.H.; Ghosh, S.; Tang, W.F.; Pang, B.B.; Liu, M.Q.; Peng, J.S.; Zhou, D.J.; Kobayashi, N. Genomic characterization of G3P[6], G4P[6] and G4P[8] human rotaviruses from Wuhan, China: Evidence for interspecies transmission and reassortment events. Infect. Genet. Evol. 2015, 33, 55–71. [Google Scholar] [CrossRef]
- Wang, Y.H.; Kobayashi, N.; Nagashima, S.; Zhou, X.; Ghosh, S.; Peng, J.S.; Hu, Q.; Zhou, D.J.; Yang, Z.Q. Full genomic analysis of a porcine-bovine reassortant G4P[6] rotavirus strain R479 isolated from an infant in China. J. Med. Virol. 2010, 82, 1094–1102. [Google Scholar] [CrossRef]
- Chen, D.Y.; Zhou, L.; Tian, Y.M.; Wu, X.; Feng, L.; Zhang, X.P.; Liu, Z.H.; Pang, S.R.; Kang, R.M.; Yu, J.F.; et al. Genetic characterization of a novel G9P[23] rotavirus A strain identified in southwestern China with evidence of a reassortment event between human and porcine strains. Arch. Virol. 2019, 164, 1229–1232. [Google Scholar] [CrossRef]
- Wu, F.T.; Bányai, K.; Jiang, B.; Liu, L.T.; Marton, S.; Huang, Y.C.; Huang, L.M.; Liao, M.H.; Hsiung, C.A. Novel G9 rotavirus strains co-circulate in children and pigs, Taiwan. Sci. Rep. 2017, 7, 40731. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Liu, L.P.; Huang, T.; Tian, Y.; Guo, X.Z.; Liu, C.X.; Huang, B.; Chen, Q.S. Complete genomic analysis of rabbit rotavirus G3P[22] in China. Arch. Virol. 2023, 168, 129. [Google Scholar] [CrossRef]
- Falkenhagen, A.; Patzina-Mehling, C.; Gadicherla, A.K.; Strydom, A.; O'Neill, H.G.; Johne, R. Generation of simian rotavirus reassortants with VP4- and VP7-encoding genome segments from human strains circulating in Africa using reverse genetics. Viruses 2020, 12, 201. [Google Scholar] [CrossRef]
- Rasebotsa, S.; Uwimana, J.; Mogotsi, M.T.; Rakau, K.; Magagula, N.B.; Seheri, M.L.; Mwenda, J.M.; Mphahlele, M.J.; Sabiu, S.; Mihigo, R.; et al. Whole-genome analyses identifies multiple reassortant rotavirus strains in Rwanda post-vaccine introduction. Viruses 2021, 13, 95. [Google Scholar] [CrossRef]
- Shrestha, J.; Shrestha, S.K.; Strand, T.A.; Dudman, S.; Dembinski, J.L.; Vikse, R.; Andreassen, A.K. Diversity of rotavirus strains in children; results from a community-based study in Nepal. Front. Med. 2021, 8, 712326. [Google Scholar] [CrossRef]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Steele, A.D.; Duque, J.; Parashar, U.D.; WHO-coordinated Global Rotavirus Surveillance Network. Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 136–141. [Google Scholar] [CrossRef]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D.; WHO-coordinated Global Rotavirus Surveillance Network. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin. Infect. Dis. 2016, 62, 96–105. [Google Scholar] [CrossRef]
- Tagbo, B.N.; Mwenda, J.M.; Armah, G.; Obidike, E.O.; Okafor, U.H.; Oguonu, T.; Ozumba, U.C.; Eke, C.B.; Chukwubuike, C.; Edelu, B.O.; et al. Epidemiology of rotavirus diarrhea among children younger than 5 Years in Enugu, South East, Nigeria. Pediatr. Infect. Dis. J. 2014, 33, 19–22. [Google Scholar] [CrossRef]
- Du, Y.X.; Chen, C.; Zhang, X.B.; Yan, D.P.; Jiang, D.X.; Liu, X.X.; Yang, M.Y.; Ding, C.; Lan, L.; Hecht, R.; et al. Global burden and trends of rotavirus infection-associated deaths from 1990 to 2019: An observational trend study. Virol. J. 2022, 19, 166. [Google Scholar] [CrossRef]
- Carossino, M.; Vissani, M.A.; Barrandeguy, M.E.; Balasuriya, U.B.R.; Parreño, V. Equine rotavirus A under the one health lens: Potential impacts on public health. Viruses 2024, 16, 130. [Google Scholar] [CrossRef]
- Komoto, S.; Wandera Apondi, E.; Shah, M.; Odoyo, E.; Nyangao, J.; Tomita, M.; Wakuda, M.; Maeno, Y.; Shirato, H.; Tsuji, T.; et al. Whole genomic analysis of human G12P[6] and G12P[8] rotavirus strains that have emerged in Kenya: Identification of porcine-like NSP4 genes. Infect. Genet. Evol. 2014, 27, 277–293. [Google Scholar] [CrossRef]
- Amimo, J.O.; Junga, J.O.; Ogara, W.O.; Vlasova, A.N.; Njahira, M.N.; Maina, S.; Okoth, E.A.; Bishop, R.P.; Saif, L.J.; Djikeng, A. Detection and genetic characterization of porcine group A rotaviruses in asymptomatic pigs in smallholder farms in East Africa: Predominance of P[8] genotype resembling human strains. Vet. Microbiol. 2015, 175, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Bwogi, J.; Jere, K.C.; Karamagi, C.; Byarugaba, D.K.; Namuwulya, P.; Baliraine, F.N.; Desselberger, U.; Iturriza-Gomara, M. Whole genome analysis of selected human and animal rotaviruses identified in Uganda from 2012 to 2014 reveals complex genome reassortment events between human, bovine, caprine and porcine strains. PLoS ONE 2017, 12, e0178855. [Google Scholar] [CrossRef]
- Maringa, W.M.; Mwangi, P.N.; Simwaka, J.; Mpabalwani, E.M.; Mwenda, J.M.; Peenze, I.; Esona, M.D.; Mphahlele, M.J.; Seheri, M.L.; Nyaga, M.M. Molecular characterisation of a rare reassortant porcine-Like G5P[6] rotavirus strain detected in an unvaccinated child in Kasama, Zambia. Pathogens 2020, 9, 663. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, F.; Esona, M.D.; Seheri, L.M.; Nyaga, M.M.; Magagula, N.B.; Mukaratirwa, A.; Mulindwa, A.; Abebe, A.; Boula, A.; Tsolenyanu, E.; et al. African Rotavirus Surveillance Network. Whole genome analysis of African G12P[6] and G12P[8] rotaviruses provides evidence of porcine-human reassortment at NSP2, NSP3, and NSP4. Front. Microbiol. 2021, 11, 604444. [Google Scholar] [CrossRef]
- Dong, H.J.; Qian, Y.; Huang, T.; Zhu, R.N.; Zhao, L.Q.; Zhang, Y.; Li, R.C.; Li, Y.P. Identification of circulating porcine-human reassortant G4P[6] rotavirus from children with acute diarrhea in China by whole genome analyses. Infect. Genet. Evol. 2013, 20, 155–162. [Google Scholar] [CrossRef]
- Kaneko, M.; Do, L.P.; Doan, Y.H.; Nakagomi, T.; Gauchan, P.; Agbemabiese, C.A.; Dang, A.D.; Nakagomi, O. Porcine-like G3P[6] and G4P[6] rotavirus A strains detected from children with diarrhoea in Vietnam. Arch. Virol. 2018, 163, 2261–2263. [Google Scholar] [CrossRef]
- Malasao, R.; Khamrin, P.; Kumthip, K.; Ushijima, H.; Maneekarn, N. Complete genome sequence analysis of rare G4P[6] rotavirus strains from human and pig reveals the evidence for interspecies transmission. Infect. Genet. Evol. 2018, 65, 357–368. [Google Scholar] [CrossRef]
- Tacharoenmuang, R.; Guntapong, R.; Upachai, S.; Singchai, P.; Fukuda, S.; Ide, T.; Hatazawa, R.; Sutthiwarakom, K.; Kongjorn, S.; Onvimala, N.; et al. Full genome-based characterization of G4P[6] rotavirus strains from diarrheic patients in Thailand: Evidence for independent porcine-to-human interspecies transmission events. Virus Genes. 2021, 57, 338–357. [Google Scholar] [CrossRef]
- Martella, V.; Bányai, K.; Ciarlet, M.; Iturriza-Gómara, M.; Lorusso, E.; De Grazia, S.; Arista, S.; Decaro, N.; Elia, G.; Cavalli, A.; et al. Relationships among porcine and human P[6] rotaviruses: Evidence that the different human P[6] lineages have originated from multiple interspecies transmission events. Virology 2006, 344, 509–519. [Google Scholar] [CrossRef]
- Papp, H.; Borzak, R.; Farkas, S.; Kisfali, P.; Lengyel, G.; Molnar, P.; Melegh, B.; Matthijnssens, J.; Jakab, F.; Martella, V.; et al. Zoonotic transmission of reassortant porcine G4P[6] rotaviruses in Hungarian pediatric patients identified sporadically over a 15 year period. Infect. Genet. Evol. 2013, 19, 71–80. [Google Scholar] [CrossRef]
- Moutelíková, R.; Dufková, L.; Kamler, J.; Drimaj, J.; Plhal, R.; Prodělalová, J. Epidemiological survey of enteric viruses in wild boars in the Czech Republic: First evidence of close relationship between wild boar and human rotavirus A strains. Vet. Microbiol. 2016, 193, 28–35. [Google Scholar] [CrossRef]
- Degiuseppe, J.I.; Beltramino, J.C.; Millán, A.; Stupka, J.A.; Parra, G.I. Complete genome analyses of G4P[6] rotavirus detected in Argentinean children with diarrhoea provides evidence of interspecies transmission from swine. Clin. Microbiol. Infect. 2013, 19, E367–E371. [Google Scholar] [CrossRef]
- Contin, R.; Arnoldi, F.; Campagna, M.; Burrone, O.R. Rotavirus NSP5 orchestrates recruitment of viroplasmic proteins. J. Gen. Virol. 2010, 91, 1782–1793. [Google Scholar] [CrossRef]
- Ianiro, G.; Micolano, R.; Conte, M.; Labianca, M.; Vaccari, G.; Monini, M. Detection of an animal-derived G4P[6] group A rotavirus strain in a symptomatic child, in Italy. Virus Res. 2019, 260, 7–11. [Google Scholar] [CrossRef]
- Wei, J.J.; Radcliffe, S.; Pirrone, A.; Lu, M.Q.; Li, Y.; Cassaday, J.; Newhard, W.; Heidecker, G.J.; Rose Ii, W.A.; He, X.; et al. A novel rotavirus reverse genetics platform supports flexible insertion of exogenous genes and enables rapid development of a high-throughput neutralization assay. Viruses 2023, 15, 2034. [Google Scholar] [CrossRef]
- Criglar, J.M.; Anish, R.; Hu, L.; Crawford, S.E.; Sankaran, B.; Prasad, B.V.V.; Estes, M.K. Phosphorylation cascade regulates the formation and maturation of rotaviral replication factories. Proc. Natl. Acad. Sci. USA 2018, 115, E12015–E12023. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, L.L.; Du, J.; Hu, X.Q.; Xie, Y.P.; Wu, J.Y.; Lin, X.C.; Yin, N.; Sun, M.S.; Li, H.J. MicroRNA-7 inhibits rotavirus replication by targeting viral NSP5 in vivo and in vitro. Viruses 2020, 12, 209. [Google Scholar] [CrossRef]
- Bellinzoni, R.C.; Mattion, N.M.; Burrone, O.; Gonzalez, A.; La Torre, J.L.; Scodeller, E.A. Isolation of group A swine rotaviruses displaying atypical electropherotypes. J. Clin. Microbiol. 1987, 25, 952–954. [Google Scholar] [CrossRef]
- Allen, A.M.; Desselberger, U. Reassortment of human rotaviruses carrying rearranged genomes with bovine rotavirus. J. Gen. Virol. 1985, 66, 2703–2714. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Rahman, M.; Martella, V.; Xuelei, Y.; De Vos, S.; De Leener, K.; Ciarlet, M.; Buonavoglia, C.; Van Ranst, M. Full genomic analysis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 provides evidence for interspecies transmission. J. Virol. 2006, 80, 3801–3810. [Google Scholar] [CrossRef] [PubMed]
- Pocock, D.H. Isolation and characterization of two group A rotaviruses with unusual genome profiles. J. Gen. Virol. 1987, 68, 653–660. [Google Scholar] [CrossRef]
- Thouless, M.E.; DiGiacomo, R.F.; Neuman, D.S. Isolation of two lapine rotaviruses: Characterization of their subgroup, serotype and RNA electropherotypes. Arch. Virol. 1986, 89, 161–170. [Google Scholar] [CrossRef]
- McIntyre, M.; Rosenbaum, V.; Rappold, W.; Desselberger, M.; Wood, D.; Desselberger, U. Biophysical characterization of rotavirus particles containing rearranged genomes. J. Gen. Virol. 1987, 68, 2961–2966. [Google Scholar] [CrossRef]
- Besselaar, T.G.; Rosenblatt, A.; Kidd, A.H. Atypical rotavirus from South African neonates. Brief report. Arch. Virol. 1986, 87, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Troupin, C.; Schnuriger, A.; Duponchel, S.; Deback, C.; Schnepf, N.; Dehee, A.; Garbarg-Chenon, A. Rotavirus rearranged genomic RNA segments are preferentially packaged into viruses despite not conferring selective growth advantage to viruses. PLoS ONE 2011, 6, e20080. [Google Scholar] [CrossRef]
- Ghosh, S.; Urushibara, N.; Taniguchi, K.; Kobayashi, N. Whole genomic analysis reveals the porcine origin of human G9P[19] rotavirus strains Mc323 and Mc345. Infect. Genet. Evol. 2012, 12, 471–477. [Google Scholar] [CrossRef]
- Matsuno, S.; Hasegawa, A.; Mukoyama, A.; Inouye, S. A candidate for a new serotype of human rotavirus. J. Virol. 1985, 54, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Heiman, E.M.; McDonald, S.M.; Barro, M.; Taraporewala, Z.F.; Bar-Magen, T.; Patton, J.T. Group A human rotavirus genomics: Evidence that gene constellations are influenced by viral protein interactions. J. Virol. 2008, 82, 11106–11116. [Google Scholar] [CrossRef]
- Nagai, M.; Shimada, S.; Fujii, Y.; Moriyama, H.; Oba, M.; Katayama, Y.; Tsuchiaka, S.; Okazaki, S.; Omatsu, T.; Furuya, T.; et al. H2 genotypes of G4P[6], G5P[7], and G9[23] porcine rotaviruses show super-short RNA electropherotypes. Vet. Microbiol. 2015, 176, 250–256. [Google Scholar] [CrossRef] [PubMed]


| Name | Sequence (5′–3′) | PCR Product Size (bp) | Annealing Temperature (°C) | Extension Time |
|---|---|---|---|---|
| VP1 | F1: CGGTACCCGGGGATCGGCTATTAAAGCTGTACAATG | 3302 | 47.8 | 3 min 25 s |
| R2: CGACTCTAGAGGATCGGTCACATCTAAGCRCTCTAATC | ||||
| VP2 | F: CGGTACCCGGGGATCGGCTATTAAAGGYTCAATGGC | 2690 | 47.8 | 2 min 45 s |
| R: CGACTCTAGAGGATCGGTCATATCTCCACARTGGG | ||||
| VP3 | F: CGGTACCCGGGGATCATGAAAGTATTAGCTTTAAGAGACA | 2507 | 64.4 | 2 min 45 s |
| R: CGACTCTAGAGGATCTCACTCAGRCATATCAAATACGG | ||||
| VP4 | F: ATGGCTTCGCTCATTTACAGACAATTG | 2360 | 56.8 | 2 min 35 s |
| R: GGTCACAACCTCTCGACATTGCTTACAGT | ||||
| VP6 | F: GGCTTTWAAACGAAGTCTTCGACAT | 1356 | 52.5 | 1 min 30 s |
| R: GGTCACATCCTCTCACTAYAYCAT | ||||
| VP7 | F: GGCTTTAAAAGAGAGAATTTC | 1062 | 48.0 | 1 min 10 s |
| R: GGTCACATCAWACARTTCTA | ||||
| NSP1 | F: TATGAAAAGTCTTGTGGAAGCCAT | 1550 | 50.6 | 1 min 45 s |
| R: ATGCTGCCTAGGCGCTACTCTAGTGCA | ||||
| NSP2 | F: GGCTTTTAAAGCGTCTCAGT | 1059 | 52.5 | 1 min 10 s |
| R: GGTCACATAAGCGCTTTCTATTCT | ||||
| NSP3 | F: CAGTGGTTGATGCTCAAGATGGAG | 1020 | 56.3 | 1 min 10 s |
| R: CTATTGTGCTCATAGAGGGTCATGTGTA | ||||
| NSP4 | F: GGCTTTTAAAAGTTCTGTTCCG | 749 | 57.5 | 50 s |
| R: GGTCACACTARGACCATTCCTTCCAT | ||||
| NSP5 | F: GGCTTTAAAAGCGCTACAGTGATGT | 667 | 64.4 | 45 s |
| R: GAGTGGGGAGCTCCCTAGTGTGCTCT |
| Gene | Length(bp) | Closely Related Strains | Country | Accession No. | nt (%) |
|---|---|---|---|---|---|
| VP7 | 1069 | HLJ/15/1 | China | KU886318 | 96.07 |
| VP4 | 2359 | RVA/Human-wt/CHN/E931/2008/G4P[6] | China | KF726034 | 96.40 |
| VP6 | 1356 | RVA/Human-wt/CHN/E931/2008/G4P[6] | China | KF726035 | 95.87 |
| VP1 | 3302 | YT | China | OR232949 | 95.00 |
| VP2 | 2717 | RVA/Pig-wt/CHN/HeNNY-01/2019/G4P[23] | China | MW575218 | 92.01 |
| VP3 | 2527 | RVA/Human-wt/CHN/R479/2004/G4P[6] | China | GU189553 | 96.87 |
| NSP1 | 1528 | RVA/Pig/China/SC11/2017/G9P[23] | China | MH624168 | 96.03 |
| NSP2 | 1044 | RVA/Pig-wt/TWN/4-1/2015/G9P[19] | China: Taiwan | KU739963 | 95.50 |
| NSP3 | 1007 | Human/LL4260/China/T1 | China | KC149929 | 95.63 |
| NSP4 | 750 | pig-wt/CHN/CN127/2021/G12P[7] | China | ON989011 | 95.47 |
| NSP5 | 962 | Hu/BEL/BE2001/2009/G9P[6] | Belgium | JQ993328 | 91.90 |
| Accession Number | Strains | 7-1a | 7-1b | 7-2 | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 87 | 91 | 94 | 96 | 97 | 98 | 99 | 100 | 104 | 123 | 125 | 129 | 130 | 291 | 201 | 211 | 212 | 213 | 238 | 242 | 143 | 145 | 146 | 147 | 148 | 190 | 217 | 221 | 264 | ||
| AB792641 | AU-1 | T | T | N | N | S | W | K | D | Q | D | A | V | D | K | Q | D | T | N | N | N | K | D | A | A | L | S | E | A | G |
| HQ650124 | DS-1 | A | N | S | D | E | W | E | N | Q | D | N | V | N | K | Q | D | V | N | N | N | R | D | N | T | S | D | I | S | G |
| K02033 | Wa | V | E | N | V | D | F | S | V | L | M | E | L | I | N | V | T | T | T | N | I | L | Y | Y | Q | Q | A | I | K | W |
| KF447866 | GX82 | T | T | S | N | E | W | K | D | Q | N | L | V | D | K | Q | N | T | N | D | T | R | V | S | G | E | S | T | S | G |
| KF041440 | GX54 | T | T | S | N | E | W | K | D | Q | N | L | V | D | K | Q | N | T | N | D | T | R | V | S | G | E | S | T | S | G |
| EU708602 | E931 | T | T | S | N | E | W | K | D | Q | N | L | V | D | K | Q | N | T | N | D | T | R | A | S | G | E | S | T | S | G |
| KX363437 | 14250_9 | T | T | S | N | E | W | K | D | Q | N | L | V | D | K | Q | N | T | N | D | T | R | A | S | G | E | S | T | S | G |
| MG781057 | CMP-011-09 | T | T | N | N | E | W | K | D | Q | N | L | V | D | K | Q | N | T | N | N | T | R | I | S | G | E | L | T | S | G |
| MW575222 | HeNNY-01 | T | T | N | N | E | W | K | D | Q | N | L | V | D | K | Q | N | T | N | D | T | R | A | S | G | E | S | T | S | G |
| KF726066 | R1954 | T | T | N | N | E | W | K | D | Q | N | L | V | D | K | Q | N | T | N | D | T | R | A | S | G | E | S | T | S | G |
| DQ873680 | R479 | T | T | S | N | E | W | K | D | Q | N | L | V | D | K | Q | N | T | N | D | T | R | A | S | G | E | S | T | G | G |
| KF726044 | E2484 | T | T | S | N | E | W | K | D | Q | N | L | I | D | K | Q | N | A | N | D | T | R | A | S | G | E | S | T | S | G |
| KP222851 | 21250 | T | T | S | N | E | W | K | D | Q | N | L | V | D | K | Q | N | T | N | D | T | R | A | S | G | E | S | T | S | G |
| KU886318 | HLJ/15/1 | T | T | G | N | E | W | K | D | Q | N | L | I | D | R | Q | N | T | G | D | T | R | I | A | G | E | S | M | N | G |
| JX498954 | HLJkd | T | T | S | N | E | W | K | D | Q | N | L | I | D | R | Q | N | T | G | D | T | R | I | A | G | E | S | M | N | G |
| KP752964 | DPRU1557 | T | T | G | N | E | W | K | D | Q | N | L | I | D | R | Q | N | A | G | D | T | R | I | A | G | Q | S | T | N | G |
| KC713876 | NoV11-N2687 | A | T | S | N | E | W | K | D | Q | N | L | V | D | R | Q | N | T | G | D | T | R | V | A | G | E | S | M | N | G |
| OQ440159 | D230-ZG | A | T | G | N | E | W | K | D | Q | N | L | I | D | R | Q | N | A | G | D | T | R | I | A | G | E | S | M | N | G |
| OQ440224 | S344-Z | A | T | G | N | E | W | K | D | Q | N | L | I | D | R | Q | N | A | G | D | T | R | I | A | G | E | S | M | N | G |
| OQ440211 | S338-Z | A | T | G | N | E | W | K | D | Q | N | L | I | D | R | Q | N | A | G | D | T | R | I | A | G | E | S | M | N | G |
| PP255809 | S2CF | A | T | G | N | E | W | K | D | Q | N | L | I | D | R | Q | N | T | G | D | T | R | I | A | G | E | S | M | N | G |
| Accession Number | Strains | 8-1 | 8-2 | 8-3 | 8-4 | 5-1 | 5-2 | 5-3 | 5-4 | 5-5 | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 146 | 148 | 150 | 188 | 190 | 192 | 193 | 194 | 195 | 196 | 180 | 183 | 113 | 114 | 115 | 116 | 125 | 131 | 132 | 133 | 135 | 87 | 88 | 89 | 384 | 386 | 388 | 393 | 394 | 398 | 440 | 441 | 434 | 459 | 429 | 306 | ||
| D10970 | AU-1 | D | L | P | G | Y | L | I | N | N | D | N | A | N | Q | N | T | Q | N | S | N | D | S | T | R | E | S | S | A | N | H | S | S | R | E | A | R | T |
| HQ650119 | DS-1 | D | S | Q | D | S | T | D | L | N | N | I | T | A | S | Q | T | N | N | E | N | N | D | N | T | N | Y | F | L | W | P | G | R | T | P | E | L | R |
| L34161 | Wa | D | S | Q | E | S | T | N | L | N | N | I | T | A | N | P | V | D | S | S | N | D | N | N | T | N | Y | F | I | W | P | G | R | T | P | E | L | R |
| MT025912 | Gottfried-V | D | N | N | D | S | T | N | L | P | D | V | T | A | P | S | Q | D | V | E | N | S | D | I | N | K | Y | F | I | W | P | G | R | T | P | E | L | R |
| MT025913 | Gottfried-A | D | N | N | D | S | T | N | L | P | D | V | T | A | P | S | Q | D | V | E | N | S | D | I | N | K | Y | F | I | W | P | G | R | T | P | E | L | R |
| JQ993319 | BE2001 | D | N | S | E | S | T | N | L | P | D | I | T | A | T | S | Q | D | T | E | N | N | S | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| KX655476 | MUL-12-117 | D | S | S | E | S | T | N | L | S | E | V | T | A | T | N | Q | R | T | E | N | N | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| LC569883 | DU2014-259 | D | S | N | E | S | T | N | L | S | E | I | T | A | A | N | G | N | T | E | N | N | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| KX363402 | 14226_39 | D | S | S | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | T | E | N | N | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| GU189554 | R479 | D | S | S | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | T | E | N | S | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| KC139780 | LL3354 | D | S | S | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | T | E | N | N | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| MG570048 | Z84 | D | S | S | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | T | E | N | N | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| KF041444 | GX54 | D | S | S | E | S | T | N | L | L | E | V | T | A | T | N | Q | S | T | E | N | N | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| KF726067 | R1954 | D | N | S | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | T | E | N | N | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| KF726034 | E931 | D | S | S | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | T | E | N | N | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| KY748310 | CMH-N016-10 | D | S | S | E | S | T | N | L | S | E | I | T | A | T | N | Q | S | T | E | N | D | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| KY748311 | CMH-N014-11 | D | S | S | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | T | E | N | N | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
| PP255810 | S2CF | D | S | S | E | S | T | N | L | S | E | V | T | A | T | N | Q | S | T | E | N | N | N | T | N | Q | Y | F | I | W | P | G | R | T | P | E | L | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Hou, X.; Xiao, G.; Liu, B.; Jia, H.; Wei, J.; Mi, X.; Guo, Q.; Wei, Y.; Zhai, S.-L. Emergence of a Novel G4P[6] Porcine Rotavirus with Unique Sequence Duplication in NSP5 Gene in China. Animals 2024, 14, 1790. https://doi.org/10.3390/ani14121790
Zhou X, Hou X, Xiao G, Liu B, Jia H, Wei J, Mi X, Guo Q, Wei Y, Zhai S-L. Emergence of a Novel G4P[6] Porcine Rotavirus with Unique Sequence Duplication in NSP5 Gene in China. Animals. 2024; 14(12):1790. https://doi.org/10.3390/ani14121790
Chicago/Turabian StyleZhou, Xia, Xueyan Hou, Guifa Xiao, Bo Liu, Handuo Jia, Jie Wei, Xiaoyun Mi, Qingyong Guo, Yurong Wei, and Shao-Lun Zhai. 2024. "Emergence of a Novel G4P[6] Porcine Rotavirus with Unique Sequence Duplication in NSP5 Gene in China" Animals 14, no. 12: 1790. https://doi.org/10.3390/ani14121790






