Lactobacillus delbrueckii Ameliorated Blood Lipids via Intestinal Microbiota Modulation and Fecal Bile Acid Excretion in a Ningxiang Pig Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Animals, Diets and Experimental Design
2.3. Sample Collection and Preparation
2.4. Determination of Lipid Contents in Serum and Tissue
2.5. Determination of Enzyme Activity Related to Fat Metabolism
2.6. RT-PCR to Determine the mRNA Expression of Genes Related to Fat Metabolism
2.7. Fecal TC and TBA Content Determination
2.8. Intestinal Bacterial Composition and Structure
2.9. Analysis of SCFA Composition in Gut Contents
2.10. Statistics Analysis
3. Results
3.1. L. delbrueckii did Not Affect the Growth Performance
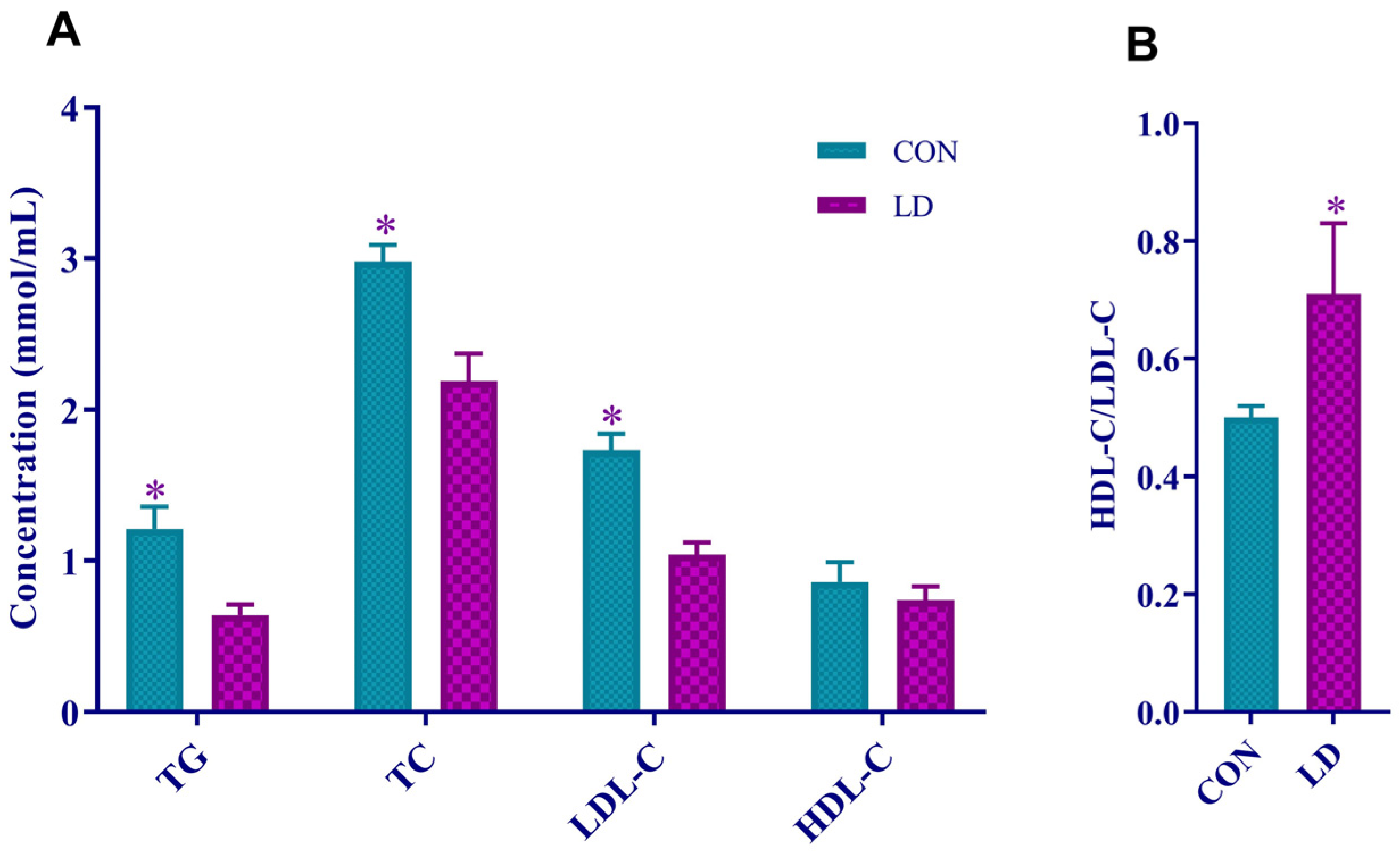
3.2. L. delbrueckii Reduced Serum Lipid Levels
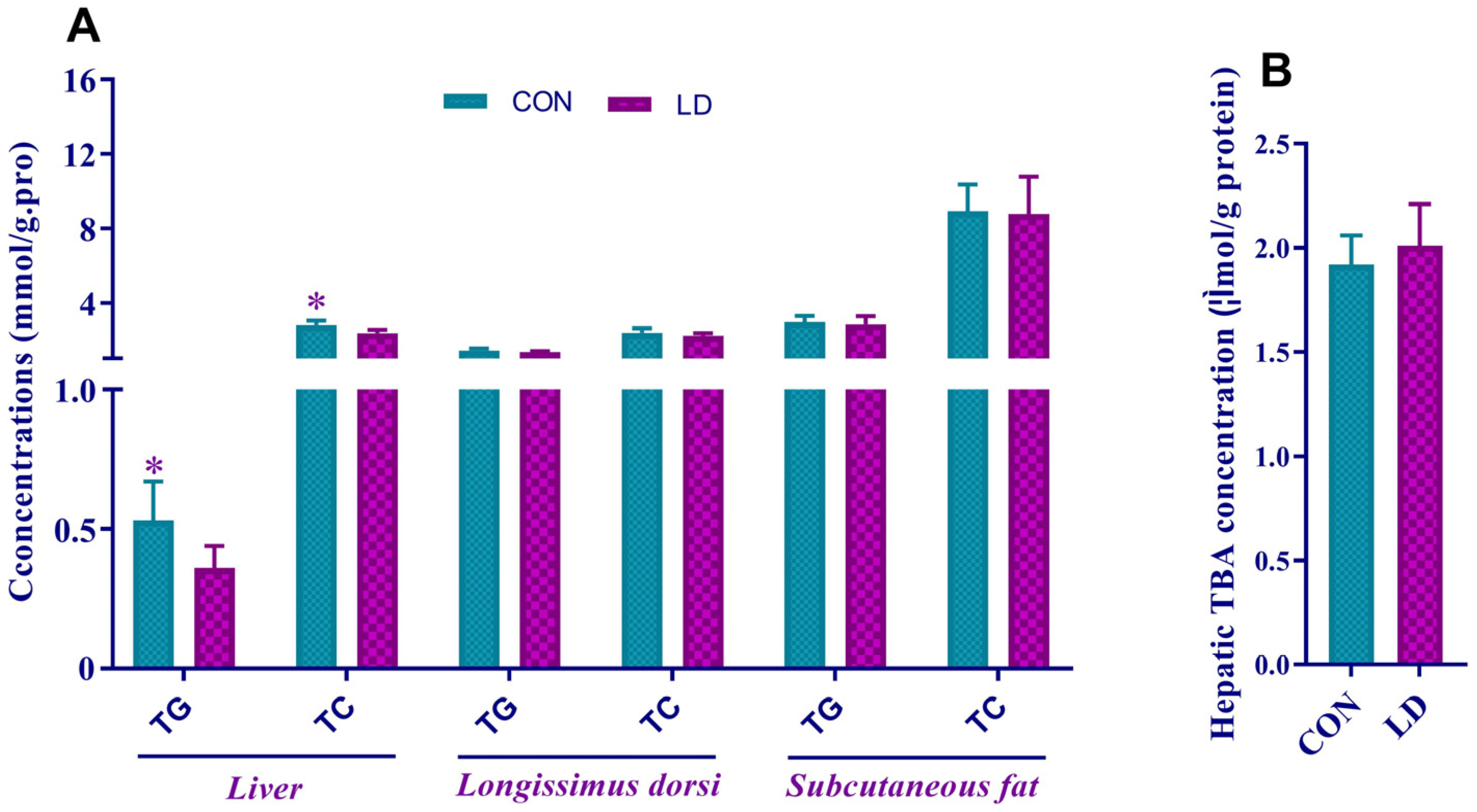
3.3. L. delbrueckii Altered Lipid Contents in Liver
3.4. L. delbrueckii Affected Hepatic Cholesterol and Bile Acid Metabolism
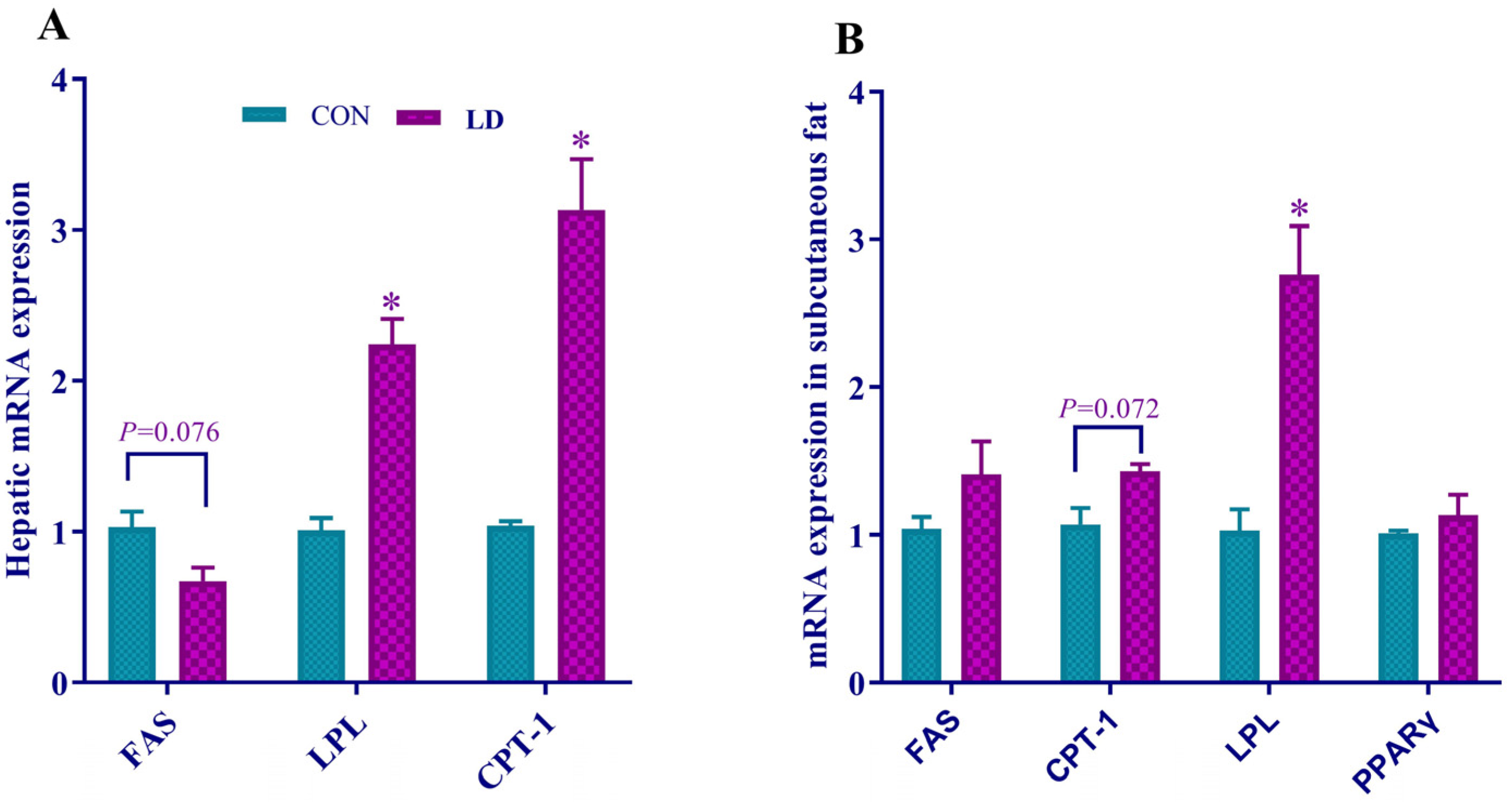
3.5. L. delbrueckii Regulated Gene Expression Related to Fatty Acid Synthesis, Lipolysis and β-oxidation in Liver and Subcutaneous Fat
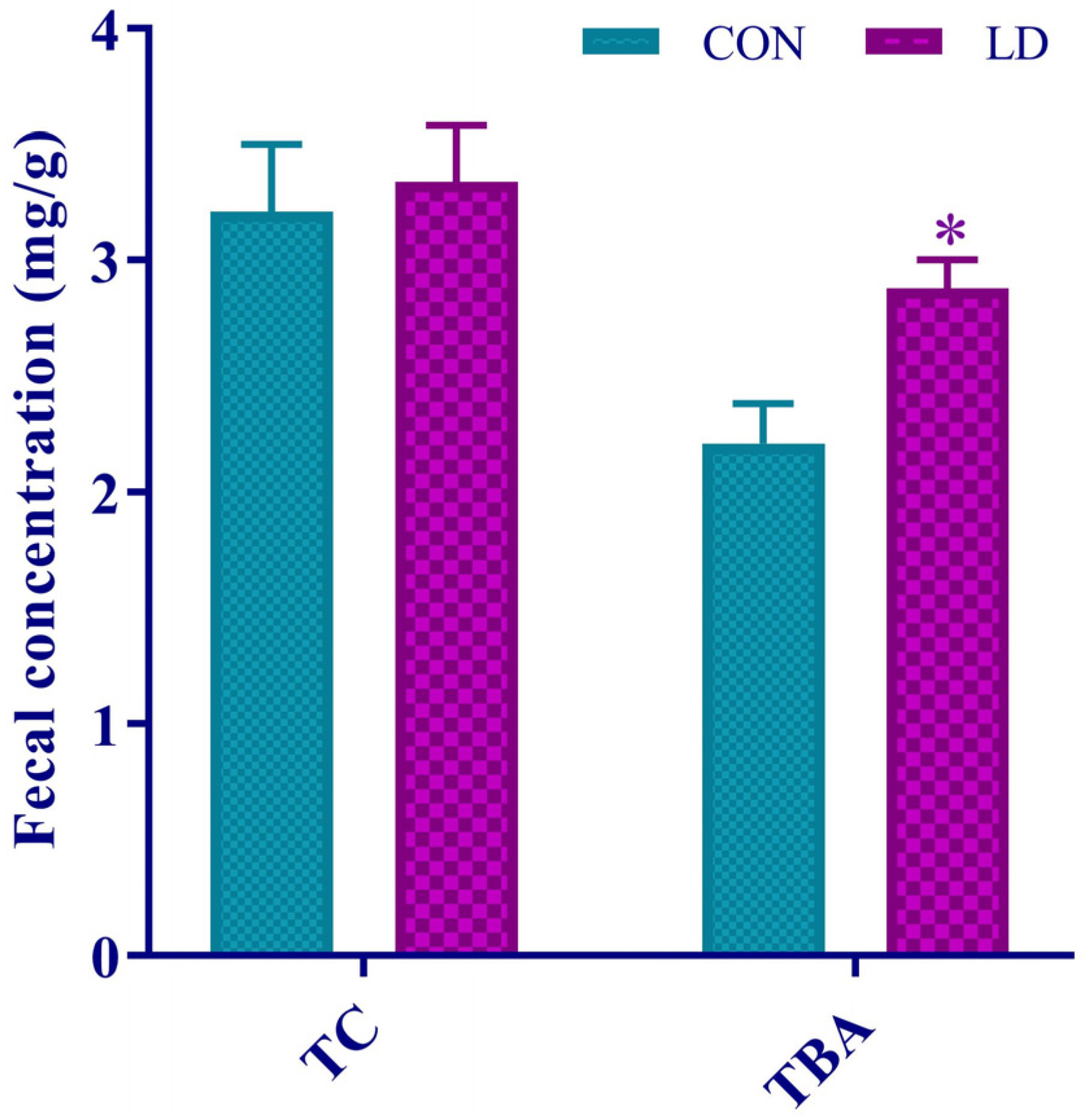
3.6. L. delbrueckii Facilitated Fecal TBA Excretion
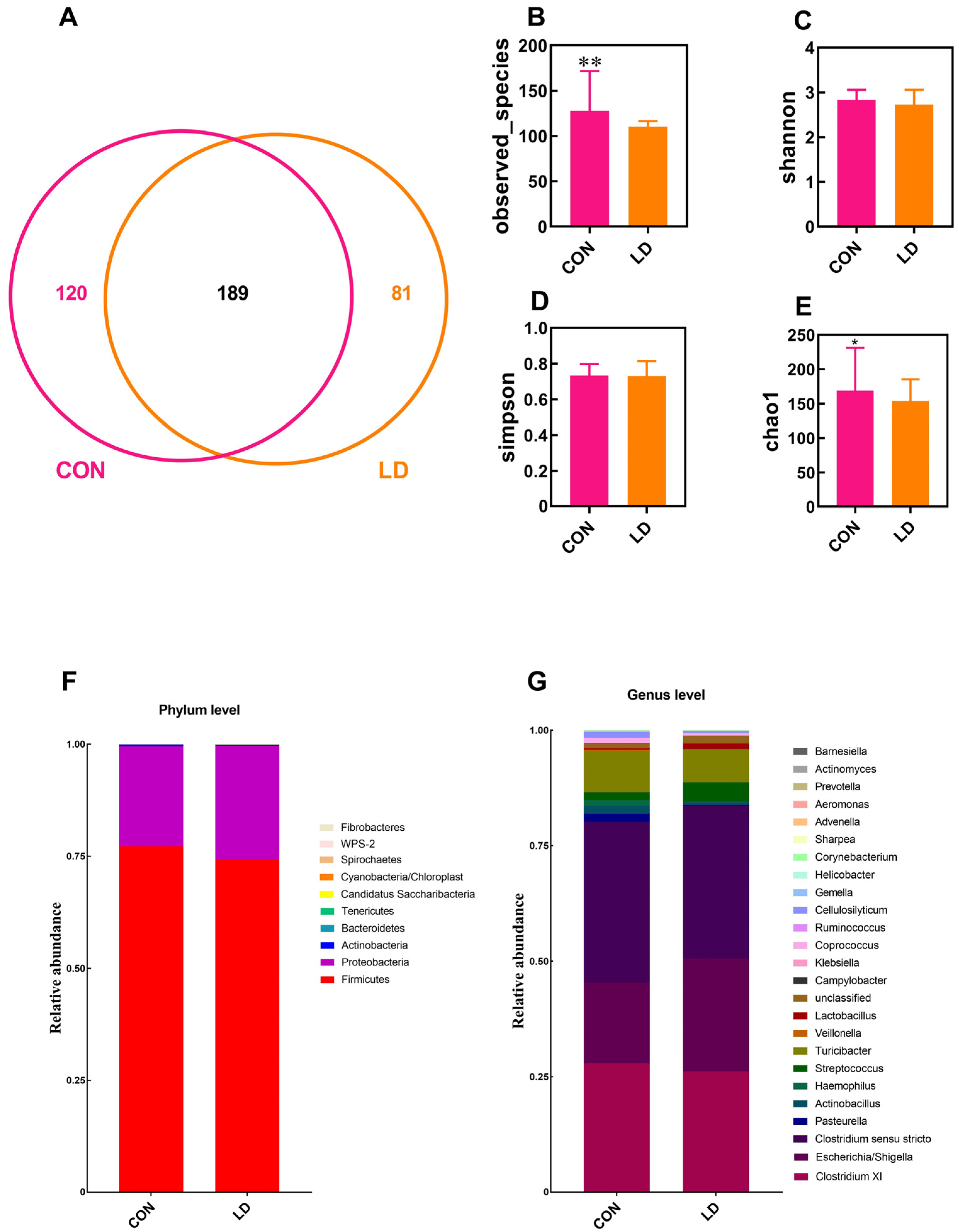
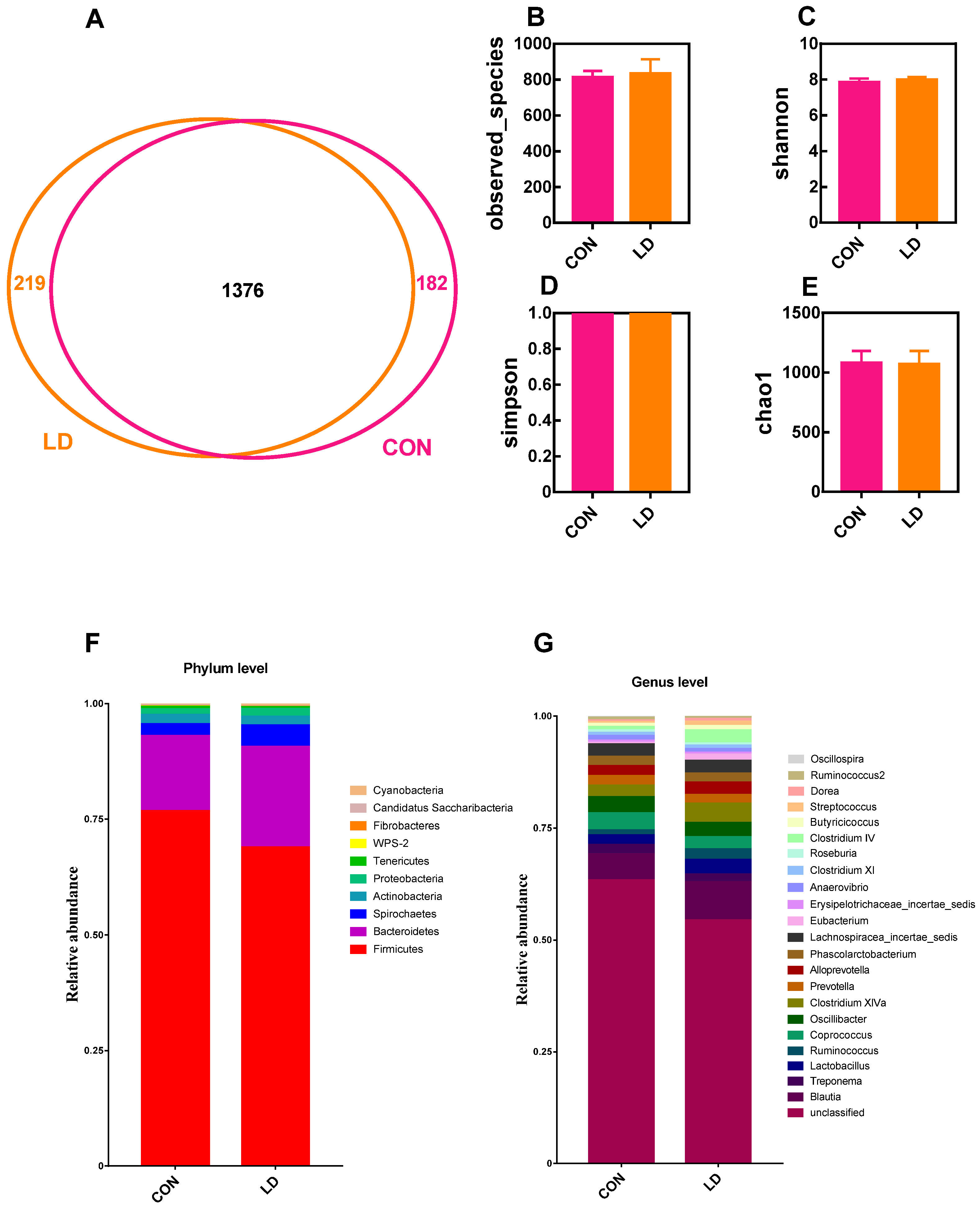
3.7. L. delbrueckii Altered Intestinal Microbiota Structure
3.8. L. delbrueckii Changes Intestinal SCFA Profiles
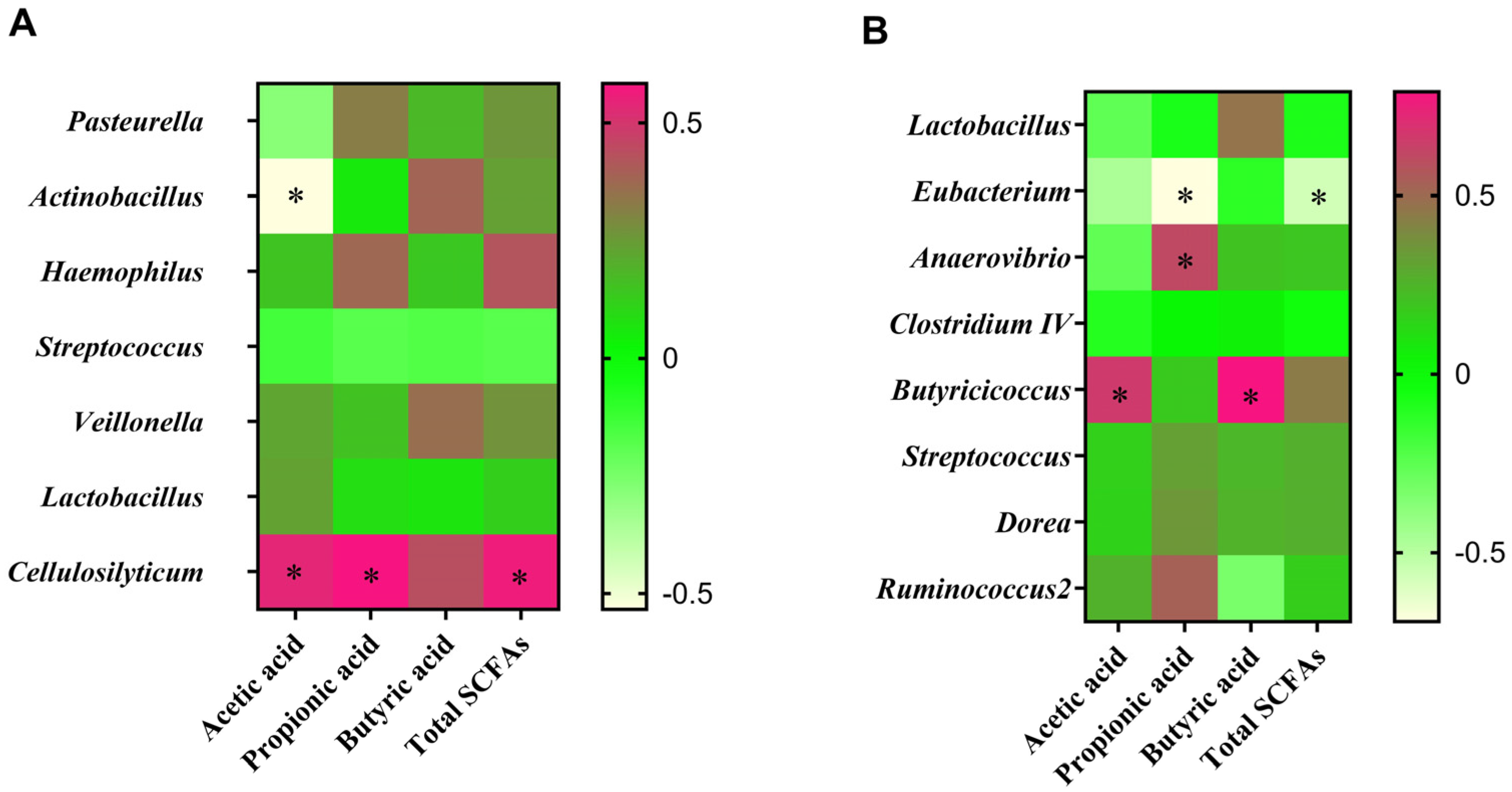
3.9. Correlation between Intestinal Microbiota and SCFA Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, F.; Wang, J.; Nicholas, S.; Qian, D.; Liu, R. A cohort study on risk factors of high-density lipoprotein cholesterol hypoli pidemia among urban chinese adults. Lipids Health Dis. 2021, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Xu, W.; Zhang, L.; Li, X.; Wang, R.; Wu, S. Impact of gut microbiota and microbiota-related metabolites on hyper lipidemia. Front. Cell Infect. Microbiol. 2021, 11, 634780. [Google Scholar] [CrossRef]
- Hou, G.; Peng, W.; Wei, L.; Li, R.; Yuan, Y.; Huang, X.; Yin, Y. Lactobacillus delbrueckii interfere with bile acid enterohepatic circulation to regulate cholesterol metabolism of growing-finishing pigs via its bile salt hydrolase activity. Front. Nutr. 2020, 7, 617676. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.Y. New guidelines and evidence for the prevention and treatment of dyslipidemia and atherosclerotic cardiovascular disease in China. Chronic Dis. Transl. Med. 2016, 3, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Walldius, G.; de Faire, U.; Alfredsson, L.; Leander, K.; Westerholm, P.; Malmström, H.; Ivert, T.; Hammar, N. Long-term risk of a major cardiovascular event by apob, apoa-1, and the Apob/Apoa-1 ratio-experience from the swedish amoris cohort: A cohort study. PLoS Med. 2021, 18, e1003853. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, M.A.; Turnquist, H.R.; Little, S.R. Biologics and their delivery systems: Trends in myocardial infarction. Adv. Drug Deliv. Rev. 2021, 173, 181–215. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Azimullah, S.; Al Ahbabi, M.M.; Jha, N.K.; Lakshmanan, V.K.; Goyal, S.N.; Ojha, S. Nootkatone, A dietary fragrant bioactive compound, attenuates dyslipidemia and intramyocardial lipid accumulation and favorably alters lipid metabolism in a rat model of myocardial injury: An in vivo and in vitro study. Molecules 2020, 25, 5656. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.F.; Yin, J.; Wei, L.K.; Li, R.; Peng, W.; Yuan, Y.; Huang, X.G.; Yin, Y.L. Lactobacillus delbrueckii might lower serum triglyceride levels via colonic microbiota modulation and scfa-mediated fat metabolism in parenteral tissues of growing-finishing pigs. Front. Vet. Sci. 2022, 9, 982349. [Google Scholar] [CrossRef]
- Ma, H.M.; Jiang, J.; He, J.; Liu, H.F.; Han, L.L.; Gong, Y.; Li, B.; Yu, Z.G.; Tang, S.G.; Zhang, Y.B.; et al. Long-read assembly of the chinese indigenous ningxiang pig genome and identification of genetic variations in fat metabolism among different breeds. Mol. Ecol. Resour. 2022, 22, 1508–1520. [Google Scholar] [CrossRef]
- Olayanju, A.; Jones, L.; Greco, K.; Goldring, C.E.; Ansari, T. Application of porcine gastrointestinal organoid units as a poten tial in vitro tool for drug discovery and development. J. Appl. Toxicol. 2019, 39, 4–15. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, X.L.; Zeng, Q.H.; Li, H.; Ma, H.M.; Jiang, J.; Rosa, G.J.M.; Gianola, D.; Tait, R.G.; Bauck, S. Genomic mating as sustainable breeding for chinese indigenous ningxiang pigs. PLoS ONE. 2020, 15, e0236629. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.M.; Li, C.Y.; Yu, Y.N.; Xing, Y.T.; Xiao, D.F.; Zhang, B. Comparison of fatty acid profile of three adipose tissues in ningxiang pigs. Anim. Nutr. 2018, 4, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, I.; Kojima, M.; Oe, M.; Ojima, K.; Muroya, S.; Chikuni, K. Comparing pig breeds with genetically low and high backfat thickness: Differences in expression of adiponectin, its receptor, and blood metabolites. Domest. Anim. Endocrin. 2019, 68, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hou, G.; Wei, L.; Peng, W.; Pan, J.; Huang, X. Effects of lactobacillus delbrueckii on serum lipid indexes, enzyme activities and genes mRNA expression related to cholesterol metabolism and fat deposition of finishing pigs. Chin. J. Anim. Nutr. 2017, 29, 3184–3192. (in Chinese). [Google Scholar]
- Li, D.F.; Wang, K.L.; Qiao, S.Y.; Jia, G.; Jiang, Z.Y.; Chen, Z.L.; Lin, Y.C.; Wu, D.; Zhu, X.M.; Xiong, B.H.; et al. Feeding Standard of Swine, the Agricultural Industry Standard of the People’s Republic of China (NY/T 65-2004); The National Standards of the People’s Republic of China: Beijing, china, 2004. (in Chinese) [Google Scholar]
- Li, R.; Chang, L.; Hou, G.; Song, Z.; Fan, Z.; He, X.; Hou, D.X. Colonic microbiota and metabolites response to different dietary protein sources in a piglet model. Front. Nutr. 2019, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Huo, D.; Peng, Q.; Pan, Y.; Jiang, S.; Liu, B.; Zhang, J. Lactobacillus Plantarum HNU082-derived improvements in the intestinal microbiome prevent the development of hyperlipidaemia. Food Funct. 2017, 8, 4508–4516. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Q.; Ren, Y.; Ruan, Z. Effect of Probiotic lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PLoS ONE 2017, 12, e0178868. [Google Scholar] [CrossRef]
- Khare, A.; Gaur, S. Cholesterol-lowering effects of lactobacillus species. Curr. Microbiol. 2020, 77, 638–644. [Google Scholar] [CrossRef]
- Xie, C.; Teng, J.; Wang, X.; Xu, B.; Niu, Y.; Ma, L.; Yan, X. Multi-omics analysis reveals gut microbiota-induced intramuscular fat deposition via regulating expression of lipogenesis-associated genes. Anim. Nutr. 2022, 9, 84–99. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Zhang, H.; Su, Y.; Zhu, W. Swine gut microbiota and its interaction with host nutrient metabolism. Anim. Nutr. 2020, 6, 410–420. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Yang, W.; Jiang, S.; Li, Y. Supplementation with exogenous catalase from penicillium notatum in the diet amliorates lipopolysaccharide-induced intestinal oxidative damage through affecting intestinal antioxidant capacity and microbiota in weaned pigs. Microbiol. Spectr. 2021, 9, e0065421. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Song, Q.; Zhu, W.; Wei, J.; Shao, D.; Liu, K.; Li, Z.; Qiu, Y.; Ma, Z.; Xia, L.; et al. Characterization of small plasmids carrying florfenicol resistance gene flor in actinobacillus pleuropneumoniae and pasteurella multocida isolates from swine in china. Front. Vet. Sci. 2023, 10, 1084491. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.; Li, L.; Zhang, L.; Genco, R.J.; Falkner, K.L.; Tettelin, H.; Rowsam, A.M.; Smiraglia, D.J.; Novak, J.M.; Diaz, P.I.; et al. Periodontal disease is associated with increased gut colonization of pathogenic haemophilusparainfluenzae in patients with crohn’s disease. Cell Rep. 2023, 42, 112120. [Google Scholar] [CrossRef]
- Helm, E.T.; Outhouse, A.C.; Schwartz, K.J.; Dekkers, J.C.M.; Lonergan, S.M.; Rauw, W.M.; Gabler, N.K. Impact of myco plasma hyopneumoniae and lawsonia intracellularis on the performance of pigs divergently selected for feed efficiency. J. Anim. Sci. 2018, 96, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wang, L.; Li, S.; Ding, Q.; Wang, Y.; Wan, X.; Ji, X.; Lou, Y.; Li, X. The correlation between dysfunctional intestinal flora and pathology feature of patients with pulmonary tuberculosis. Front. Cell Infect. Microbiol. 2022, 12, 1090889. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, R.; Liu, M.; Wang, H.; Hou, S.; Hou, G.; Huang, X. Effects of lactobacillus delbrueckii on performance, carcass traits and meat quality of Ningxiang pigs. Chin. J. Anim. Nutr. 2017, 29, 4562–4569. (in Chinese). [Google Scholar]
- Torres-Pitarch, A.; Gardiner, G.E.; Cormican, P.; Rea, M.; Crispie, F.; O’Doherty, J.V.; Cozannet, P.; Ryan, T.; Lawlor, P.G. Effect of cereal soaking and carbohydrase supplementation on growth, nutrient digestibility and intestinal microbiota in liquid-fed grow-finishing pigs. Sci. Rep. 2020, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; An, M.; Zhang, W.; Li, K.; Kulyar, M.F.; Duan, K.; Zhou, H.; Wu, Y.; Wan, X.; Li, J.; et al. Integrated bacteria-fungi diversity analysis reveals the gut microbial changes in buffalo with mastitis. Front. Vet. Sci. 2022, 9, 918541. [Google Scholar] [CrossRef]
- Li, R.; Hou, G.; Jiang, X.; Song, Z.; Fan, Z.; Hou, D.X.; He, X. Different dietary protein sources in low protein diets regulate colonic microbiota and barrier function in a piglet model. Food Funct. 2019, 10, 6417–6428. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Ma, Y.; Cai, S.; Yang, L.; Fan, Y.; Zeng, X.; Qiao, S. Lactobacillus reuteri improves the development and maturation of fecal microbiota in piglets through mother-to-infant microbe and metabolite vertical transmission. Microbiome 2022, 10, 211. [Google Scholar] [CrossRef]
- Gao, Q.; Sun, G.; Duan, J.; Luo, C.; Yangji, C.; Zhong, R.; Chen, L.; Zhu, Y.; Wangdui, B.; Zhang, H. Alterations in gut microbiota improve scfa production and fiber utilization in tibetan pigs fed alfalfa diet. Front. Microbiol. 2022, 13, 969524. [Google Scholar] [CrossRef]
- Zhu, B.; Zhai, Y.; Ji, M.; Wei, Y.; Wu, J.; Xue, W.; Tao, W.W.; Wu, H. Alismaorientalis beverage treats atherosclerosis by regulating gut microbiota in ApoE(−/−) mice. Front. Pharmacol. 2020, 11, 570555. [Google Scholar] [CrossRef]
- Jin, H.; Leng, Q.; Zhang, C.; Zhu, Y.; Wang, J. P-cymene prevent high-fat diet-associated colorectal cancer by improving the structure of intestinal flora. J. Cancer 2021, 12, 4355–4361. [Google Scholar] [CrossRef]
- Lu, K.; He, Y.; Wu, C.; Bao, J. Moderate hyperglycemia-preventive effect and mechanism of action of periplaneta americana oligosaccharides in streptozotocin-induced diabetic mice. Nutrients 2022, 14, 4620. [Google Scholar] [CrossRef]
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, A.B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe 2020, 28, 245–257.e6. [Google Scholar] [CrossRef]
- Wang, Y.; Gunewardena, S.; Li, F.; Matye, D.J.; Chen, C.; Chao, X.; Jung, T.; Zhang, Y.; Czerwinski, M.; Ni, H.M.; et al. An FGF15/19-TFEB regulatory loop controls hepatic cholesterol and bile acid homeostasis. Nat. Commun. 2020, 11, 3612. [Google Scholar] [CrossRef]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. FAT SIGNALS—Lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy ho meostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]

| Items | 30–50 kg | 50–80 kg |
|---|---|---|
| Ingredients | ||
| Corn | 51.30 | 45.50 |
| Soybean meal (CP, 43%) | 12.70 | 8.20 |
| Paddy rice | 15.70 | 23.50 |
| Rice bran meal | 18.00 | 20.50 |
| Limestone | 0.70 | 0.70 |
| CaHPO4 | 0.80 | 0.80 |
| NaCl | 0.30 | 0.30 |
| Premix 1 | 0.50 | 0.50 |
| Total | 100.00 | 100.00 |
| Calculated nutritional levels | ||
| Digestible energy, DE MJ/kg | 13.07 | 12.64 |
| Crude protein, CP | 13.51 | 12.01 |
| Standardized ileal digestible lysine, SID Lys | 0.53 | 0.43 |
| Standardized ileal digestible methionine, SID Met | 0.22 | 0.20 |
| Standardized ileal digestible threonine, SID Thr | 0.40 | 0.34 |
| Standardized ileal digestible tryptophan, SID Trp | 0.11 | 0.09 |
| Calcium, Ca | 0.57 | 0.56 |
| Available phosphorus, AP | 0.42 | 0.44 |
| Analyzed nutritional levels | ||
| Gross energy, GE MJ/kg | 14.87 | 14.31 |
| Crude protein, CP | 13.72 | 12.27 |
| Ether extract, EE | 2.42 | 2.35 |
| Gene | Accession Number | Sequences (5′-3′) | Product Size (pb) |
|---|---|---|---|
| CPT-1 | NM_001129805.1 | F: GACAAGTCCTTCACCCTCATCGC | 117 |
| R: GGGTTTGGTTTGCCCAGACAG | |||
| PPARγ | NM_214379.1 | F: ACTTTATGGAGCCCAAGTTCG | 108 |
| R: GCAGCAAATTGTCTTGAATGTCC | |||
| LPL | NM_214286.1 | F: CACATTCACCAGAGGGTC | 126 |
| R: TCATGGGAGCACTTCACG | |||
| FAS | NM_213839.1 | F: TTTTCCCTGGCACTGGCTACCTG | 81 |
| R: TGCAGCGTCACGTCCTCAAACAC | |||
| GAPDH | NM_001206359.1 | F: ATGGTGAAGGTCGGAGTGAAC | 235 |
| R: CTCGCTCCTGGAAGATGGT |
| Items | CON | LD | p-Value |
|---|---|---|---|
| Ileal digesta | |||
| Acetic acid | 0.671 ± 0.165 a | 0.374 ± 0.198 b | 0.018 |
| Propionic acid | 0.039 ± 0.029 | 0.060 ± 0.043 | 0.329 |
| Butyric acid | 0.067 ± 0.060 | 0.074 ± 0.057 | 0.837 |
| Total SCFAs | 0.775 ± 0.232 | 0.506 ± 0.200 | 0.058 |
| Colonic digesta | |||
| Acetic acid | 0.509 ± 0.177 | 0.680 ± 0.149 | 0.101 |
| Propionic acid | 0.532 ± 0.098 | 0.319 ± 0.250 | 0.080 |
| Butyric acid | 0.239 ± 0.060 a | 0.597 ± 0.238 b | 0.045 |
| Total SCFAs | 1.280 ± 0.259 | 1.610 ± 0.581 | 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, G.; Wei, L.; Li, R.; Chen, F.; Yin, J.; Huang, X.; Yin, Y. Lactobacillus delbrueckii Ameliorated Blood Lipids via Intestinal Microbiota Modulation and Fecal Bile Acid Excretion in a Ningxiang Pig Model. Animals 2024, 14, 1801. https://doi.org/10.3390/ani14121801
Hou G, Wei L, Li R, Chen F, Yin J, Huang X, Yin Y. Lactobacillus delbrueckii Ameliorated Blood Lipids via Intestinal Microbiota Modulation and Fecal Bile Acid Excretion in a Ningxiang Pig Model. Animals. 2024; 14(12):1801. https://doi.org/10.3390/ani14121801
Chicago/Turabian StyleHou, Gaifeng, Liangkai Wei, Rui Li, Fengming Chen, Jie Yin, Xingguo Huang, and Yulong Yin. 2024. "Lactobacillus delbrueckii Ameliorated Blood Lipids via Intestinal Microbiota Modulation and Fecal Bile Acid Excretion in a Ningxiang Pig Model" Animals 14, no. 12: 1801. https://doi.org/10.3390/ani14121801
APA StyleHou, G., Wei, L., Li, R., Chen, F., Yin, J., Huang, X., & Yin, Y. (2024). Lactobacillus delbrueckii Ameliorated Blood Lipids via Intestinal Microbiota Modulation and Fecal Bile Acid Excretion in a Ningxiang Pig Model. Animals, 14(12), 1801. https://doi.org/10.3390/ani14121801







