Osteomyelitis in Pig Carcasses at a Portuguese Slaughterhouse: Association with Tail-Biting and Teeth Resection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Descriptive Data Analysis
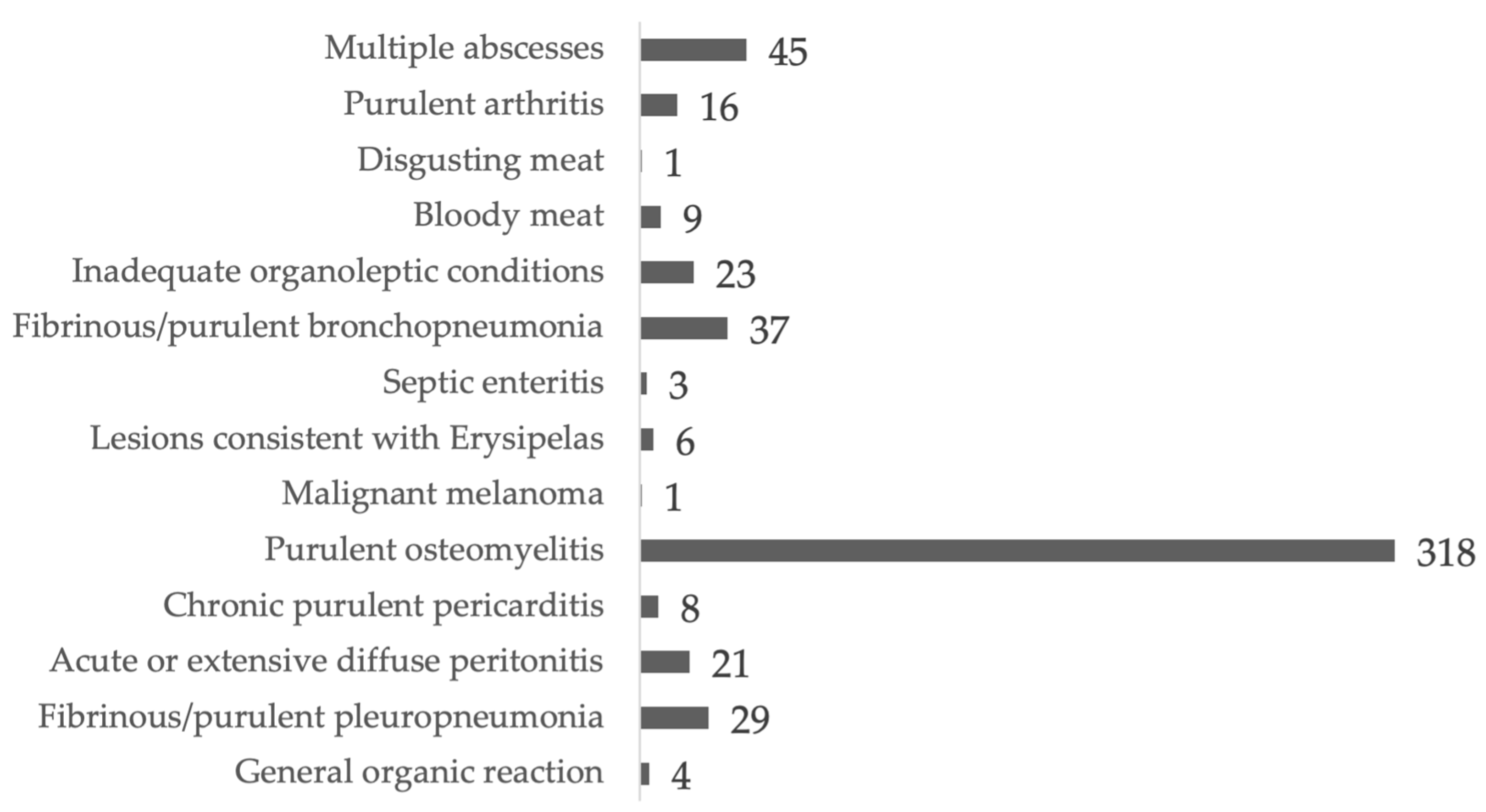
3.2. Univariate Analysis of Carcasses Condemned for Osteomyelitis
3.3. Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gieling, F.; Peters, S.; Erichsen, C.; Richards, R.G.; Zeiter, S.; Moriarty, T.F. Bacterial osteomyelitis in veterinary orthopaedics: Pathophysiology, clinical presentation and advances in treatment across multiple species. Vet. J. 2019, 250, 44–54. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, M.; Silva, V.; Poeta, P.; Corbera, J.A.; Tejedor-Junco, M.T. Microbiological aspects of osteomyelitis in veterinary medicine: Drawing parallels to the infection in human medicine. Vet. Q. 2022, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bækbo, A.K.; Petersen, J.V.; Larsen, M.H.; Alban, L. The food safety value of de-boning finishing pig carcasses with lesions indicative of prior septicemia. Food Control 2016, 69, 177–184. [Google Scholar] [CrossRef]
- Patel, M.; Rojavin, Y.; Jamali, A.A.; Wasielewski, S.J.; Salgado, C.J. Animal models for the study of osteomyelitis. Semin. Plast. Surg. 2009, 23, 148–154. [Google Scholar] [CrossRef][Green Version]
- Vieira-Pinto, M.; Azevedo, J.; Poeta, P.; Pires, I.; Ellebroek, L.; Lopes, R.; Veloso, M.; Alban, L. Classification of Vertebral Osteomyelitis and Associated Judgment Applied during Post-Mortem Inspection of Swine Carcasses in Portugal. Foods 2020, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diez, J.; Coelho, A. Causes and Factors Related to Pig Carcass Condemnation. Vet. Med. 2014, 59, 194–201. [Google Scholar] [CrossRef]
- Martínez, J.; Jaro, P.J.; Aduriz, G.; Gómez, E.A.; Peris, B.; Corpa, J.M. Carcass Condemnation Causes of Growth Retarded Pigs at Slaughter. Vet. J. 2007, 174, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, P.; Pallarés, F.J.; Gómez, M.A.; Bernabé, A.; Gómez, S.; Seva, J. Importance of the knowledge of pathological processes for risk-based inspection in pig slaughterhouses (Study of 2002 to 2016). Asian-Australas J. Anim. Sci. 2018, 31, 1818. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Commission Implementing Regulation (EU) 2019/627 of 15 March 2019, laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls. Off. J. Eur. Union 2019, 131, 51–100. [Google Scholar]
- Ninčáková, S.; Večerek, V.; Válková, L.; Voslářová, E.; Kaluža, M.; Zavřelová, V. Health status of slaughtered animals as indicated by post-mortem inspection at slaughterhouses. Acta Vet. Brno 2022, 91, 99–106. [Google Scholar] [CrossRef]
- Vecerek, V.; Voslarova, E.; Semerad, Z.; Passantino, A. The health and welfare of pigs from the perspective of post mortem findings in slaughterhouses. Animals 2020, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Brandt, P.; Aaslyng, M.D. Welfare measurements of finishing pigs on the day of slaughter: A review. Meat. Sci. 2015, 103, 13–23. [Google Scholar] [CrossRef] [PubMed]
- De Luca, S.; Zanardi, E.; Alborali, G.L.; Ianieri, A.; Ghidini, S. Abattoir-based measures to assess swine welfare: Analysis of the methods adopted in European slaughterhouses. Animals 2021, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Čobanović, N.; Stajković, S.; Blagojević, B.; Betić, N.; Dimitrijević, M.; Vasilev, D.; Karabasil, N. The effects of season on health, welfare, and carcass and meat quality of slaughter pigs. Int. J. Biometeorol. 2020, 64, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, O.A.; Faucitano, L.; Coldebella, A.; Ludke, J.V.; Peloso, J.V.; dalla Roza, D.; da Costa, M.P. Effects of the season of the year, truck type, and location on truck on skin bruises and meat quality in pigs. Livest. Sci. 2007, 107, 29–36. [Google Scholar] [CrossRef]
- Harley, S.; More, S.J.; O’Connell, N.E.; Hanlon, A.; Teixeira, D.; Boyle, L. Evaluating the Prevalence of Tail biting and Carcase Condemnations in Slaughter Pigs in the Republic and Northern Ireland, and the Potential of Abattoir Meat Inspection as a Welfare Surveillance Tool. Vet. Rec. 2012, 171, 621. [Google Scholar] [CrossRef] [PubMed]
- Kongsted, H.; Sørensen, J.T. Lesions found at routine meat inspection on finishing pigs are associated with production system. Vet. J. 2017, 223, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Valkova, L.; Vecerek, V.; Voslarova, V.; Kaluza, M.; Takacova, D. The Welfare of Cattle, Sheep, Goats and Pigs from the Perspective of Traumatic Injuries Detected at Slaughterhouse Post mortem Inspection. Animals 2021, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- Vom Brocke, A.L.; Karnholz, C.; Madey-Rindermann, D.; Gauly, M.; Leeb, C.; Winckler, C.; Schrader, L.; Dippel, S. Tail Lesions in Fattening Pigs: Relationships with Post-mortem Meat Inspection and Influence of a Tail biting Management Tool. Animal 2019, 13, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Schrøder-Petersen, D.L.; Simonsen, H.B. Tail biting in pigs. Vet. J. 2001, 162, 196–210. [Google Scholar] [CrossRef]
- Kritas, S.K.; Morrison, R.B. Relationships between tail biting in pigs and disease lesions and condemnations at slaughter. Vet. Rec. 2007, 160, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.M.F.; Bernardi, M.L.; Coelho, C.F.; Almeida, M.; Morales, O.E.; Mores, T.J.; Borowski, S.M.; Barcellos, D.E.S.N. Influence of tail biting on weight gain, lesions and condemnations at slaughter of finishing pigs. Pesq. Vet. Bras. 2012, 32, 967–974. [Google Scholar] [CrossRef]
- Smulders, D.; Hautekiet, V.; Verbeke, G.; Geers, R. Tail and ear biting lesions in pigs: An epidemiological study. Anim. Welf. 2008, 17, 61–69. [Google Scholar] [CrossRef]
- Heinonen, M.; Orro, T.; Kokkonen, T.; Munsterhjelm, C.; Peltoniemi, O.; Valros, A. Tail biting induces a strong acute phase response and tail-end inflammation in finishing pigs. Vet. J. 2010, 184, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.L.; Harley, S.; Hanlon, A.; O’Connell, N.E.; More, S.J.; Manzanilla, E.G.; Boyle, L.A. Study on the association between tail lesion score, cold carcass weight, and viscera condemnations in slaughter pigs. Front. Vet. Sci. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Boyle, L.A.; Edwards, S.A.; Bolhuis, J.E.; Pol, F.; Šemrov, M.Z.; Schütze, S.; Nordgreen, J.; Bozakova, N.; Sossidou, E.N.; Valros, A. The evidence for a causal link between disease and damaging behavior in pigs. Front. Vet. Sci. 2022, 8, 771682. [Google Scholar] [CrossRef] [PubMed]
- Van Staaveren, N.; Vale, A.P.; Manzanilla, E.G.; Teixeira, D.L.; Leonard, F.C.; Hanlon, A.; Boyle, L.A. Relationship between Tail Lesions and Lung Health in Slaughter Pigs. Prev. Vet. Med. 2016, 127, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Rue, J.; Sansac, C.; Brunel, G.; Prunier, A. Long-term detrimental effects of tooth clipping or grinding in piglets: A histological approach. Anim. Welf. 2004, 13, 27–32. [Google Scholar] [CrossRef]
- Prunier, A.; Devillers, N.; Herskin, M.; Sandercock, D.; Sinclair, A.; Tallet, C.; von Borell, E. Husbandry interventions in suckling piglets, painful consequences and mitigation. In The Suckling and Weaned Piglet; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2020; pp. 107–138. [Google Scholar] [CrossRef]
- Anonymous. Commission Directive 2001/93/EC of 9 November 2001 amending Directive 91/630/EEC laying down minimum standards for the protection of pigs. Off. J. Eur. Union 2001, L 316, 0036–0038. [Google Scholar]
- Fredriksen, B.; i Furnols, M.F.; Lundström, K.; Migdal, W.; Prunier, A.; Tuyttens, F.A.; Bonneau, M. Practice on castration of piglets in Europe. Animal 2009, 3, 1480–1487. [Google Scholar] [CrossRef]
- Gallois, M.; Le Cozler, Y.; Prunier, A. Influence of tooth resection in piglets on welfare and performance. Prev. Vet. Med. 2005, 69, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Holyoake, P.K.; Broek, D.J.; Callinan, A.P. The effects of reducing the length of canine teeth in sucking pigs by clipping or grinding. Aust. Vet. J. 2004, 82, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.A. Welfare implications of invasive piglet husbandry procedures, methods of alleviation and alternatives: A review. N. Z. Vet. J. 2015, 63, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhou, B.; Li, H.; Liang, T.; Chu, Q.; Schinckel, A.P.; Li, Y.; Xu, F. Effects of tail docking and/or teeth clipping on behavior, lesions, and physiological indicators of sows and their piglets. Anim. Sci. J. 2019, 90, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Reese, D.; Straw, B.E. Teeth Clipping—Have You Tried to Quit? Neb. Swine Rep. 2005, 33, 12–13. Available online: https://digitalcommons.unl.edu/coopext_swine/33 (accessed on 2 June 2024).
- DGAV. Análise Exploratória dos Dados de Abate de Ungulados Para Consumo Humano em Portugal Entre Janeiro de 2011 e Dezembro de 2019. 2020. Available online: https://www.dropbox.com/s/26p3sukb6vx6koc/Dados%20de%20abates%20e%20reprova%C3%A7%C3%B5es%20Ungulados%202011%20a%202019.pdf?dl=0 (accessed on 26 April 2023).
- Voslarova, E.; Vecerek, V.; Passantino, A.; Chloupek, P.; Bedanova, I. Transport losses in finisher pigs: Impact of transport distance and season of the year. Asian-Australas J. Anim. Sci. 2017, 30, 119. [Google Scholar] [CrossRef]
- Driessen, B.; Van Beirendonck, S.; Buyse, J. Effects of transport and lairage on the skin damage of pig carcasses. Animals 2020, 10, 575. [Google Scholar] [CrossRef]
- Fraile, L.; Alegre, A.; López-Jiménez, R.; Nofrarías, M.; Segalés, J. Risk factors associated with pleuritis and cranio-ventral pulmonary consolidation in slaughter-aged pigs. Vet. J. 2010, 184, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Bottacini, M.; Scollo, A.; Contiero, B.; Mazzoni, C.; Pace, V.; Gottardo, F. Prevalence of fibrinous pericarditis in heavy pigs (170 kg) and its association with other pluck lesions at slaughter inspection. Vet. J. 2021, 273, 105680. [Google Scholar] [CrossRef]
- Beirendonck, S.V.; Driessen, B.; Verbeke, G.; Permentier, L.; Van de Perre, V.; Geers, R. Improving survival, growth rate, and animal welfare in piglets by avoiding teeth shortening and tail docking. J. Vet. Behav. 2012, 7, 88–93. [Google Scholar] [CrossRef]
- Araújo, A.A.; Cidral, J.C.; Silvano, E.; Lafin, N.A.; Marson, E.P. Avaliação da Prática do Corte dos Dentes dos Leitões na Maternidade. Edu. br. 2013. Available online: https://professor.pucgoias.edu.br/SiteDocente/admin/arquivosUpload/4753/material/Corte%20dos%20Dentes%20dos%20Leit%C3%B5es.pdf (accessed on 28 April 2023).
- Koller, F.L.; Borowski, S.M.; Asanome, W.; Hein, G.; Lagemann, F.L.; Driemeier, D.; de Barcellos, D.E.S. Abscessos dentários periapicais em leitões com síndrome multissistêmica do definhamento. Pesqui. Vet. Bras. 2008, 28, 271–274. [Google Scholar] [CrossRef]
- Fertner, M.; Denwood, M.; Birkegård, A.C.; Stege, H.; Boklund, A. Associations between Antibacterial Treatment and the Prevalence of Tail biting-Related Sequelae in Danish Finishers at Slaughter. Front. Vet. Sci. 2017, 4, 182. [Google Scholar] [CrossRef] [PubMed]
- Ninios, T.; Lundén, J.; Korkeala, H.; Fredriksson-Ahomaa, M. Meat Inspection and Control in the Slaughterhouse, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; p. 35. [Google Scholar] [CrossRef]

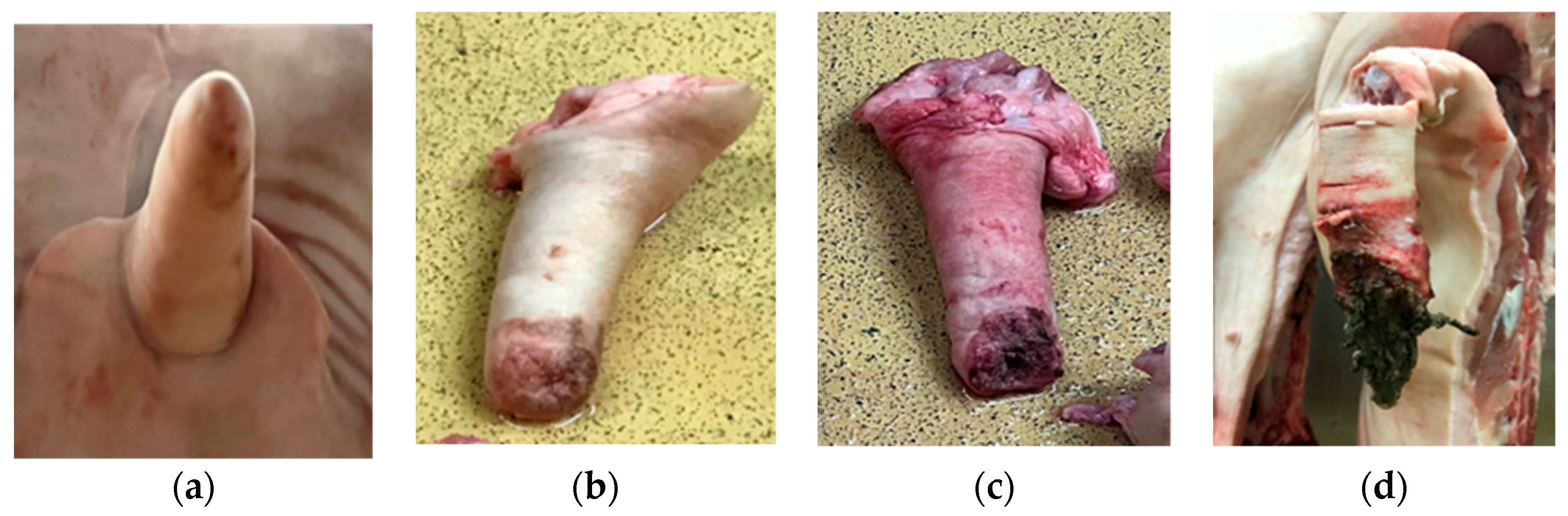
| Variable | Classification | Abs. Freq. * (CCO) | Rel. Freq. ** (CCO) |
|---|---|---|---|
| Season | Spring | 111 | 34.90% |
| Winter | 72 | 22.64% | |
| Autumn | 66 | 20.75% | |
| Summer | 69 | 21.69% | |
| Origin | Portugal | 245 | 77.04% |
| France | 14 | 4.40% | |
| Spain | 59 | 18.55% | |
| Sex | Female | 220 | 69.18% |
| Male | 98 | 30.81% |
| Classification | Abs. Freq. * | Rel. Freq. ** | |
|---|---|---|---|
| Cardiorespiratory lesions | Pleurisy | 115 | 36.16% |
| No pleurisy | 203 | 63.83% | |
| Pneumonia | 32 | 10.06% | |
| No pneumonia | 286 | 89.93% | |
| Pericarditis | 52 | 16.35% | |
| No pericarditis | 266 | 83.64% | |
| Tail-biting | Tail-biting lesions grade 1 | 26 | 8.17% |
| Tail-biting lesions grade 2 | 26 | 8.17% | |
| Tail-biting lesions grade 3 | 56 | 17.61% | |
| No tail-biting lesions | 210 | 66.03% | |
| Teeth resection | Intact | 39 | 12.26% |
| Clipped | 226 | 71.06% | |
| Ground | 53 | 16.66% |
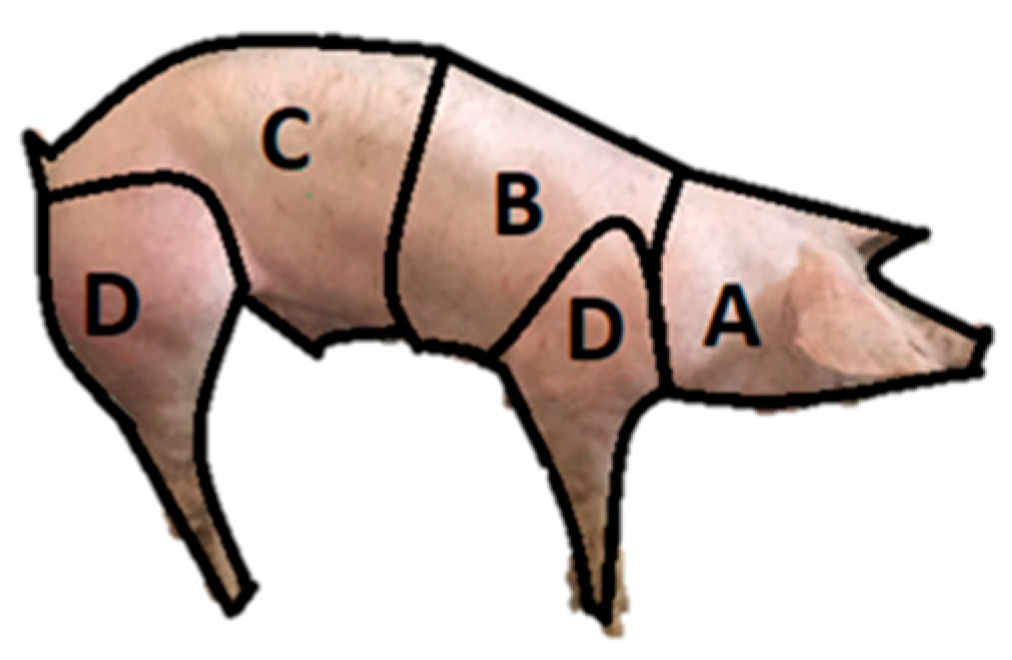
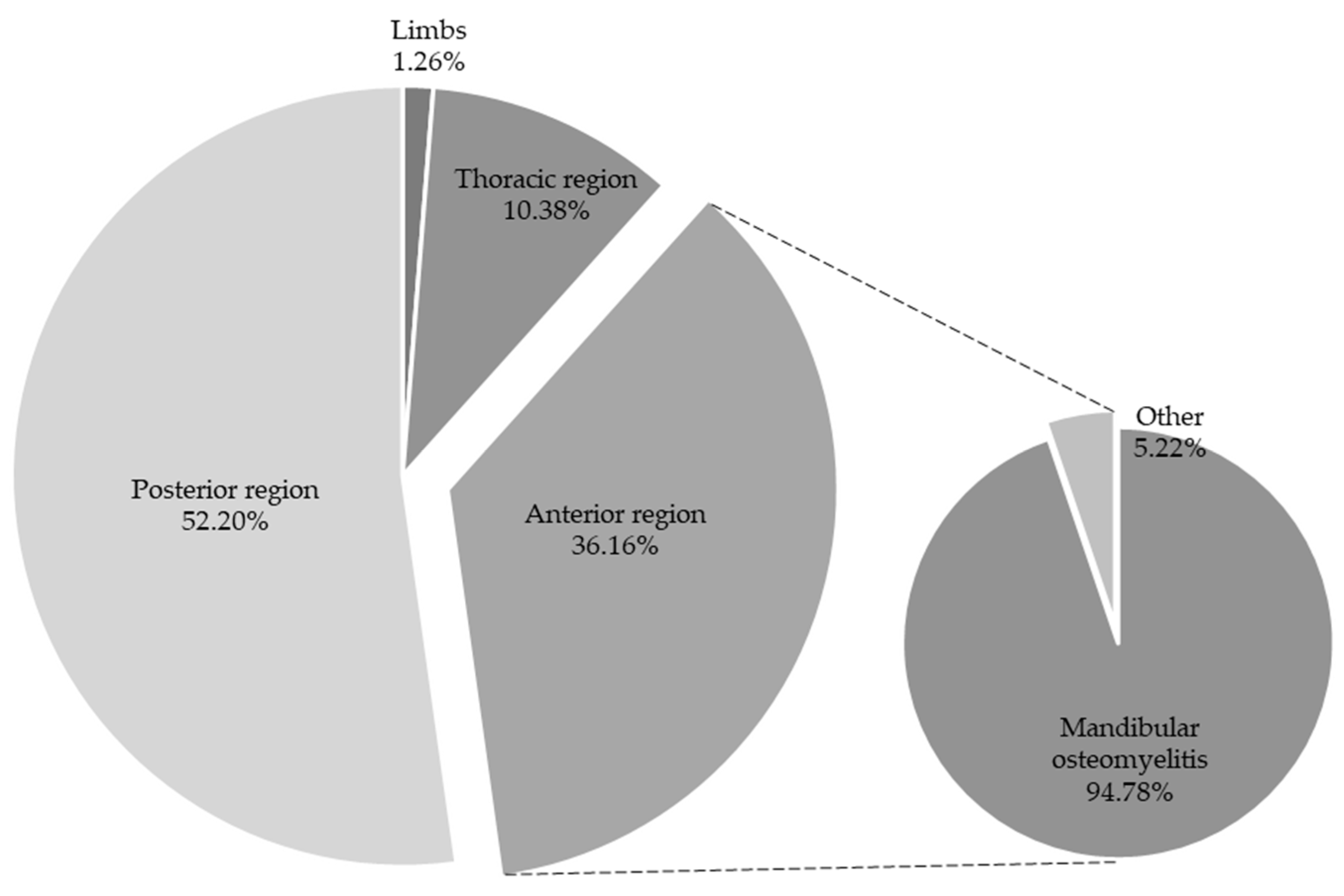
| Location | Grade 1 | Grade 2 | Grade 3 | Total Lesions | No Lesions | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abs. Rel. * | Rel. Freq. ** | Abs. Rel. * | Rel. Freq. ** | Abs. Rel. * | Rel. Freq. ** | Abs. Rel. * | Rel. Freq. ** | Abs. Rel. * | Rel. Freq. ** | |
| Anterior region | 6 | 1.88% | 7 | 2.20% | 6 | 1.88% | 19 | 5.97% | 96 | 30.18% |
| Limbs | 0 | 0.00% | 1 | 0.31% | 1 | 0.31% | 2 | 0.62% | 2 | 0.62% |
| Posterior region | 16 | 5.03% | 17 | 5.34% | 46 | 14.46% | 79 | 24.84% | 87 | 27.35% |
| Thoracic region | 4 | 1.25% | 1 | 0.31% | 3 | 0.94% | 8 | 2.51% | 25 | 7.86% |
| Location | Intact Teeth | Clipped Teeth | Ground Teeth | |||
|---|---|---|---|---|---|---|
| Abs. Freq. * | Rel. Freq. ** | Abs. Freq. * | Rel. Freq. ** | Abs. Freq. * | Rel. Freq. ** | |
| Anterior region | 8 | 2.51% | 92 | 28.93% | 15 | 4.71% |
| Limb | 0 | 0.00% | 3 | 0.94% | 1 | 0.31% |
| Posterior region | 25 | 7.86% | 113 | 35.53% | 28 | 8.80% |
| Thoracic region | 6 | 1.88% | 18 | 5.66% | 9 | 2.83% |
| Estimate | Std. Error | p-Value | OR | |
|---|---|---|---|---|
| (Intercept) | 0.27 | 0.36 | 0.46 | 1.303544 |
| Season winter (vs. spring) | 0.15 | 0.29 | 0.60 | 1.164103 |
| Season autumn (vs. spring) | 0.66 | 0.33 | 0.04584 * | 1.943729 |
| Season summer (vs. spring) | −0.72 | 0.27 | 0.00716 ** | 0.487798 |
| Origin France (vs. Portugal) | 0.30 | 0.51 | 0.56 | 1.347112 |
| Origin Spain (vs. Portugal) | 0.35 | 0.37 | 0.35 | 1.416778 |
| Pleurisy | −1.18 | 0.25 | p < 0.0001 *** | 0.306115 |
| Pericarditis | −0.30 | 0.29 | 0.29 | 0.738054 |
| Tail-biting lesion grade 1 | −0.14 | 0.33 | 0.66 | 0.865161 |
| Tail-biting lesion grade 2 | 1.74 | 0.54 | 0.00128 ** | 5.713419 |
| Tail-biting lesion grade 3 | 2.90 | 0.62 | p < 0.0001 *** | 18.14192 |
| Clipped teeth | 1.00 | 0.33 | 0.00262 ** | 2.709421 |
| Ground teeth | −0.50 | 0.44 | 0.26 | 0.609405 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teiga-Teixeira, P.; Alves Rodrigues, M.; Moura, D.; Teiga-Teixeira, E.; Esteves, A. Osteomyelitis in Pig Carcasses at a Portuguese Slaughterhouse: Association with Tail-Biting and Teeth Resection. Animals 2024, 14, 1794. https://doi.org/10.3390/ani14121794
Teiga-Teixeira P, Alves Rodrigues M, Moura D, Teiga-Teixeira E, Esteves A. Osteomyelitis in Pig Carcasses at a Portuguese Slaughterhouse: Association with Tail-Biting and Teeth Resection. Animals. 2024; 14(12):1794. https://doi.org/10.3390/ani14121794
Chicago/Turabian StyleTeiga-Teixeira, Pedro, Melissa Alves Rodrigues, Dina Moura, Eduardo Teiga-Teixeira, and Alexandra Esteves. 2024. "Osteomyelitis in Pig Carcasses at a Portuguese Slaughterhouse: Association with Tail-Biting and Teeth Resection" Animals 14, no. 12: 1794. https://doi.org/10.3390/ani14121794
APA StyleTeiga-Teixeira, P., Alves Rodrigues, M., Moura, D., Teiga-Teixeira, E., & Esteves, A. (2024). Osteomyelitis in Pig Carcasses at a Portuguese Slaughterhouse: Association with Tail-Biting and Teeth Resection. Animals, 14(12), 1794. https://doi.org/10.3390/ani14121794






