GnRH Vaccine Could Suppress Serum Testosterone in Stallion Mules
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. GnRH Vaccination and Clinical Examination for Adverse Effects
2.3. Samples and Data Collection
2.4. Serum Anti-GnRH Antibody Analysis
2.5. Serum Testosterone Analysis
2.6. Statistical Analysis
3. Results
3.1. Anti-GnRH Antibody Concentration
3.2. Serum Testosterone Concentration
3.3. Behavioral Response, Changes in Total Scrotal Width, and Body Weight
3.4. Clinical Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLean, A.; Varnum, A.; Ali, A.; Heleski, C.; Navas Gonzalez, F.J. Comparing and contrasting knowledge on mules and hinnies as a tool to comprehend their behavior and improve their welfare. Animals 2019, 9, 488. [Google Scholar] [CrossRef]
- Lagos, J.; Rojas, M.; Rodrigues, J.B.; Tadich, T. Perceptions and attitudes towards mules in a group of soldiers. Animals 2021, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Heaton, K.; Ragle, C.; Godderidge, M.T.; Farrell, A.; Tibary, A. Estrous behavior in mules—An owner’s perspective. J. Equine Vet. Sci. 2018, 60, 109–112. [Google Scholar] [CrossRef]
- Desta, T.T.; Teklemariam, H.; Mulugeta, T. The insights of smallholder farmers on special attributes of the genetically robust mule. Biodiversitas 2022, 23, 3561–3566. [Google Scholar] [CrossRef]
- Mason, B.J.; Newton, J.R.; Payne, R.J.; Pilsworth, R.C. Costs and complications of equine castration: A UK practice-based study comparing ‘standing nonsutured’ and ‘recumbent sutured’ techniques. Equine Vet. J. 2005, 37, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, I.; Watson, J.L.; Kass, P.H.; Spier, S.J. Incidence, management, and outcome of complications of castration in equids: 324 cases (1998–2008). J. Am. Vet. Med. Assoc. 2013, 242, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.P.; Chapuis, R.J.J.; de Fourmestraux, C.; Geffroy, O.J. Complications and risk factors of castration with primary wound closure: Retrospective study in 159 horses. Can. Vet. J. 2017, 58, 466–471. [Google Scholar] [PubMed]
- Owens, C.D.; Hughes, K.J.; Hilbert, B.J.; Heller, J.; Nielsen, S.; Trope, G.D. Survey of equine castration techniques, preferences and outcomes among Australian veterinarians. Aust. Vet. J. 2018, 96, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Rosanowski, S.M.; MacEoin, F.; Graham, R.J.T.Y.; Riggs, C.M. Open standing castration in Thoroughbred racehorses in Hong Kong: Prevalence and severity of complications 30 days post-castration. Equine Vet. J. 2018, 50, 327–332. [Google Scholar] [CrossRef]
- Koenig, J.B.; Sinclair, M.; Sorge, U.S. Comparison of the use of a braided multifilament transfixation suture for field castration with other castration techniques. Equine Vet. Educ. 2019, 31, 427–431. [Google Scholar] [CrossRef]
- Baldwin, C.M. A review of prevention and management of castration complications. Equine Vet. Educ. 2024, 36, 97–106. [Google Scholar] [CrossRef]
- Vilar, J.M.; Batista, M.; Carrillo, J.M.; Rubio, M.; Sopena, J.; Álamo, D. Histological, cytogenetic and endocrine evaluation in twenty-five unilateral cryptorchid horses. J. Appl. Anim. Res. 2018, 46, 441–444. [Google Scholar] [CrossRef]
- Stout, T.A.; Colenbrander, B. Suppressing reproductive activity in horses using GnRH vaccines, antagonists or agonists. Anim. Reprod. Sci. 2004, 82–83, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Stout, T.A.E. Modulating reproductive activity in stallions: A review. Anim. Reprod. Sci. 2005, 89, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Pedersen, H.G.; Sandøe, P. Beyond castration and culling: Should we use non-surgical, pharmacological methods to control the sexual behavior and reproduction of animals? J. Agric. Environ. Ethics 2018, 31, 197–218. [Google Scholar] [CrossRef]
- Burger, D.; Janett, F.; Vidament, M.; Stump, R.; Fortier, G.; Imboden, I.; Thun, R. Immunization against GnRH in adult stallions: Effects on semen characteristics, behaviour, and shedding of equine arteritis virus. Anim. Reprod. Sci. 2006, 94, 107–111. [Google Scholar]
- Schulman, M.L.; Botha, A.E.; Muenscher, S.B.; Annandale, C.H.; Guthrie, A.J.; Bertschinger, H.J. Reversibility of the effects of GnRH -vaccination used to suppress reproductive function in mares. Equine Vet. J. 2013, 45, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Dordas-Perpinya, M.; Gorréguès, M.; Gervasoni, M.A.; Berder, C.; Thorin, C.; Jaillardon, L.; Bruyas, J.F. What is the effect of anti- GnRH immunization on plasmatic levels of anti mullerian hormone? J. Equine Vet. Sci. 2018, 66, 26–28. [Google Scholar] [CrossRef]
- Khumsap, S.; Thitaram, C.; Somgird, C. GnRH vaccine could suppress serum progesterone level in Thai pony mares; A preliminary study. Vet. Integr. Sci. 2020, 18, 43–51. [Google Scholar]
- Khonmee, J.; Brown, J.L.; Li, M.Y.; Somgird, C.; Boonprasert, K.; Norkaew, T.; Punyapornwithaya, V.; Lee, W.M.; Thitaram, C. Effect of time and temperature on stability of progestagens, testosterone and cortisol in Asian elephant blood stored with and without anticoagulant. Conserv. Physiol. 2019, 7, coz031. [Google Scholar]
- Janett, F.; Stump, R.; Burger, D.; Thun, R. Suppression of testicular function and sexual behavior by vaccination against GnRH (Equity) in the adult stallion. Anim. Reprod. Sci. 2009, 115, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Botha, A.E.; Birrell, J.; Schulman, M.L.; du Plessis, L.; Laver, P.N.; Soley, J.T.; Bertschinger, H.J. Effects of the GnRH vaccine Improvac on testicular tissue of young stallions. Anim. Reprod. Sci. 2016, 169, 97. [Google Scholar] [CrossRef]
- Bailly-Chouriberry, L.; Loup, B.; Popot, M.A.; Dreau, M.L.; Garcia, P.; Bruyas, J.F.; Bonnaire, Y. Two complementary methods to control gonadotropin-releasing hormone vaccination (Improvac) misuse in horseracing: Enzyme-linked immunosorbent assay test in plasma and steroidomics in urine. Drug Test. Anal. 2017, 9, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Miszczak, F.; Burger, D.; Ferry, B.; Legrand, L.; Fortier, G.; Laine, A.-L.; Vabret, A.; Pronost, S.; Vidament, M. Anti-GnRH vaccination of stallions shedding equine arteritis virus in their semen: A field study. Vet. Arh. 2020, 90, 543–556. [Google Scholar] [CrossRef]
- Rocha, J.M.; Ferreira-Silva, J.C.; Veloso Neto, H.F.; Moura, M.T.; Ferreira, H.N.; Silva Júnior, V.A.; Manso Filho, H.C.; Oliveira, M.A.L. Immunocastration in donkeys: Clinical and physiological aspects. Pferdeheilkunde Equine Med. 2018, 34, 12–16. [Google Scholar] [CrossRef]
- Horohov, D.W.; Adams, A.A.; Chambers, T.M. Immunosenescence of the equine immune system. J. Comp. Pathol. 2010, 142 (Suppl. S1), S78–S84. [Google Scholar] [CrossRef] [PubMed]
- Guasti, P.N.; Papa, P.M.; Schmith, R.A.; Camargo, L.S.; Andrade, L.R.P., Jr.; Silva, L.F.M.C.; Freitas-DellAqua, C.P.; Souza, F.F.; Papa, F.O. Serum testosterone levels and seminal plasma proteins in castrated stallions. J. Equine Vet. Sci. 2018, 66, 64–65. [Google Scholar] [CrossRef]
- Hannan, M.A.; Murase, H.; Sato, F.; Tsogtgerel, M.; Kawate, N.; Nambo, Y. Age related and seasonal changes of plasma concentrations of insulin-like peptide 3 and testosterone from birth to early-puberty in Thoroughbred male horses. Theriogenology 2019, 132, 212–217. [Google Scholar] [CrossRef]
- Burns, P.J.; Jawad, M.J.; Weld, J.M.; Kaufman, W.C.; Witherspoon, D.M.; Wilson, E.A.; Douglas, R.H. Effects of season, age and increased photoperiod on reproductive hormone concentrations and testicular diameters in Thoroughbred stallions. J. Equine Vet. Sci. 1984, 4, 202–208. [Google Scholar] [CrossRef]
- Inoue, J.; Cerbito, W.A.; Oguri, N.; Matsuzawa, T.; Sato, K. Serum levels of testosterone and oestrogens in normal and infertile stallions. Int. J. Androl. 1993, 16, 155–158. [Google Scholar] [CrossRef]
- Rota, A.; Sgorbini, M.; Panzani, D.; Bonelli, F.; Baragli, P.; Ille, N.; Gatta, D.; Sighieri, C.; Casini, L.; Maggiorelli, M. Met al. Effect of housing system on reproductive behaviour and on some endocrinological and seminal parameters of donkey stallions. Reprod. Domest. Anim. 2018, 53, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Waddington, B.; Penitente-Filho, J.M.; Neves, J.; Pinho, R.O.; Chaya, A.Y.; Maitan, P.P.; Silveira, C.O.; Neves, M.G.; Guimarães, S.E.F.; de Carvalho, G.R.; et al. Testosterone serum profile, semen characteristics and testicular biometry of Mangalarga Marchador stallions in a tropical environment. Reprod. Domest. Anim. 2017, 52, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Rong, R.; Chandley, A.C.; Song, J.; McBeath, S.; Tan, P.P.; Bai, Q.; Speed, R.M. A fertile mule and hinny in China. Cytogenet. Cell Genet. 1988, 47, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.W.; Shah, S.A.H.; Qureshi, I.Z. Effect of kisspeptin-10, LH and HCG on serum testosterone concentrations in stallions, donkeys and mules. Theriogenology 2017, 102, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Coryn, M.; De Morr, A.; Bouters, R.; Vandeplassche, M. Clinical, morphological and endocrinological aspects of cryptorchidism in the horse. Theriogenology 1981, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.E. Testosterone concentrations in normal and cryptorchid horses. Response to human chorionic gonadotrophin. Anim. Reprod. Sci. 1989, 18, 43–50. [Google Scholar] [CrossRef]
- Hejmej, A.; Bilińska, B. The effects of cryptorchidism on the regulation of steroidogenesis and gap junctional communication in equine testes. Endokrynol. Pol. 2008, 59, 112–118. [Google Scholar] [PubMed]
- Claes, A.; Ball, B.A.; Corbin, C.J.; Conley, A.J. Age and season affect serum testosterone concentrations in cryptorchid stallions. Vet. Rec. 2013, 173, 168. [Google Scholar] [CrossRef] [PubMed]
- Miragaya, M.H.; Neild, D.M.; Alonso, A.E. A Review of reproductive biology and biotechnologies in donkeys. J. Equine Vet. Sci. 2018, 65, 55–61. [Google Scholar] [CrossRef]
- Elhay, M.; Newbold, A.; Britton, A.; Turley, P.; Dowsett, K.; Walker, J. Suppression of behavioural and physiological oestrus in the mare by vaccination against GnRH. Aust. Vet. J. 2007, 85, 39–45. [Google Scholar] [CrossRef]
- Imboden, I.; Janett, F.; Burger, D.; Crowe, M.A.; Hässig, M.; Thun, R. Influence of immunization against GnRH on reproductive cyclicity and estrous behavior in the mare. Theriogenology 2006, 66, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Botha, A.; Schulman, M.; Bertschinger, H.; Guthrie, A.; Annandale, C.B.; Hughes, S. The use of a GnRH vaccine to suppress mare ovarian activity in a large group of mares under field conditions. Wildl. Res. 2008, 35, 548–554. [Google Scholar] [CrossRef]
- Gershwin, L.J. Adverse reactions to vaccination: From anaphylaxis to autoimmunity. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 279–290. [Google Scholar] [CrossRef] [PubMed]
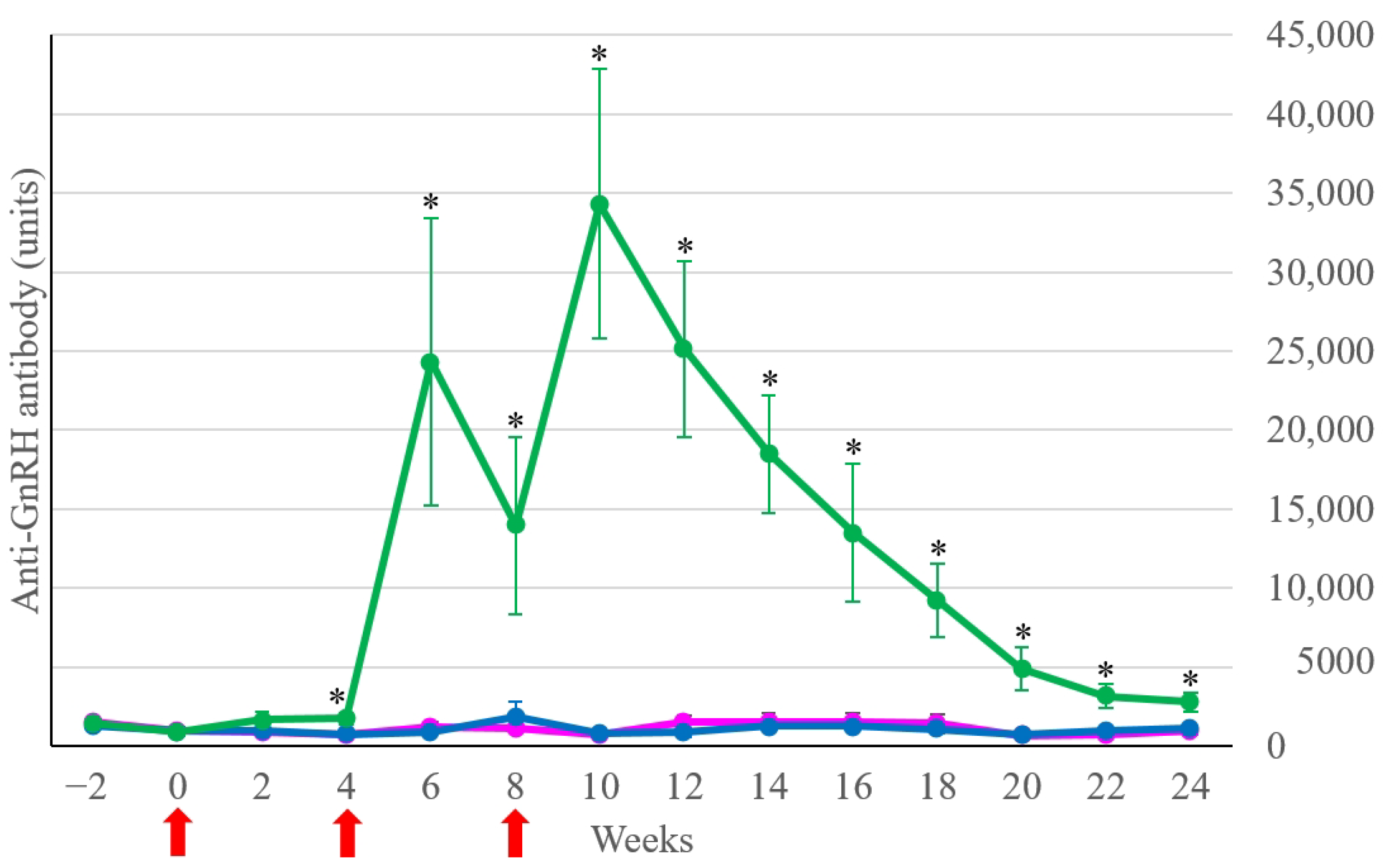
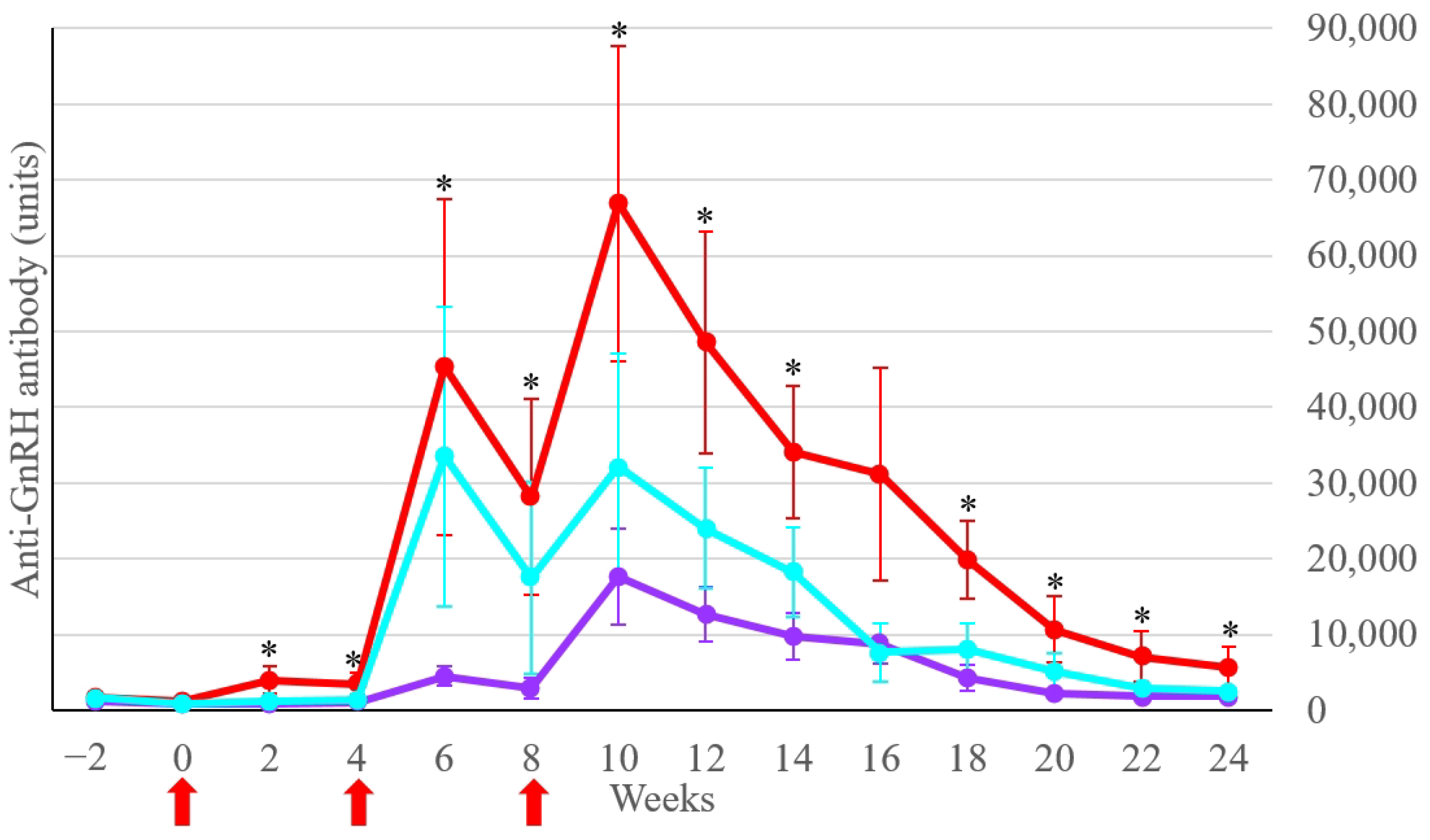
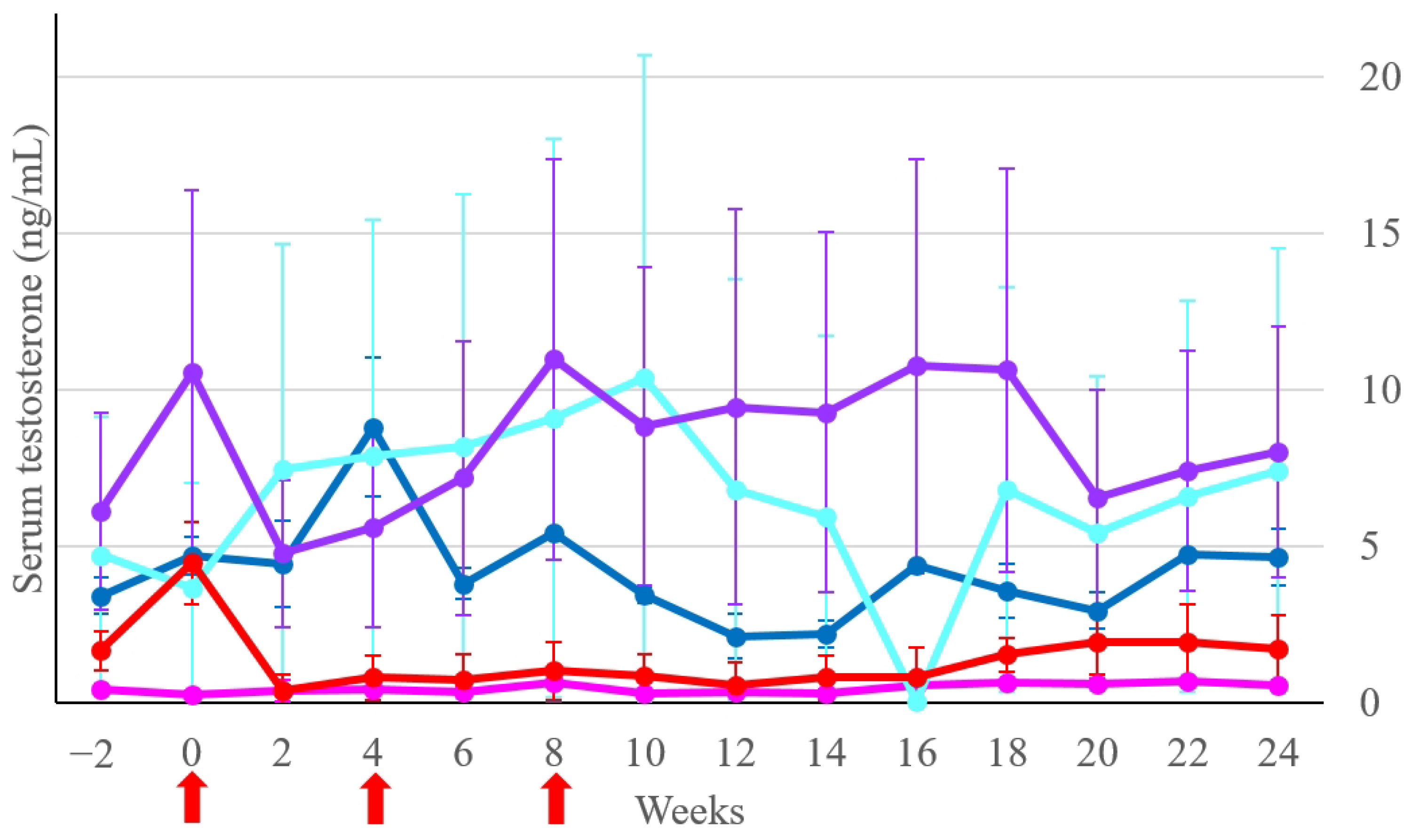
| Group | Week 2 | Week 6 | Week 12 | Week 18 | Week 22 | Week 24 |
|---|---|---|---|---|---|---|
| Control-intact group (n = 2) | ||||||
| Stood still for one min | 1.0 (0–2) | 1.5 (0–3) | 1.5 (1–2) | 0.5 (0–1) | 0.5 (0–1) | 0.0 (0–0) |
| Palpation at scrotal area | 1.0 (0–2) | 1.5 (1–2) | 0.5 (0–1) | 0.5 (0–1) | 0.5 (0–1) | 1.5 (0–3) |
| Blood collection | 1.0 (0–2) | 0.0 (0–0) | 0.5 (0–1) | 0.0 (0–0) | 0.0 (0–0) | 0.0 (0–0) |
| Control-castrated group (n = 5) | ||||||
| Stood still for one min | 0.5 (0–1) | 2.0 (1–3) | 1.0 (1–1) | 1.5 (0–3) | 1.0 (0–3) | 0.8 (0–3) |
| Palpation at scrotal area | 0.3 (0–1) | 0.8 (0–1) | 0.3 (0–1) | 0.0 (0–0) | 0.0 (0–0) | 0.8 (0–3) |
| Blood collection | 0.8 (0–1) | 0.3 (0–1) | 0.3 (0–1) | 0.0 (0–0) | 0.0 (0–0) | 0.0 (0–0) |
| Treatment group (n = 17) | ||||||
| Stood still for one min | 1.1 (0–2) | 0.8 (0–2) | 1.0 (0–2) | 1.3 (0–2) | 0.6 (0–2) | 0.7 (0–2) |
| Palpation at scrotal area | 0.7 (0–2) | 0.3 (0–1) | 0.2 (0–1) | 0.1 (0–1) | 0.0 * (0–0) | 0.5 (0–2) |
| Blood collection | 0.7 (0–2) | 0.1 * (0–1) | 0.0 * (0–0) | 0.1 * (0–2) | 0.0 * (0–0) | 0.3 * (0–3) |
| Adverse Effects | First Vaccine | Second Vaccine | Third Vaccine |
|---|---|---|---|
| Rectal temperature > 39 °C | 6, 10, 18 | 5, 9, 13, 14, 16, 17 | 6, 7, 11, 14 |
| 39.5 ± 0.3 °C | 39.4 ± 0.4 °C | 39.5 ± 0.3 °C | |
| Injection site swelling | |||
| Score 1 (<2 cm) | 16 | 7, 11 | 12, 15, 18 |
| Score 2 (>2 cm) | - | 9, 14, 16 | 1, 6, 7, 11, 13, 16, 17 |
| Pain response | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumsap, S.; Tangtrongsup, S.; Towiboon, P.; Somgird, C. GnRH Vaccine Could Suppress Serum Testosterone in Stallion Mules. Animals 2024, 14, 1800. https://doi.org/10.3390/ani14121800
Khumsap S, Tangtrongsup S, Towiboon P, Somgird C. GnRH Vaccine Could Suppress Serum Testosterone in Stallion Mules. Animals. 2024; 14(12):1800. https://doi.org/10.3390/ani14121800
Chicago/Turabian StyleKhumsap, Siriporn, Sahatchai Tangtrongsup, Patcharapa Towiboon, and Chaleamchat Somgird. 2024. "GnRH Vaccine Could Suppress Serum Testosterone in Stallion Mules" Animals 14, no. 12: 1800. https://doi.org/10.3390/ani14121800





