Optimization of Fair Arterial Spin Labeling Magnetic Resonance Imaging (ASL-MRI) for Renal Perfusion Quantification in Dogs: Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs
2.1.1. MRI
2.1.2. T1 Blood
2.1.3. Blood-Tissue Water Partition Coefficient
2.2. Anesthesia
2.3. MRI
2.3.1. Scan Protocol
2.3.2. ASL
2.3.3. T1 Blood
2.4. Post-Processing
2.4.1. ASL
2.4.2. T1 Blood
2.4.3. Blood-Tissue Water Partition Coefficient
2.5. Statistics
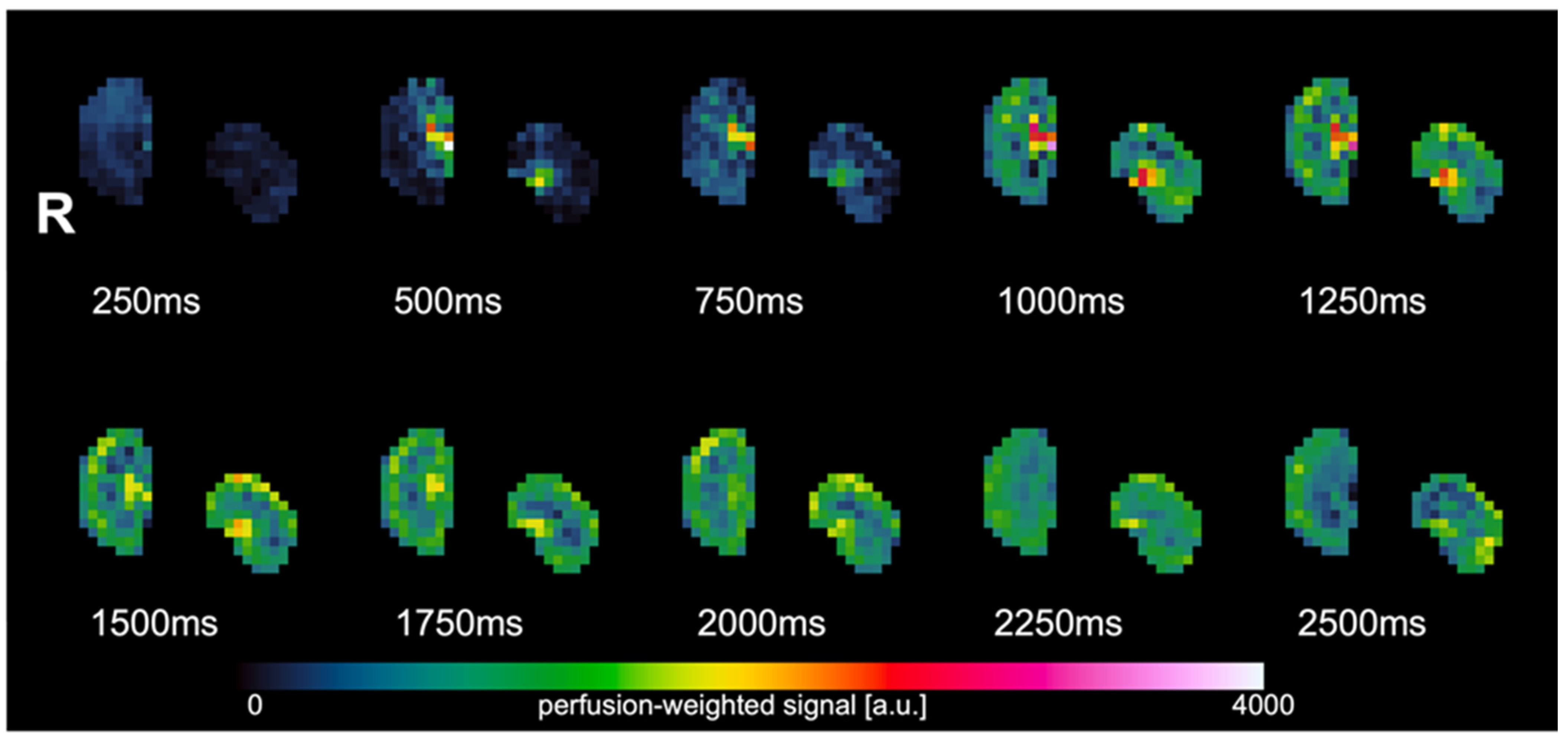
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Just, A. Mechanisms of renal blood flow autoregulation: Dynamics and contributions. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R1–R17. [Google Scholar] [CrossRef] [PubMed]
- Ross, L. Acute Kidney Injury in Dogs and Cats. Vet. Clin. N. Am.-Small Anim. Pract. 2011, 41, 1–14. [Google Scholar] [CrossRef]
- Mahmoud, H.; Buchanan, C.; Francis, S.T.; Selby, N.M. Imaging the kidney using magnetic resonance techniques: Structure to function. Curr. Opin. Nephrol. Hypertens. 2016, 25, 487–493. [Google Scholar] [CrossRef]
- Lankadeva, Y.R.; Okazaki, N.; Evans, R.G.; Bellomo, R.; May, C.N. Renal Medullary Hypoxia: A New Therapeutic Target for Septic Acute Kidney Injury? Semin. Nephrol. 2019, 39, 543–553. [Google Scholar] [CrossRef]
- Ohara, Y.; Yabuki, A.; Nakamura, R.; Ichii, O.; Mizukawa, H.; Yokoyama, N.; Yamato, O. Renal Infiltration of Macrophages in Canine and Feline Chronic Kidney Disease. J. Comp. Pathol. 2019, 170, 53–59. [Google Scholar] [CrossRef]
- Selby, N.M.; Williams, J.P.; Phillips, B.E. Application of dynamic contrast enhanced ultrasound in the assessment of kidney diseases. Curr. Opin. Nephrol. Hypertens. 2021, 30, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.C.; Germano, G.; Huang, S.C.; Hawkins, R.A.; Hansen, H.W.; Robert, M.J.; Buxton, D.B.; Schelbert, H.R.; Kurtz, I.; Phelps, M.E. A new noninvasive quantification of renal blood flow with N-13ammonia, dynamic positron emission tomography and a twocompartment model. J. Am. Soc. Nephrol. 1992, 3, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Lerman, L.O.; Bell, M.R.; Lahera, V.; Rumberger, J.A.; Sheedy, P.F.; Fueyo, A.S.; Romero, J.C. Quantification of global and regional renal blood flow with electron beam computed tomography. Am. J. Hypertens. 1994, 7, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Odudu, A.; Francis, S.T.; McIntyre, C.W. MRI for the assessment of organ perfusion in patients with chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 647–654. [Google Scholar] [CrossRef]
- Zhang, J.L.; Rusinek, H.; Chandarana, H.; Lee, V.S. Functional MRI of the kidneys. J. Magn. Reason. Imaging 2013, 37, 282–293. [Google Scholar] [CrossRef]
- Zhang, J.L.; Lee, V.S. Renal Perfusion Imaging by MRI. J. Magn. Reason. Imaging 2020, 52, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Nery, F.; Gordon, I.; Thomas, D.L. Non-Invasive Renal Perfusion Imaging Using Arterial Spin Labeling MRI: Challenges and Opportunities. Diagnostics 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Odudu, A.; Nery, F.; Harteveld, A.A.; Evans, R.G.; Pendse, D.; Buchanan, C.E.; Francis, S.T.; Fernandez-Seara, M.A. Arterial spin labelling MRI to measure renal perfusion: A systematic review and statement paper. Nephrol. Dial. Transplant. 2018, 33, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Schraml, C.; Schwenzer, N.F.; Claussen, C.D.; Martirosian, P. Examination of tissue perfusion by arterial spin labeling (ASL). Curr. Radiol. Rep. 2013, 1, 93–101. [Google Scholar] [CrossRef]
- Garteiser, P.; Bane, O.; Doblas, S.; Friedli, I.; Hectors, S.; Pagé, G.; Van Beers, B.E.; Waterton, J.C. Experimental Protocols for MRI Mapping of Renal T1. In Preclinical MRI of the Kidney: Methods and Protocols; Pohlmann, A., Niendorf, T., Eds.; Humuna Press: New York, NY, USA, 2021; pp. 383–402. [Google Scholar]
- van Osch, M.J.P.; Teeuwisse, W.M.; Chen, Z.; Suzuki, Y.; Helle, M.; Schmid, S. Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow. J. Cereb. Blood Flow. Metab. 2018, 38, 1461–1480. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.T.; Zimine, I.; Ho, Y.C.L.; Golay, X. Non-invasive measurement of perfusion: A critical review of arterial spin labelling techniques. Br. J. Radiol. 2006, 79, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Gillis, K.A.; McComb, C.; Foster, J.E.; Taylor, A.H.M.; Patel, R.K.; Morris, S.T.W.; Jardine, A.G.; Schneider, M.P.; Roditi, G.H.; Delles, C.; et al. Inter-study reproducibility of arterial spin labeling magnetic resonance imaging for measurement of renal perfusion in healthy volunteers at 3 Tesla. BMC Nephrol. BMC Nephrol. 2014, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Heusch, P.; Wittsack, H.J.; Blondin, D.; Ljimani, A.; Nguyen-Quang, M.; Martirosian, P.; Zenginli, H.; Bilk, P.; Kröpil, P.; Heusner, T.A.; et al. Functional evaluation of transplanted kidneys using arterial spin labeling MRI. J. Magn. Reason. Imaging 2014, 40, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Yang, X.; Wang, X.; Zhang, J.; Fang, J.; Jiang, X. Hemodynamic Effects of Furosemide on Renal Perfusion as Evaluated by ASL-MRI. Acad. Radiol. 2012, 19, 1194–1200. [Google Scholar] [CrossRef]
- Tan, H.; Koktzoglou, I.; Prasad, P.V. Renal perfusion imaging with two-dimensional navigator gated arterial spin labeling. Magn. Reason. Med. 2014, 71, 570–579. [Google Scholar] [CrossRef]
- Karger, N.; Biederer, J.; Lüsse, S.; Grimm, J.; Steffens, J.C.; Heller, M.; Glüer, C.C. Quantitation of renal perfusion using arterial spin labeling with FAIR-UFLARE. Magn. Reason. Imaging 2000, 18, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Bones, I.K.; Bos, C.; Moonen, C.; Hendrikse, J.; Van Stralen, M. Workflow for automatic renal perfusion quantification using ASL-MRI and machine learning. Magn. Reason. Med. 2021, 87, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, Z.; Zuo, P.; Pfeuffer, J.; Li, Y.; Liu, F.; Liu, R. Diagnostic Value of Renal Perfusion in Patients With Chronic Kidney Disease Using 3D Arterial Spin Labeling. J. Magn. Reason. Imaging 2017, 46, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Shim, W.H.; Yoon, S.K.; Oh, J.Y.; Kim, J.K.; Jung, H.; Matsuda, T.; Kim, D. Measurement of arterial transit time and renal blood flow using pseudocontinuous ASL MRI with multiple post-labeling delays: Feasibility, reproducibility, and variation. J. Magn. Reason. Imaging 2017, 46, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Nery, F.; Buchanan, C.E.; Harteveld, A.A.; Odudu, A.; Bane, O.; Cox, E.F.; Derlin, K.; Gach, H.M.; Golay, X.; Gutberlet, M.; et al. Consensus-based technical recommendations for clinical translation of renal ASL MRI. Magn. Reason. Mater. Phys. Biol. Med. 2020, 33, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Kudomi, N.; Koivuviita, N.; Liukko, K.E.; Oikonen, V.J.; Tolvanen, T.; Iida, H.; Tertti, R.; Metsärinne, K.; Iozzo, P.; Nuutila, P. Parametric renal blood flow imaging using [15O]H2O and PET. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Herscovitch, P.; Raichle, M.E. What Is the Correct Value for the Brain-Blood Partition Coefficient for Water? J. Cereb. Blood Flow Metab. 1985, 5, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Kommer, S.; Fembek, S.; Dröszler, U.; Körner, T.; Berg, A.; Schmid, A.I.; Moser, E.; Meyerspeer, M. Reproducible phantom for quality assurance in abdominal MRI focussing kidney imaging. Front. Phys. 2022, 10, 993241. [Google Scholar] [CrossRef]
- Wang, J.; Licht, D.J.; Jahng, G.H.; Liu, C.S.; Rubin, J.T.; Haselgrove, J.; Zimmerman, R.A.; Detre, J.A. Pediatric perfusion imaging using pulsed arterial spin labeling. J. Magn. Reason. Imaging. 2003, 18, 404–413. [Google Scholar] [CrossRef]
- Karakuzu, A.; Boudreau, M.; Duval, T.; Boshkovski, T.; Leppert, I.; Cabana, J.-F.; Gagnon, I.; Beliveau, P.; Pike, G.; Cohen-Adad, J.; et al. qMRLab: Quantitative MRI analysis, under one umbrella. J. Open Source Softw. 2020, 5, 2343. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, L.-P. Ventilatory changes of pulmonary capillary blood volume assessed by arterial density. J. Appl. Physiol. 1986, 61, 1724–1731. [Google Scholar] [CrossRef]
- Bourgès-Abella, N.H.; Gury, T.D.; Geffré, A.; Concordet, D.; Thibault-Duprey, K.C.; Dauchy, A.; Trumel, C. Reference intervals, intraindividual and interindividual variability, and reference change values for hematologic variables in laboratory beagles. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 17–24. [Google Scholar]
- Baik, S.J. Distribution of Dog Erythrocyte Density and Relationship to Cell Suspension Viscosity. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2002. [Google Scholar]
- Clausen, G.; Hope, A.; Aukland, K. Partition of 125I-iodoantipyrine among erythrocytes, plasma, and renal cortex in the dog. Acta Physiol. Scand. 1979, 107, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Kenner, T.; Moser, M.; Hinghofer-Szalkay, H.; Mohl, W. Indirect Determination of Fluid Filtration and Reabsorption in the Microcirculation of the Myocardium. Biomed. Eng./Biomed. Tech. 1984, 29, 108–116. [Google Scholar] [CrossRef]
- Piskunowicz, M.; Hofmann, L.; Zuercher, E.; Bassi, I.; Milani, B.; Stuber, M.; Narkiewicz, K.; Vogt, B.; Burnier, M.; Pruijm, M. A new technique with high reproducibility to estimate renal oxygenation using BOLD-MRI in chronic kidney disease. Magn. Reason. Imaging 2015, 33, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Milani, B.; Ansaloni, A.; Sousa-Guimaraes, S.; Vakilzadeh, N.; Piskunowicz, M.; Vogt, B.; Stuber, M.; Burnier, M.; Pruijm, M. Reduction of cortical oxygenation in chronic kidney disease: Evidence obtained with a new analysis method of blood oxygenation level-dependent magnetic resonance imaging. Nephrol. Dial. Transplant. 2017, 32, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Sanmiguel Serpa, L.C.; Hillaert, A.; Pullens, P. Absolute quantification of renal ASL in dogs. In Proceedings of the ISMRM ISMRT Annual Meeting and Exhibition, Toronto, ON, Canada, 3–8 June 2023. [Google Scholar]
- Zhang, J.L.; Morrell, G.; Rusinek, H.; Sigmund, E.E.; Chandarana, H.; Lerman, L.O.; Prasad, P.V.; Niles, D.; Artz, N.; Fain, S.; et al. New magnetic resonance imaging methods in nephrology. Kidney Int. 2014, 85, 768–778. [Google Scholar] [CrossRef]
- Kiefer, C.; Schroth, G.; Gralla, J.; Diehm, N.; Baumgartner, I.; Husmann, M. A Feasibility Study on Model-based Evaluation of Kidney Perfusion Measured by Means of FAIR Prepared True-FISP Arterial Spin Labeling (ASL) on a 3-T MR Scanner. Acad. Radiol. 2009, 16, 79–87. [Google Scholar] [CrossRef]
- Niles, D.J.; Artz, N.S.; Djamali, A.; Sadowski, E.A.; Grist, T.M.; Fain, S.B. Longitudinal Assessment of Renal Perfusion and Oxygenation in Transplant Donor-Recipient Pairs Using Arterial Spin Labeling and Blood Oxygen Level-Dependent Magnetic Resonance Imaging. Investig. Radiol. 2016, 51, 113–120. [Google Scholar] [CrossRef]
- Dong, J.; Yang, L.; Su, T.; Yang, X.D.; Chen, B.; Zhang, J.; Wang, X.Y.; Jiang, X.X. Quantitative assessment of acute kidney injury by noninvasive arterial spin labeling perfusion MRI: A pilot study. Sci. China Life Sci. 2013, 56, 745–750. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Y.; Li, X.; Chen, J.; Zhang, Y.; Zhang, L.; Wen, J. Early detection of subclinical pathology in patients with stable kidney graft function by arterial spin labeling. Eur. Radiol. Eur. Radiol. 2021, 31, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Niendorf, T. Preclinical MRI of the Kidney; Humana Press: New York, NY, USA, 2021; pp. 1–713. [Google Scholar]
- Wu, W.C.; St Lawrence, K.S.; Licht, D.J.; Wang, D.J.J. Quantification issues in arterial spin labeling perfusion magnetic resonance imaging. Top Magn. Reason. Imaging 2010, 21, 65–73. [Google Scholar]
- Lu, H.; Clingman, C.; Golay, X.; Van Zijl, P.C.M. Determining the longitudinal relaxation time (T1) of blood at 3.0 tesla. Magn. Reason. Med. 2004, 52, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Artz, N.S.; Wentland, A.L.; Sadowski, E.A.; Djamali, A.; Grist, T.M.; Seo, S.; Fain, S.B. Comparing kidney perfusion using noncontrast arterial spin labeling MRI and microsphere methods in an interventional swine model. Investig. Radiol. 2011, 46, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wentland, A.L.; Artz, N.S.; Fain, S.B.; Grist, T.M.; Djamali, A.; Sadowski, E.A. MR measures of renal perfusion, oxygen bioavailability and total renal blood flow in a porcine model: Noninvasive regional assessment of renal function. Nephrol. Dial. Transplant. 2012, 27, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, E.M.; Dickerson, J.W. The effect of growth and function on the chemical composition of soft tissues. Biochem. J. 1960, 77, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Juillard, L.; Janier, M.F.; Fouque, D.; Lionnet, M.; Le Bars, D.; Cinotti, L.; Barthez, P.; Gharib, C.; Laville, M. Renal blood flow measurement by positron emission tomography using 15 O-labeled water. Kidney Int. 2000, 57, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Yang, X.; Wang, X.; Zhang, J.; Fang, J.; Jiang, X. The serial effect of iodinated contrast media on renal hemodynamics and oxygenation as evaluated by ASL and BOLD MRI. Contrast. Media Mol. Imaging 2012, 7, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Lew, S.K.; Yong, C.X.; Tan, J.; Wang, D.J.J.; Chuang, K.H. Quantitative mouse renal perfusion using arterial spin labeling. NMR Biomed. 2013, 26, 1225–1232. [Google Scholar] [CrossRef]
- Winter, J.D.; St. Lawrence, K.S.; Margaret Cheng, H.L. Quantification of renal perfusion: Comparison of arterial spin labeling and dynamic contrast-enhanced MRI. J. Magn. Reason. Imaging 2011, 34, 608–615. [Google Scholar] [CrossRef]
- Alsop, D.C.; Detre, J.A.; Golay, X.; Günther, M.; Hendrikse, J.; Hernandez-garcia, L.; Lu, H.; Macintosh, B.J.; Parkes, L.M.; Smits, M.; et al. Recommended Implementation of Arterial Spin-Labeled Perfusion MRI for Clinical Applications: A Consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. Magn. Reason. Med. 2015, 73, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Vajda, Z.; Stødkilde-Jørgensen, H.; Nielsen, S.; Frøkiær, J. Furosemide increases water content in renal tissue. Am. J. Physiol.-Ren. Physiol. 2007, 292, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Kundel, H.L.; Schlakman, B.; Joseph, P.M.; Fishman, J.E.; Summers, R. Water Content and NMR Relaxation Time Gradients in the Rabbit Kidney. Investig. Radiol. 1986, 21, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Václavu, L.; van der Land, V.; Heijtel, D.F.R.; van Osch, M.J.P.; Cnossen, M.H.; Majoie, C.B.L.M.; Bush, A.; Wood, J.C.; Fijnvandraat, K.J.; Mutsaerts, H.J.M.M.; et al. In Vivo T1 of Blood Measurements in Children with Sickle Cell Disease Improve Cerebral Blood Flow Quantification from Arterial Spin-Labeling MRI. Am. J. Neuroradiol. 2016, 37, 1727–1732. [Google Scholar] [CrossRef] [PubMed]

| Dog | Breed | Age | Body Weight (kg) | Sex |
|---|---|---|---|---|
| 1 | Mixed breed | 3 years 9 months | 42.4 | Male C. |
| 2 | Bernese Mountain Dog | 7 years | 32 | Male |
| 3 | Italian Greyhound | 12 years | 9 | Male C. |
| 4 | Cavalier King Charles Spaniel | 4 years | 10.5 | Female S. |
| Layer | Inline RBF (Mean ± SD) | Corrected RBF (Mean ± SD) |
|---|---|---|
| 1 | 216.21 ± 104.00 | 157.73 ± 67.20 |
| 2 | 294.40 ± 108.36 | 216.42 ± 69.25 |
| 3 | 360.50 ± 78.43 | 251.11 ± 54.02 |
| 4 | 313.83 ± 99.14 | 209.20 ± 65.76 |
| 5 | 253.66 ± 113.71 | 168.48 ± 77.92 |
| 6 | 194.90 ± 85.37 | 129.58 ± 60.67 |
| 7 | 143.19 ± 76.23 | 93.24 ± 56.72 |
| 8 | 137.35 ± 76.91 | 90.38 ± 58.05 |
| 9 | 117.15 ± 54.46 | 77.43 ± 42.82 |
| 10 | 135.55 ± 54.73 | 95.45 ± 46.29 |
| 11 | 112.39 ± 43.61 | 81.52 ± 35.42 |
| 12 | 127.78 ± 38.98 | 94.92 ± 26.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillaert, A.; Sanmiguel Serpa, L.C.; Xu, Y.; Hesta, M.; Bogaert, S.; Vanderperren, K.; Pullens, P. Optimization of Fair Arterial Spin Labeling Magnetic Resonance Imaging (ASL-MRI) for Renal Perfusion Quantification in Dogs: Pilot Study. Animals 2024, 14, 1810. https://doi.org/10.3390/ani14121810
Hillaert A, Sanmiguel Serpa LC, Xu Y, Hesta M, Bogaert S, Vanderperren K, Pullens P. Optimization of Fair Arterial Spin Labeling Magnetic Resonance Imaging (ASL-MRI) for Renal Perfusion Quantification in Dogs: Pilot Study. Animals. 2024; 14(12):1810. https://doi.org/10.3390/ani14121810
Chicago/Turabian StyleHillaert, Amber, Luis Carlos Sanmiguel Serpa, Yangfeng Xu, Myriam Hesta, Stephanie Bogaert, Katrien Vanderperren, and Pim Pullens. 2024. "Optimization of Fair Arterial Spin Labeling Magnetic Resonance Imaging (ASL-MRI) for Renal Perfusion Quantification in Dogs: Pilot Study" Animals 14, no. 12: 1810. https://doi.org/10.3390/ani14121810
APA StyleHillaert, A., Sanmiguel Serpa, L. C., Xu, Y., Hesta, M., Bogaert, S., Vanderperren, K., & Pullens, P. (2024). Optimization of Fair Arterial Spin Labeling Magnetic Resonance Imaging (ASL-MRI) for Renal Perfusion Quantification in Dogs: Pilot Study. Animals, 14(12), 1810. https://doi.org/10.3390/ani14121810







