Effects of Heat Stress on Goat Production and Mitigating Strategies: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Heat Stress in Goat Production
2.3. Data Collection
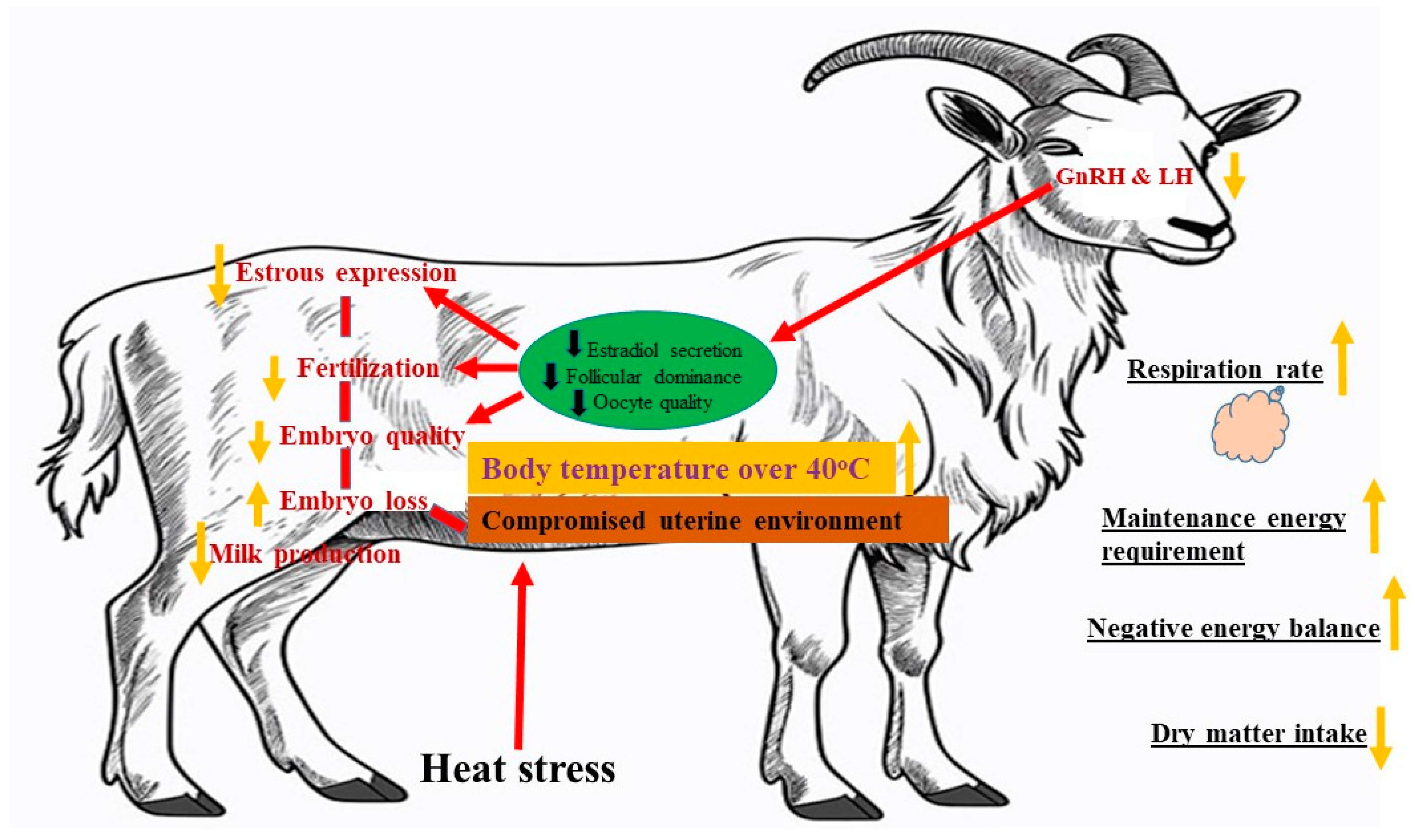
2.4. Reproductive Performance
2.5. Meat Quality and Carcass Characteristics
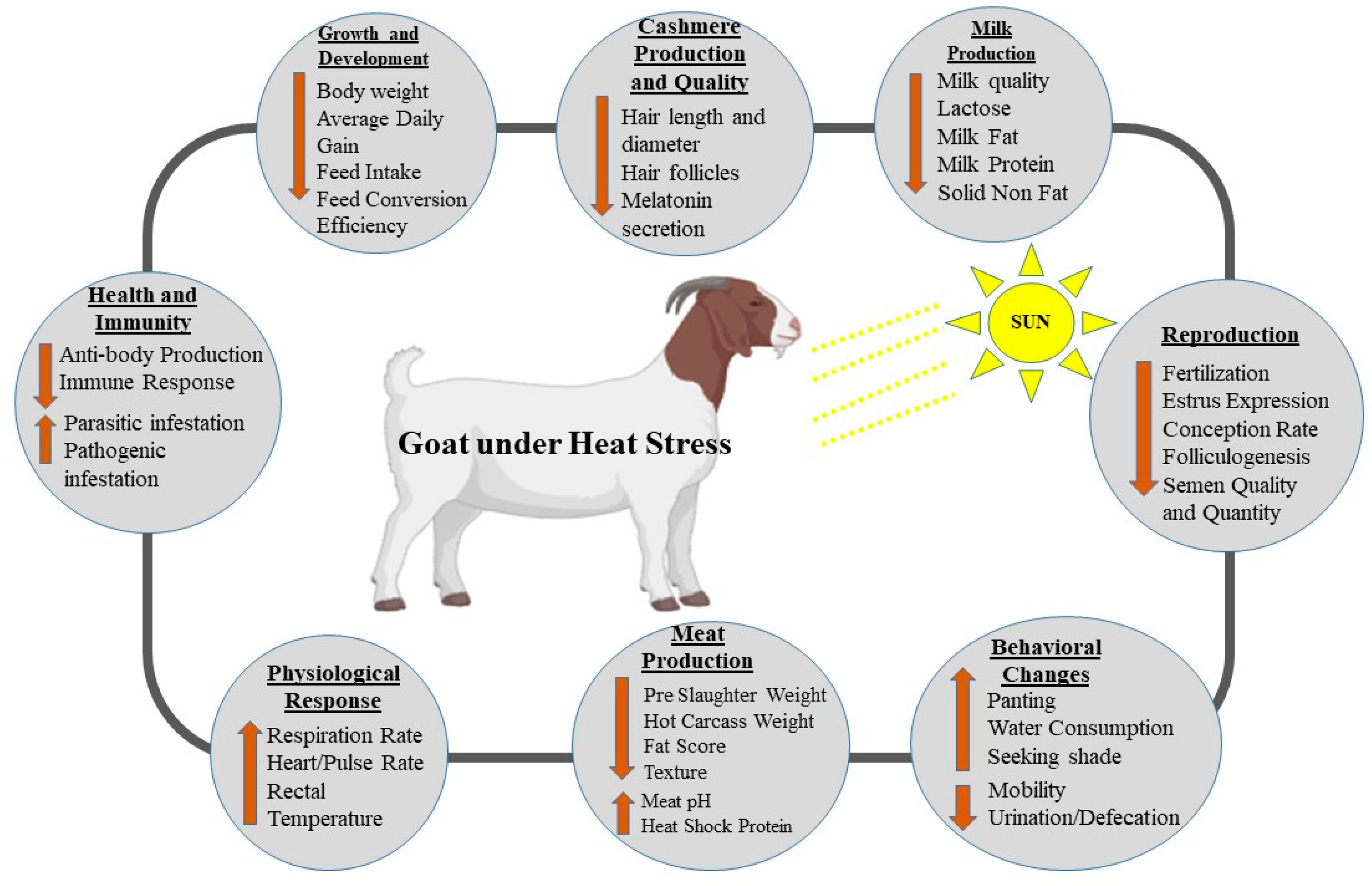
3. Effects of Heat Stress on the Production of Goat
3.1. Behavioral Responses of Goats to Heat Stress
3.2. Effects on Growth and Development
3.3. Effects of Heat Stress on Reproductive Performance of Goats
3.4. Heat Stress Effects on Physiological Responses
3.5. Effects of Heat Stress on Health and Immunity
3.6. Effects of Heat Stress on Milk Production and Quality
3.7. Effects of Heat Stress on Meat Quality and Carcass Characteristics
3.8. Effects of Heat Stress on Cashmere/Wool Production and Quality
3.9. Genetic Adaptations of Goats under Heat Stress
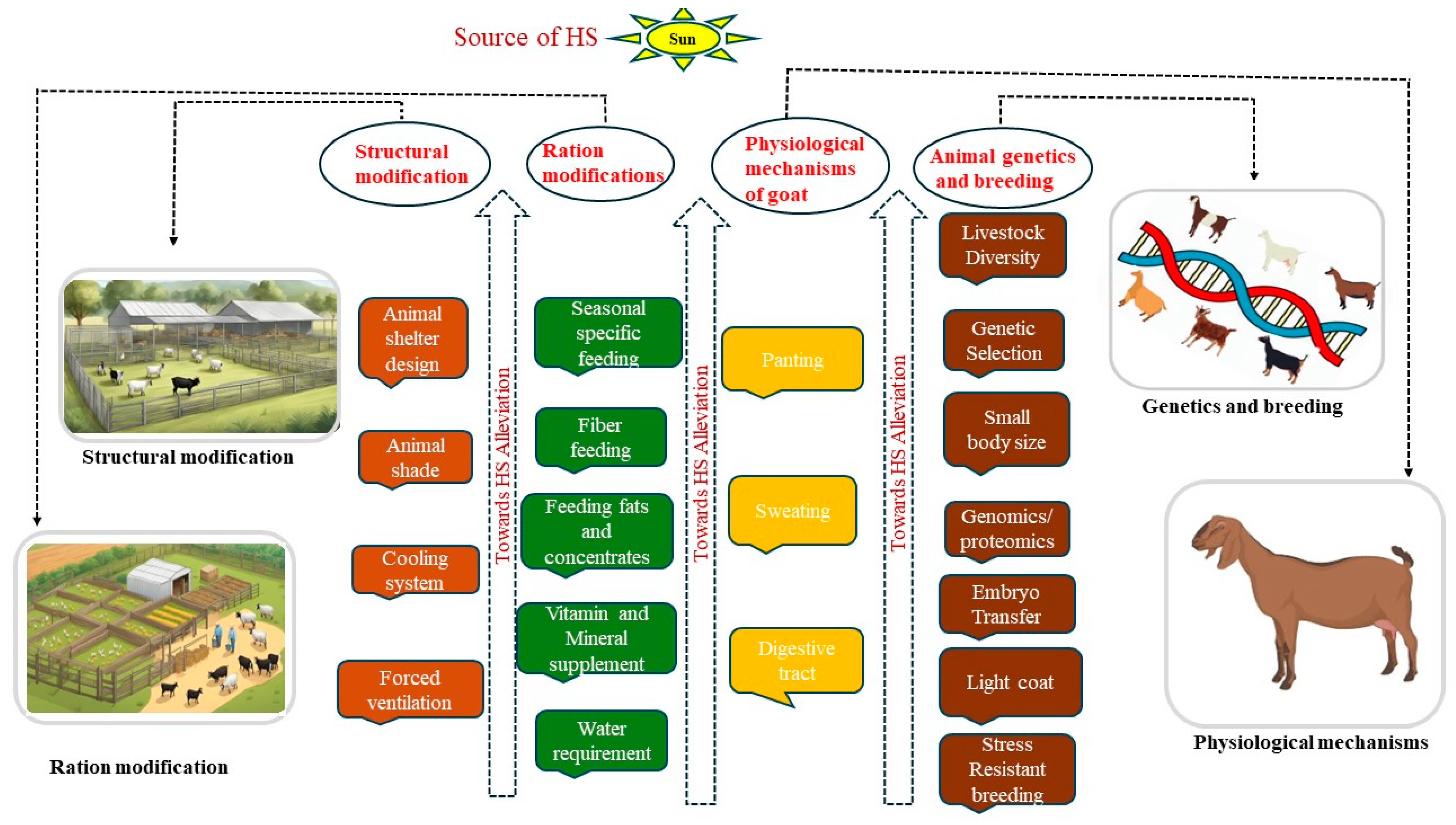
4. Mitigation Strategies in Alleviating Heat Stress
5. Conclusions
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, M.; Kumar, S.; Dangi, S.; Jangir, B. Physiological, Biochemical and Molecular Responses to Thermal Stress in Goats. Int. J. Livest. Res. 2013, 3, 27–38. [Google Scholar] [CrossRef]
- Salem, H. Ben Nutritional Management to Improve Sheep and Goat Performances in Semiarid Regions. Rev. Bras. Zootec. 2010, 39, 337–347. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U.; Prosser, C.G. Recent Advances in Exploiting Goat’s Milk: Quality, Safety and Production Aspects. Small Rumin. Res. 2010, 89, 110–124. [Google Scholar] [CrossRef]
- Dwyer, C.M. The Ethology of Domestic Animals: An Introductory Text, 2nd ed.; Jensen, P., Ed.; CABI: Cambridge, MA, USA, 2009. [Google Scholar]
- Zeder, M.A. Domestication and Early Agriculture in the Mediterranean Basin: Origins, Diffusion, and Impact. Proc. Natl. Acad. Sci. USA 2008, 105, 11597–11604. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U. The Interrelationships between Lactose Intolerance and the Modern Dairy Industry: Global Perspectives in Evolutional and Historical Backgrounds. Nutrients 2015, 7, 7312–7331. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, H.; Smith, T. Feeding Strategies to Increase Small Ruminant Production in Dry Environments. Small Rumin. Res. 2008, 77, 174–194. [Google Scholar] [CrossRef]
- Okoruwa, M.I. Effect of Heat Stress on Thermoregulatory, Live Bodyweight and Physiological Responses of Dwarf Goats in Southern Nigeria. Eur. Sci. J. 2014, 10, 255–264. [Google Scholar]
- Banerjee, D.; Upadhyay, R.C.; Chaudhary, U.B.; Kumar, R.; Singh, S.; Ashutosh, G.J.M.; Polley, S.; Mukherjee, A.; Das, T.K.; De, S. Seasonal Variation in Expression Pattern of Genes under HSP70: Seasonal Variation in Expression Pattern of Genes under HSP70 Family in Heat- and Cold-Adapted Goats (Capra hircus). Cell Stress Chaperones 2014, 19, 401–408. [Google Scholar] [CrossRef]
- Kojo, I. Effect of Coat Colour, Ecotype, Location and Sex on Hair Density of West African Dwarf (WAD) Goats in Northern Ghana. Sky J. Agric. Res. 2014, 3, 25–30. [Google Scholar]
- Silanikove, N. Effects of Heat Stress on the Welfare of Extensively Managed Domestic Ruminants. Livest. Prod. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Silanikove, N. Effects of Water Scarcity and Hot Environment on Appetite and Digestion in Ruminants: A Review. Livest. Prod. Sci. 1992, 30, 175–194. [Google Scholar] [CrossRef]
- Silanikove, N.; Koluman, D.N. Impact of Climate Change on the Dairy Industry in Temperate Zones: Predications on the Overall Negative Impact and on the Positive Role of Dairy Goats in Adaptation to Earth Warming. Small Rumin. Res. 2015, 123, 27–34. [Google Scholar] [CrossRef]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of Climate Changes on Animal Production and Sustainability of Livestock Systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Al-Dawood, A. Adoption of Agricultural Innovations: Investigating Current Status and Barriers to Adoption of Heat Stress Management in Small Ruminants in Jordan. J. Agric. Environ. Sci 2015, 15, 388–398. [Google Scholar]
- Ominski, K.H.; Kennedy, A.D.; Wittenberg, K.M.; Moshtaghi Nia, S.A. Physiological and Production Responses to Feeding Schedule in Lactating Dairy Cows Exposed to Short-Term, Moderate Heat Stress. J. Dairy Sci. 2002, 85, 730–737. [Google Scholar] [CrossRef]
- Ingraham, R.H.; Gillette, D.D.; Wagner, W.D. Relationship of Temperature and Humidity to Conception Rate of Holstein Cows in Subtropical Climate. J. Dairy Sci. 1974, 57, 476–481. [Google Scholar] [CrossRef]
- Silanikove, N. The Physiological Basis of Adaptation in Goats to Harsh Environments. Small Rumin. Res. 2000, 35, 181–193. [Google Scholar] [CrossRef]
- Collier, R.J.; Beede, D.K.; Thatcher, W.W.; Israel, L.A.; Wilcox, C.J. Influences of Environment and Its Modification on Dairy Animal Health and Production. J. Dairy Sci. 1982, 65, 2213–2227. [Google Scholar] [CrossRef]
- Hafez, E.S.E. Behavioral Adaptation. In Adaptation of Domestic Animals; Hafez, E.S.E., Ed.; Lea Febiger: Philadelphia, PA, USA, 1968; pp. 202–214. [Google Scholar]
- Facanha, D.A.E.; Oliveira, M.G.C.; Guilhermino, M.G.; Costa, W.P.; Paula, V.V. Hemogasometric Parameters of Brazilian Native Goats under Thermal Stress Conditions. In Proceedings of the XI International Conference on Goats, Gran Canaria, Spain, 23–27 September 2012; p. 72. [Google Scholar]
- Cain, J.W.; Krausman, P.R.; Rosenstock, S.S.; Turner, J.C. Mechanisms of Thermoregulation and Water Balance in Desert Ungulates. Wildl. Soc. Bull. 2006, 34, 570–581. [Google Scholar] [CrossRef]
- Johnson, H.D. Physiological Responses and Productivity of Cattle. In Stress Physiology in Livestock; Yousef, M.K., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1985; Volume II Ungulates, pp. 3–24. [Google Scholar]
- Carolyn, L. Stull; University of California: Davis, CA, USA, 1997. [Google Scholar]
- Alam, M.; Hashem, M.; Rahman, M.; Hossain, M.; Haque, M.; Sobhan, Z.; Islam, M. Effect of Heat Stress on Behavior, Physiological and Blood Parameters of Goat. Progress. Agric. 2013, 22, 37–45. [Google Scholar] [CrossRef]
- Shilja, S.; Sejian, V.; Bagath, M.; Mech, A.; David, C.G.; Kurien, E.K.; Varma, G.; Bhatta, R. Adaptive Capability as Indicated by Behavioral and Physiological Responses, Plasma HSP70 Level, and PBMC HSP70 MRNA Expression in Osmanabadi Goats Subjected to Combined (Heat and Nutritional) Stressors. Int. J. Biometeorol. 2016, 60, 1311–1323. [Google Scholar] [CrossRef]
- West, J.W. Nutritional Strategies for Managing the Heat-Stressed Dairy Cow. J. Anim. Sci. 1999, 77 (Suppl. 2), 21–35. [Google Scholar] [CrossRef] [PubMed]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat Stress in Lactating Dairy Cows: A Review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- National Research Council (US). NRC Nutrient Requirements of Small Ruminants, Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007; p. 384. [Google Scholar]
- Hamzaoui, S.; Salama, A.A.K.; Albanell, E.; Such, X.; Caja, G. Physiological Responses and Lactational Performances of Late-Lactation Dairy Goats under Heat Stress Conditions. J. Dairy Sci. 2013, 96, 6355–6365. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.E.; Kay, J.K.; VanBaale, M.J.; Baumgard, L.H. Calculating and Improving Energy Balance During Times of Nutrient Limitations. In Proceedings of the 20th Annual Southwest Nutrition Conference Management Conference, Tempe, AZ, USA, 24–25 February 2005. [Google Scholar]
- Salama, A.A.K.; Caja, G.; Hamzaoui, S.; Badaoui, B.; Castro-Costa, A.; Façanha, D.A.E.; Guilhermino, M.M.; Bozzi, R. Different Levels of Response to Heat Stress in Dairy Goats. Small Rumin. Res. 2014, 121, 73–79. [Google Scholar] [CrossRef]
- Ocak, S.; Darcan, N.; Çankaya, S.; İnal, T.C. Physiological and Biochemical Responses in German Fawn Kids Subjected to Cooling Treatments under Mediterranean Climate Conditions. Turk. J. Vet. Anim. Sci. 2009, 33, 455–461. [Google Scholar] [CrossRef]
- Salama, A.A.K.; Hamzaoui, S.; Caja, G. Responses of Dairy Goats to Heat Stress and Strategies to Alleviate Its Effects. In Proceedings of the XI International Conference on Goats, Gran Canaria, Spain, 23–27 September 2012; Volume 15. [Google Scholar]
- Ahmed, M.M.M.; El Kheir, I.M. Thermoregulation and Water Balance as Affected by Water and Food Restrictions in Sudanese Desert Goats Fed Good-Quality and Poor-Quality Diets. Trop. Anim. Health Prod. 2004, 36, 191–204. [Google Scholar] [CrossRef]
- Caulfield, M.P.; Cambridge, H.; Foster, S.F.; McGreevy, P.D. Heat Stress: A Major Contributor to Poor Animal Welfare Associated with Long-Haul Live Export Voyages. Vet. J. 2014, 199, 223–228. [Google Scholar] [CrossRef]
- Geraseev, L.C.; Perez, J.R.O.; Carvalho, P.A.; Oliveira, R.P.D.; Quintão, F.A.; Lima, A.L. Efeitos Das Restrições Pré e Pós-Natal Sobre o Crescimento e o Desempenho de Cordeiros Santa Inês Do Nascimento Ao Desmame. Rev. Bras. Zootec. 2006, 35, 245–251. [Google Scholar] [CrossRef]
- Grant, A.L.; Helfericht, W. Growth Regulation in Farm Animals; Elsevier: New York, NY, USA, 1991. [Google Scholar]
- da Souza, P.T.; Salles, M.G.F.; de Araújo, A.A. Impacto Do Estresse Térmico Sobre a Fisiologia, Reprodução e Produção de Caprinos. Cienc. Rural 2012, 42, 1888–1895. [Google Scholar] [CrossRef]
- Araújo, T.G.P. Influência de Fatores de Ambiente Sobre Características de Crescimento e de Sobrevivência Em Cabritos Da Raça Boer. Ph.D. Thesis, Universidade Federal da Paraíba, Areia, Brazil, 2008. [Google Scholar]
- Nóbrega, J.E.D., Jr.; Riet-Correa, F.; Nóbrega, R.S.; Medeiros, J.M.D.; Vasconcelos, J.S.D.; Simões, S.V.D.; Tabosa, I.M. Mortalidade Perinatal de Cordeiros No Semiárido Da Paraíba. Pesqui. Vet. Bras. 2005, 25, 171–178. [Google Scholar] [CrossRef]
- Vieira, M.J. Criação de Cabras: Técnica Prática Lucrativa; Nobel: São Paulo, Brazil, 2004. [Google Scholar]
- Biffani, S.; Martins Filho, R.; Giorgetti, A.; Bozzi, R.; Lima, F.D.A.M. Fatores Ambientais e Genéticos Sobre o Crescimento Ao Ano e Ao Sobreano de Bovinos Nelore, Criados No Nordeste Do Brasil. Rev. Bras. Zootec. 2008, 28, 468–473. [Google Scholar] [CrossRef]
- Phillips, C.J.C. The Effects of Forage Provision and Group Size on the Behavior of Calves. J. Dairy Sci. 2004, 87, 1380–1388. [Google Scholar] [CrossRef]
- Shaat, I.; Galal, S.; Mansour, H. Genetic Trends for Lamb Weights in Flocks of Egyptian Rahmani and Ossimi Sheep. Small Rumin. Res. 2004, 51, 23–28. [Google Scholar] [CrossRef]
- Pragna, P.; Sejian, V.; Bagath, M.; Krishnan, G.; Archana, P.R.; Soren, N.M.; Beena, V.; Bhatta, R. Comparative Assessment of Growth Performance of Three Different Indigenous Goat Breeds Exposed to Summer Heat Stress. J. Anim. Physiol. Anim. Nutr. 2018, 102, 825–836. [Google Scholar] [CrossRef]
- Zhang, M.; Warner, R.D.; Dunshea, F.R.; DiGiacomo, K.; Joy, A.; Abhijith, A.; Osei-Amponsah, R.; Hopkins, D.L.; Ha, M.; Chauhan, S.S. Impact of Heat Stress on the Growth Performance and Retail Meat Quality of 2nd Cross (Poll Dorset × (Border Leicester × Merino)) and Dorper Lambs. Meat Sci. 2021, 181, 108581. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Ponnampalam, E.N.; Celi, P.; Hopkins, D.L.; Leury, B.J.; Dunshea, F.R. High Dietary Vitamin E and Selenium Improves Feed Intake and Weight Gain of Finisher Lambs and Maintains Redox Homeostasis under Hot Conditions. Small Rumin. Res. 2016, 137, 17–23. [Google Scholar] [CrossRef]
- Nicolás-López, P.; Macías-Cruz, U.; Mellado, M.; Correa-Calderón, A.; Meza-Herrera, C.A.; Avendaño-Reyes, L. Growth Performance and Changes in Physiological, Metabolic and Hematological Parameters Due to Outdoor Heat Stress in Hair Breed Male Lambs Finished in Feedlot. Int. J. Biometeorol. 2021, 65, 1451–1459. [Google Scholar] [CrossRef]
- Bridi, A.M. Adaptação e Aclimatação Animal; UEL: Londrina, Brazil, 2010. [Google Scholar]
- Silva, R.G. Introdução à Bioclimatologia Animal; Nobel: São Paulo, Brazil, 2000. [Google Scholar]
- Cruz, L.V.; Angrimani, D.D.S.; Rui, B.R.; Silva, M.A. Efeito Do Estresse Térmico Na Produção Leiteira: Revisão de Literatura. Rev. Cient. Electrôn. Med. Vet. 2011, 16, 1–18. [Google Scholar]
- de La Salles, A.Y.F.; Batista, L.F.; de Souza, B.B.; da Silva, A.F.; de Barros Correia, É.L. Growth and Reproduction Hormones of Ruminants Subjected to Heat Stress. J. Anim. Behav. Biometeorol. 2017, 5, 7–12. [Google Scholar] [CrossRef]
- Hafez, E.S.E.; Hafez, B. Reprodução Animal, 7th ed.; Manole: São Paulo, Brazil, 2004. [Google Scholar]
- Ozawa, M.; Tabayashi, D.; Latief, T.A.; Shimizu, T.; Oshima, I.; Kanai, Y. Alterations in Follicular Dynamics and Steroidogenic Abilities Induced by Heat Stress during Follicular Recruitment in Goats. Reproduction 2005, 129, 621–630. [Google Scholar] [CrossRef]
- Da Silva, E.M.N.; De Souza, B.B.; De Sousa, O.B.; Silva, G.D.A.; De Freitas, M.M.S. Avaliação Da Adaptabilidade de Caprinos Ao Semiárido Através de Parâmetros Fisiológicos e Estruturas Do Tegumento. Rev. Caatinga 2010, 23, 142–148. [Google Scholar]
- Salviano, M.B.; Souza, J.D. Avaliação Andrológica e Tecnologia Do Sêmen Caprino. Rev. Bras. Reprod. Anim. 2010, 32, 159–167. [Google Scholar]
- de Assis Silva, G.; de Souza, B.B.; Neto, J.A.; da Silva, E.M.N.; Silva, A.K.B. Efeito Das Épocas Do Ano e de Turno Sobre Os Parâmetros Fisiológicos e Seminais de Caprinos No Semiárido Paraibano. Agropecu. Cient. No Semiarido 2005, 1, 7–14. [Google Scholar]
- Salles, M.G.F. Parâmetros Fisiológicos e Reprodutivos de Machos Caprinos Saanen Criados Em Clima Tropical. Ph.D. Thesis, Universidade Estadual do Ceará, Fortaleza, Brazil, 2010. [Google Scholar]
- Rivier, C.; Rivest, S. Effect of Stress on the Activity of the Hypothalamic-Pituitary-Gonadal Axis: Peripheral and Central Mechanisms. Biol. Reprod. 1991, 45, 523–532. [Google Scholar] [CrossRef]
- Nunes, J.F.; Ciríaco, A.L.T.; Suassuna, U. Produção e Reprodução de Caprinos e Ovinos, 2nd ed.; Editora Gráfica: Fortaleza, Brazil, 1997. [Google Scholar]
- Shehab-El-Deen, M.A.M.M.; Fadel, M.S.; Van Soom, A.; Saleh, S.Y.; Maes, D.; Leroy, J.L.M.R. Circadian Rhythm of Metabolic Changes Associated with Summer Heat Stress in High-Producing Dairy Cattle. Trop. Anim. Health Prod. 2010, 42, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Corassin, C.H. Determinação e Avaliação de Fatores Que Afetam a Produtividade de Vacas Leiteiras: Aspectos Sanitários e Reprodutivos. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2004. [Google Scholar]
- El-Sayed, A.I.M.; Farghaly, H.A.M.; Eid, S.Y.; El-Zaher, H.M. Effect of Heat Stress on Reproductive and Productive Traits in Baladi and Crossbred Goat Does under Subtropical Conditions. J. Nucl. Technol. Appl. Sci. 2018, 6, 31–45. [Google Scholar] [CrossRef]
- Abdel-Hafez, M.A.M. Studies on Reproductive Performance in Sheep. Ph.D. Thesis, Zagazig University, Zagazig, Egypt, 2002. [Google Scholar]
- Aboul-Naga, A.M.; Aboul-Ela, M. Performance of Sub-Tropical Egyptian Sheep Breeds, European Breeds and Their Crosses. 1. Egyptian Sheep Breeds. World Rev. Anim. 1987, 23, 75–82. [Google Scholar]
- Marai, I.F.M.; El-Darawany, A.A.; Abou-Fandoud, E.I.; Abdel-Hafez, M.A.M. Reproductive Traits and the Physiological Background of the Seasonal Variations in Egyptian Suffolk Ewes under the Conditions of Egypt. Ann. Arid Zone 2004, 43, 177–183. [Google Scholar]
- Curtis, S.E. Environmental Management in Animal Agriculture; Iowa State University Press: Ames, IA, USA, 1983. [Google Scholar]
- Marai, I.F.M.; El-Darawany, A.A.; Abou-Fandoud, E.I.; Abdel-Hafez, M.A. Serum Blood Components during Pre-Estrus, Estrus and Pregnancy Phases in Egyptian Suffolk as Affected by Heat Stress, under the Conditions of Egypt. In Proceedings of the 1st International Conference on Enhancement of Small Ruminant Production, Cairo, Egypt, 7–9 February 2006; pp. 47–62. [Google Scholar]
- Shimizu, T.; Ohshima, I.; Ozawa, M.; Takahashi, S.; Tajima, A.; Shiota, M.; Miyazaki, H.; Kanai, Y. Heat stress diminishes gonadotropin receptor expression and enhances susceptibility to apoptosis of rat granulosa cells. Reproduction 2005, 129, 463–472. [Google Scholar] [CrossRef]
- Chandra, V.; Sejian, V.; Sharma, G.T. Strategies to Improve Livestock Reproduction Under the Changing Climate Scenario. In Climate Change Impact on Livestock: Adaptation and Mitigation; Springer: Berlin/Heidelberg, Germany, 2015; pp. 425–439. [Google Scholar]
- Helal, A.; Hashem, A.L.S.; Abdel-Fattah, M.S.; El-Shaer, H.M. Effect of Heat Stress on Coat Characteristics and Physiological Responses of Balady and Damascus Goats in Sinai, Egypt. Am. J. Agric. Environ. Sci. 2010, 7, 60–69. [Google Scholar]
- Adedeji, T.A. of A.S. Effect of Some Qualitative Traits and Non-Genetic Factors on Heat Tolerance Attributes of Extensively Reared West African Dwarf (WAD) Goats. Int. J. Appl. Agric. Apic. Res. 2012, 8, 68–81. [Google Scholar]
- Kadim, I.T.; Mahgoub, O.; Al-Marzooqi, W.; Al-Ajmi, D.S.; Al-Maqbali, R.S.; Al-Lawati, S.M. The Influence of Seasonal Temperatures on Meat Quality Characteristics of Hot-Boned, m. Psoas Major and Minor, from Goats and Sheep. Meat Sci. 2008, 80, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ramesh, K.; Hyder, I.; Uniyal, S.; Yadav, V.P.; Panda, R.P.; Maurya, V.P.; Singh, G.; Kumar, P.; Mitra, A.; et al. Effect of Melatonin Administration on Thyroid Hormones, Cortisol and Expression Profile of Heat Shock Proteins in Goats (Capra hircus) Exposed to Heat Stress. Small Rumin. Res. 2013, 112, 216–223. [Google Scholar] [CrossRef]
- Sophia, I.; Sejian, V.; Bagath, M.; Bhatta, R. Quantitative Expression of Hepatic Toll-like Receptors 1–10 MRNA in Osmanabadi Goats during Different Climatic Stresses. Small Rumin. Res. 2016, 141, 11–16. [Google Scholar] [CrossRef]
- Aggarwal, A.; Upadhyay, R. Heat Stress and Immune Function. In Heat Stress and Animal Productivity; Springer: New Delhi, India, 2013; pp. 113–136. [Google Scholar]
- Carroll, J.A.; Burdick, N.C.; Chase, C.C.; Coleman, S.W.; Spiers, D.E. Influence of Environmental Temperature on the Physiological, Endocrine, and Immune Responses in Livestock Exposed to a Provocative Immune Challenge. Domest. Anim. Endocrinol. 2012, 43, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat Stress Causes Immune Abnormalities via Massive Damage to Effect Proliferation and Differentiation of Lymphocytes in Broiler Chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef]
- Yadav, V.P.; Dangi, S.S.; Chouhan, V.S.; Gupta, M.; Dangi, S.K.; Singh, G.; Maurya, V.P.; Kumar, P.; Sarkar, M. Expression Analysis of NOS Family and HSP Genes during Thermal Stress in Goat (Capra hircus). Int. J. Biometeorol. 2016, 60, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The Impact of Heat Stress on the Immune System in Dairy Cattle: A Review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Rashamol, V.P.; Sejian, V.; Bagath, M.; Krishnan, G.; Beena, V.; Bhatta, R. Effect of Heat Stress on the Quantitative Expression Patterns of Different Cytokine Genes in Malabari Goats. Int. J. Biometeorol. 2019, 63, 1005–1013. [Google Scholar] [CrossRef]
- Madhusoodan, A.P.; Sejian, V.; Afsal, A.; Bagath, M.; Krishnan, G.; Savitha, S.T.; Rashamol, V.P.; Devaraj, C.; Bhatta, R. Differential Expression Patterns of Candidate Genes Pertaining to Productive and Immune Functions in Hepatic Tissue of Heat-Stressed Salem Black Goats. Biol. Rhythm Res. 2021, 52, 809–820. [Google Scholar] [CrossRef]
- Zambom, M.A.; Alcalde, C.R.; Martins, E.N.; dos Santos, G.T.; Macedo, F.d.A.F.d.; Horst, J.A.; da Veiga, D.R. Curva de Lactação e Qualidade Do Leite de Cabras Saanen Recebendo Rações Com Diferentes Relações Volumoso: Concentrado. Rev. Bras. Zootec. 2005, 34, 2515–2521. [Google Scholar] [CrossRef]
- Pereira, G.M.; Souza, B.B.D.; Silva, A.M.D.A.; Roberto, J.V.B.; Silva, C.M.B.D.A. Avaliação Do Comportamento Fisiológico De Caprinos Da Raça Saanen No Semiárido Paraibano. Rev. Verde Agroecol. Desenvolv. Sustent. 2011, 6, 195–199. [Google Scholar]
- Costa, R.G.; Queiroga, R.D.C.R.E.; Pereira, R.A.G. Influência Do Alimento Na Produção e Qualidade Do Leite de Cabra. Rev. Bras. Zootec. 2009, 38, 307–321. [Google Scholar] [CrossRef]
- Viana, M.P.; da Rocha Medeiros, A.; de Souza, B.B. Efeitos Do Estresse Térmico Sobre a Fisiologia, Produção e Reprodução de Caprinos Effect of Thermal Stress on the Physiology, Production and Reproduction of Goats. ACSA Agropecu. Cient. No Semiarido 2013, 9, 1–8. [Google Scholar]
- Baccari Júnior, F.; Gonçalves, H.C.; Muniz, L.M.R.; Polastre, R.; Head, H.H. Milk Production, Serum Concentrations of Thyroxine and Some Physiological Responses of Saanen-Native Goats during Thermal Stress. Vet. Zootec. 1996, 8, 9–14. [Google Scholar]
- Juaréz, M. Physico-Chemical Characteristics of Goat’s Milk as Distinct from Those of Cow’s Milk. Bull. Int. Dairy Fed. 1986, 202, 54–67. [Google Scholar]
- Souza, B.B.; Silva, G.A.; Zotti, C.A.; e Silva, E.M.N. Termografia: Avaliação a Adaptação de Caprinos Leiteiros e Conforto Térmico Das Instalações. Disponível em FARMPOINT Ovinos e Caprinos. Disponível em. 2011. Available online: http://www.cstr.ufcg.edu.br/bioclimatologia/artigos_tecnicos/termografia_avaliacao_adaptacao.pdf (accessed on 12 April 2020).
- Darcan, N.; Güney, O. Alleviation of Climatic Stress of Dairy Goats in Mediterranean Climate. Small Rumin. Res. 2008, 74, 212–215. [Google Scholar] [CrossRef]
- de Souza, B.B.; de Souza, E.D.; Cezar, M.F.; de Souza, W.H.; dos Santos, J.R.S.; Benicio, T.M.A. Temperatura Superficial e Índice de Tolerância Ao Calor de Caprinos de Diferentes Grupos Raciais No Semi-Árido Nordestino. Ciênc. Agrotecnol. 2008, 32, 275–280. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Jones, S.D.M.; Stanley, R.W. The Use of Electrolyte Solutions for Reducing Transport Stress. J. Anim. Sci. 1997, 75, 258–265. [Google Scholar] [CrossRef]
- Rana, M.; Hashem, M.; Akhter, S.; Habibullah, M.; Islam, M.; Biswas, R. Effect of Heat Stress on Carcass and Meat Quality of Indigenous Sheep of Bangladesh. Bangladesh J. Anim. Sci. 2014, 43, 147–153. [Google Scholar] [CrossRef]
- Hashem, M.; Hossain, M.; Rana, M.; Hossain, M.; Islam, M.; Saha, N. Effect of Heat Stress on Blood Parameter, Carcass and Meat Quality of Black Bengal Goat. Bangladesh J. Anim. Sci. 2013, 42, 57–61. [Google Scholar] [CrossRef]
- Newton, K.G.; Gill, C.O. The Microbiology of DFD Fresh Meats: A Review. Meat Sci. 1981, 5, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T.; Lubach, G.R.; Coe, C.L. Prenatal Stress Alters Bacterial Colonization of the Gut in Infant Monkeys. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Verbrugghe, E.; Boyen, F.; Gaastra, W.; Bekhuis, L.; Leyman, B.; Van Parys, A.; Haesebrouck, F.; Pasmans, F. The Complex Interplay between Stress and Bacterial Infections in Animals. Vet. Microbiol. 2012, 155, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Braber, S.; Garssen, J.; Wichers, H.J.; Folkerts, G.; Fink-Gremmels, J.; Varasteh, S. Beyond Heat Stress: Intestinal Integrity Disruption and Mechanism-Based Intervention Strategies. Nutrients 2020, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, J.A.; Flores, J.A.; Duarte, G.; Vielma, J.; Hernández, H.; Bedos, M.; Fitz-Rodríguez, G.; Fernández, I.G.; López-Sebastián, A.; Gómez-Brunet, A.; et al. Out-of-Season Control of Reproduction in Subtropical Goats without Exogenous Hormonal Treatments. Small Rumin. Res. 2014, 121, 7–11. [Google Scholar] [CrossRef]
- McDonald, B.J.; Hoey, W.A. Effect of Photo-Translation on Fleece Growth in Cashmere Goats. Aust. J. Agric. Res. 1987, 38, 765–777. [Google Scholar] [CrossRef]
- Cong, Y.; Deng, H.; Feng, Y.; Chen, Q.; Sun, Y. Melatonin Implantation from Winter Solstice Could Extend the Cashmere Growth Phase Effectively. Small Rumin. Res. 2011, 99, 48–53. [Google Scholar] [CrossRef]
- Liu, B.; Gao, F.; Guo, J.; Wu, D.; Hao, B.; Li, Y.; Zhao, C. A Microarray-Based Analysis Reveals That a Short Photoperiod Promotes Hair Growth in the Arbas Cashmere Goat. PLoS ONE 2016, 11, e0147124. [Google Scholar] [CrossRef]
- Wu, Z.; Duan, C.; Li, Y.; Duan, T.; Mo, F.; Zhang, W. Melatonin Implantation during the Non-Growing Period of Cashmere Increases the Cashmere Yield of Female Inner Mongolian Cashmere Goats by Increasing Fiber Length and Density. Span. J. Agric. Res. 2018, 16, e06SC01-01. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Sun, H.Z.; Li, S.L.; Sang, D.; Zhang, C.H.; Jin, L.; Antonini, M.; Zhao, C.F. Effects of Photoperiod on Nutrient Digestibility, Hair Follicle Activity and Cashmere Quality in Inner Mongolia White Cashmere Goats. Asian-Australas. J. Anim. Sci. 2019, 32, 541–547. [Google Scholar] [CrossRef]
- Aleena, J.; Sejian, V.; Bagath, M.; Krishnan, G.; Beena, V.; Bhatta, R. Resilience of Three Indigenous Goat Breeds to Heat Stress Based on Phenotypic Traits and PBMC HSP70 Expression. Int. J. Biometeorol. 2018, 62, 1995–2005. [Google Scholar] [CrossRef]
- Afsal, A.; Sejian, V.; Bagath, M.; Devaraj, C.; Bhatta, R. Heat Stress and Livestock Adaptation: Neuro-Endocrine Regulation. Int. J. Vet. Anim. Med. 2018, 2, 1–7. [Google Scholar]
- Battini, M.; Vieira, A.; Barbieri, S.; Ajuda, I.; Stilwell, G.; Mattiello, S. Invited Review: Animal-Based Indicators for on-Farm Welfare Assessment for Dairy Goats. J. Dairy Sci. 2014, 97, 6625–6648. [Google Scholar] [CrossRef]
- Carabaño, M.J.; Ramón, M.; Menéndez-Buxadera, A.; Molina, A.; Díaz, C. Selecting for Heat Tolerance. Anim. Front. 2019, 9, 62–68. [Google Scholar] [CrossRef]
- Yakubu, A.; Salako, A.E.; De Donato, M.; Peters, S.O.; Takeet, M.I.; Wheto, M.; Okpeku, M.; Imumorin, I.G. Association of SNP Variants of MHC Class II DRB Gene with Thermo-Physiological Traits in Tropical Goats. Trop. Anim. Health Prod. 2017, 49, 323–333. [Google Scholar] [CrossRef]
- Khan, Z.A.; Mishra, C.; Dige, M. Association of Novel Polymorphisms in Caprine SOD3 Gene with Physiological and Biochemical Parameters. Biol. Rhythm Res. 2021, 52, 759–773. [Google Scholar] [CrossRef]
- Angel, S.P.; Bagath, M.; Sejian, V.; Krishnan, G.; Bhatta, R. Expression Patterns of Candidate Genes Reflecting the Growth Performance of Goats Subjected to Heat Stress. Mol. Biol. Rep. 2018, 45, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.R. Ruminant Heat Stress: Effect on Production and Means of Alleviation. J. Anim. Sci. 1983, 57, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Osei-Amponsah, R.; Chauhan, S.S.; Leury, B.J.; Cheng, L.; Cullen, B.; Clarke, I.J.; Dunshea, F.R. Genetic Selection for Thermotolerance in Ruminants. Animals 2019, 9, 948. [Google Scholar] [CrossRef]
- Pereira, A.M.F.; Titto, E.L.; Infante, P.; Titto, C.G.; Geraldo, A.M.; Alves, A.; Leme, T.M.; Baccari, F.; Almeida, J.A. Evaporative Heat Loss in Bos Taurus: Do Different Cattle Breeds Cope with Heat Stress in the Same Way? J. Therm. Biol. 2014, 45, 87–95. [Google Scholar] [CrossRef]
- Cardoso, C.C.; Peripolli, V.; Amador, S.A.; Brandão, E.G.; Esteves, G.I.F.; Sousa, C.M.Z.; França, M.F.M.S.; Gonçalves, F.G.; Barbosa, F.A.; Montalvão, T.C.; et al. Physiological and Thermographic Response to Heat Stress in Zebu Cattle. Livest. Sci. 2015, 182, 83–92. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of Heat Stress on Animal Physiology, Metabolism, and Meat Quality: A Review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Silanikove, N. Why Goats Raised on Harsh Environment Perform Better than Other Domesticated Animals. Options Mediterr. 1997, 34, 185–194. [Google Scholar]
- Kandemir, C.; Koşum, N.; Taşkin, T. Effects Of Heat Stress On Physiological Traits In Sheep. Maced. J. Anim. Sci. 2013, 3, 25–29. [Google Scholar] [CrossRef]
- Al-Tamimi, H.J. Thermoregulatory Response of Goat Kids Subjected to Heat Stress. Small Rumin. Res. 2007, 71, 280–285. [Google Scholar] [CrossRef]
- Berger, Y.; Billon, P.; Bocquier, F.; Caja, G.; Cannas, A.; McKusick, B.; Marnet, P.-G.; Thomas, D. Principles of Sheep Dairying in North America; University of Wisconsin-Extension: Madison, WI, USA, 2004; p. 156. [Google Scholar]
- Hammadi, M.; Fehem, A.; Harrabi, H.; Ayeb, N.; Khorchani, T.; Salama, A.A.K.; Casals, R.; Such, X.; Caja, G. Shading Effects on Respiratory Rate and Rectal Temperature in Tunisian Local Goat Kids during Summer Season. In Proceedings of the XI International Conference on Goats, Gran Canaria, Spain, 23–27 September 2012; Volume 127. [Google Scholar]
- Muller, C.J.C.; Botha, J.A.; Smith, W.A. Effect of Shade on Various Parameters of Friesian Cows in a Mediterranean Climate in South Africa. 3. Behaviour. S. Afr. J. Anim. Sci. 1994, 24, 61–66. [Google Scholar]
- Onyewotu, L.O.Z.; Stigter, C.J.; Abdullahi, A.M.; Ariyo, J.A.; Oladipo, E.O.; Owonubi, J.J. Reclamation of Desertified Farmlands and Consequences for Its Farmers in Semiarid Northern Nigeria: A Case Study of Yambawa Rehabilitation Scheme. Arid. Land Res. Manag. 2003, 17, 85–101. [Google Scholar] [CrossRef]
- Bond, T.; Kelly, C.; Garrett, W.; Hahn, L. Evaluation of Materials for Livestock Shades. Calif. Agric. 1961, 15, 7–8. [Google Scholar]
- Bond, T.E.; Givens, S.R.M.A.R.L. Givens Influence of Surroundings on Radiant Heat Load of Animals. Trans. ASAE 1969, 12, 246–248. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S. Effect of Management Strategies on Reducing Heat Stress of Feedlot Cattle: Feed and Water Intake. J. Anim. Sci. 2004, 82, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Soto-Navarro, S.A.; Krehbiel, C.R.; Duff, G.C.; Galyean, M.L.; Brown, M.S.; Steiner, R.L. Influence of Feed Intake Fluctuation and Frequency of Feeding on Nutrient Digestion, Digesta Kinetics, and Ruminal Fermentation Profiles in Limit-Fed Steers. J. Anim. Sci. 2000, 78, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Atrian, P.; Shahryar, H.A. Heat Stress in Dairy Cows. Res. Zool. 2012, 2, 31–37. [Google Scholar]
- Ayo, J.O.; Minka, N.S.; Mamman, M. Excitability Scores of Goats Administered Ascorbic Acid and Transported during Hot-Dry Conditions. J. Vet. Sci 2006, 7, 127–131. [Google Scholar] [CrossRef]
- Ghanem, A.M.; Jaber, L.S.; Abi Said, M.; Barbour, E.K.; Hamadeh, S.K. Physiological and Chemical Responses in Water-Deprived Awassi Ewes Treated with Vitamin C. J. Arid Environ. 2008, 72, 141–149. [Google Scholar] [CrossRef]
- Sivakumar, A.V.N.; Singh, G.; Varshney, V.P. Antioxidants Supplementation on Acid Base Balance during Heat Stress in Goats. Asian-Australas. J. Anim. Sci. 2010, 23, 1462–1468. [Google Scholar] [CrossRef]
- Kobeisy, S. Effect of Vitamin C and E on Rectal Temperature and Respiratory Rates in Heat Stressed Goats. Assiut Vet. Med. J. 1997, 37, 120–132. [Google Scholar]
- Kim, E.S.; Elbeltagy, A.R.; Aboul-Naga, A.M.; Rischkowsky, B.; Sayre, B.; Mwacharo, J.M.; Rothschild, M.F. Multiple Genomic Signatures of Selection in Goats and Sheep Indigenous to a Hot Arid Environment. Heredity 2016, 116, 255–264. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Martínez, L.; Aréchiga, C.; Bañuelos, R.; Rincón, R.M.; Urrutia, J.; Salinas, H.; Mellado, M. Circannual Identification and Quantification of Constitutive Heat Shock Proteins (HSP 70) in Goats. J. Appl. Anim. Res. 2006, 29, 9–12. [Google Scholar] [CrossRef]
- Onzima, R.B.; Upadhyay, M.R.; Doekes, H.P.; Brito, L.F.; Bosse, M.; Kanis, E.; Groenen, M.A.M.; Crooijmans, R.P.M.A. Genome-Wide Characterization of Selection Signatures and Runs of Homozygosity in Ugandan Goat Breeds. Front. Genet. 2018, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Sharma, A.; Sodhi, M.; Thakur, K.; Kataria, R.S.; Niranjan, S.K.; Bharti, V.K.; Kumar, P.; Giri, A.; Kalia, S.; et al. Transcriptome Analysis of Circulating PBMCs to Understand Mechanism of High Altitude Adaptation in Native Cattle of Ladakh Region. Sci. Rep. 2018, 8, 7681. [Google Scholar] [CrossRef] [PubMed]

| Aspect of Goats Production | Articles Identified | Source |
|---|---|---|
| Behavioral response | 25 | Google Scholar, Sci-hub |
| Growth and development | 12 | ResearchGate, Google Scholar |
| Reproductive performance | 21 | ResearchGate, Sci-hub, Web of Science |
| Physiological Response | 6 | Google Scholar, ResearchGate, |
| Health and immunity | 8 | Research Gate, Sci-hub, Google Scholar |
| Milk quantity and quality | 10 | Web of Science, Google Scholar, Sci-hub |
| Meat quality and carcass characteristics | 8 | Google, scholar, Sci-hub |
| Cashmere production | 6 | ResearchGate, Google Scholar |
| Genetic adaptability | 12 | Research Gate, Sci-hub, Google Scholar |
| Breed | HS Condition | Body Weight Change | Observation | References |
|---|---|---|---|---|
| Osmanabadi goats Malabari goats Salem Black Goats | Summer exposure: 73.5 to 86.5 THI Shed feeding: 69.9 to 74.9 THI 45-d feeding | ADG: Osmanabadi: Exposure—39.63 g Shed 48.02 g Malabari: Exposure—25.00 g Shed: 39.29 g Shalem Black: Exposure—21.03 g Shed 34.53 g | Heat stress significantly reduced the body weight gain among all heat-exposed groups, but the reduction in feed intake of the heat stress group was not significant (except for Malabari goats) | [46] |
| Poll Dorset × (Border Leicester × Merino) lambs Dorper lambs | HS: 28 °C to 38 °C, 40% to 60% RH cyclic TN: 18 °C to 21 °C, 45% to 55% RH 2-wk study | ADG: Dorper: HS—50.6 g TN: 5.95 g 2nd cross: HS—92.3 g TN 101.0 g | Two weeks of cyclic HS had a significant negative influence on feed intake and body weight gain of wool breed lambs (2nd cross), but the impact of HS was not significant for the hair breed (Dorper lambs). | [47] |
| White Suffolk × Merino × Border Leicester lambs (42 ± 2.0 kg; 7 mo) | HS: 28 °C to 40 °C, 30% to 40% RH TN: 18 °C to 21 °C, 40% to 50% RH | Feed intake: HS 959 g/d TN 1266 g/d | One week of HS significantly impacted 2nd cross lambs’ feed intake compared with the TN group. | [48] |
| Dorper × Katahdin male lambs (34.6 ± 1.4 kg; 4.5 mo) | Summer: 28.3 ± 4.0 °C, 77.2 ± 5.4 THI Winter: 19.2 ± 2.6 °C, 64.0 ± 3.0 THI 30-d study | ADG: Dorper × Katahdin: Summer 226 g Winter 302 g | The average body weight gain and feed efficiency of the summer group were significantly lower than those of the winter group. | [49] |
| Breeds | Genes | Function | Reference |
|---|---|---|---|
| Baraki goat | FGF2, GNAI3, PLCB1 | Thermo-tolerance (melanogenesis) | [134] |
| Mexico goat | HSP-70 | Thermo-tolerant | [135] |
| Baraki goat | MYH, TRHDE, ALDH1A3 | Energy and digestive metabolism | [134] |
| Uganda goat | 1L10RB and IL23A | Immune response | [136] |
| Baraki goat | GRIA1, IL2, IL7, IL21, IL1R1 | Nervous and autoimmune response | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danso, F.; Iddrisu, L.; Lungu, S.E.; Zhou, G.; Ju, X. Effects of Heat Stress on Goat Production and Mitigating Strategies: A Review. Animals 2024, 14, 1793. https://doi.org/10.3390/ani14121793
Danso F, Iddrisu L, Lungu SE, Zhou G, Ju X. Effects of Heat Stress on Goat Production and Mitigating Strategies: A Review. Animals. 2024; 14(12):1793. https://doi.org/10.3390/ani14121793
Chicago/Turabian StyleDanso, Felix, Lukman Iddrisu, Shera Elizabeth Lungu, Guangxian Zhou, and Xianghong Ju. 2024. "Effects of Heat Stress on Goat Production and Mitigating Strategies: A Review" Animals 14, no. 12: 1793. https://doi.org/10.3390/ani14121793
APA StyleDanso, F., Iddrisu, L., Lungu, S. E., Zhou, G., & Ju, X. (2024). Effects of Heat Stress on Goat Production and Mitigating Strategies: A Review. Animals, 14(12), 1793. https://doi.org/10.3390/ani14121793






