Histological and Molecular Biological Changes in Canine Skin Following Acute Radiation Therapy-Induced Skin Injury
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Animals
2.2. Irradiation and Skin Biopsies
2.3. Extraction of RNA, cDNA Synthesis, and RT–qPCR
2.4. Histological Assessment
2.5. Immunohistochemistry (IHC) Analysis
2.6. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
2.7. Statistical Analysis
3. Results
3.1. Observable Change of Radiation-Induced Skin Injury
3.2. Histological Changes of Skin Tissues for 9 Weeks Post-RT
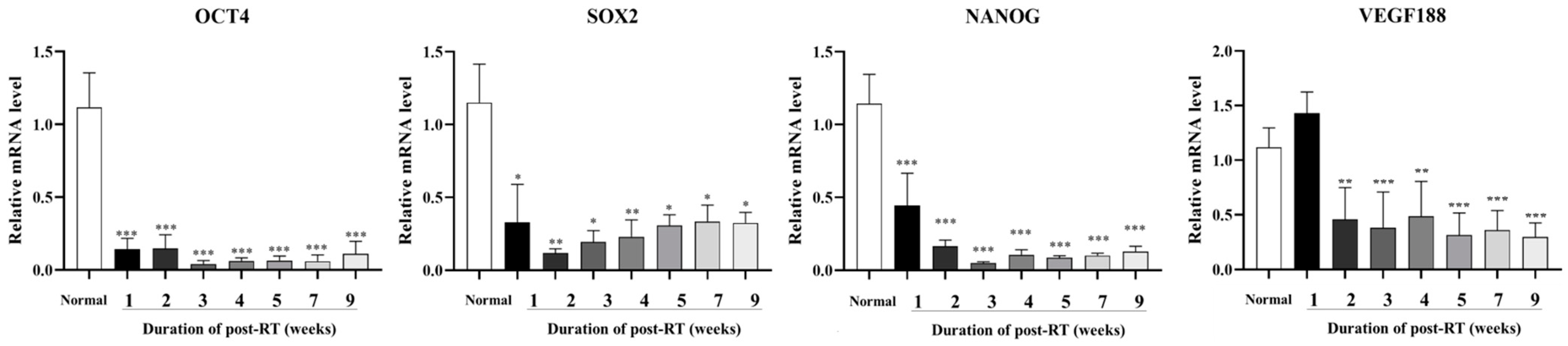
3.3. Expression of Cancer Stem Cells and Angiogenesis-Related Factors Post-RT
3.4. Expression of Inflammation and Keratinocyte Differentiation Markers Post-RT
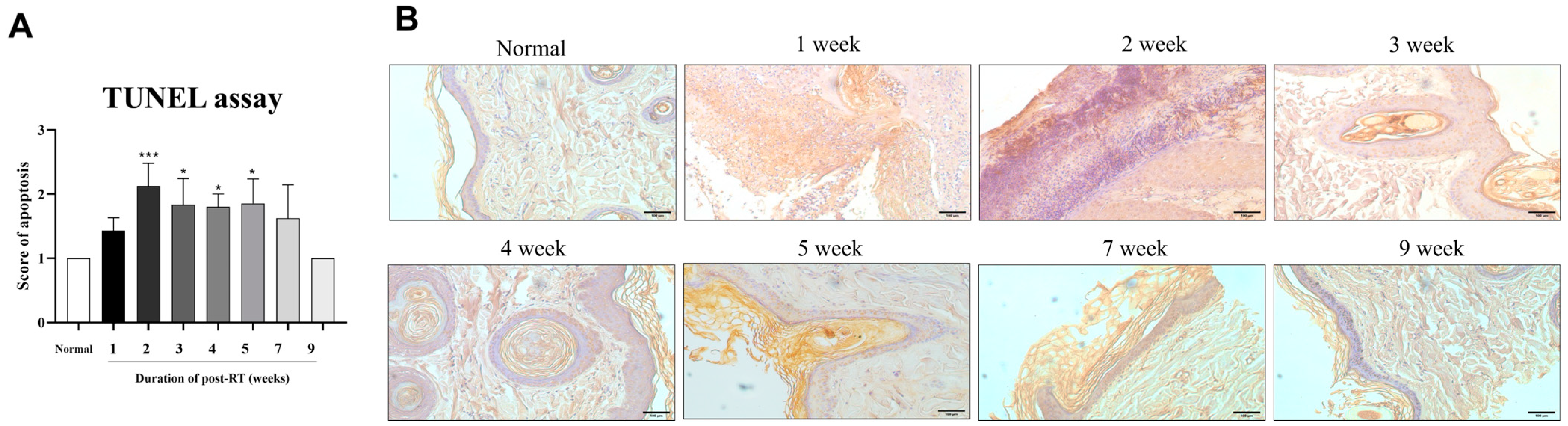
3.5. Radiation-Induced Apoptosis in Irradiated Skin Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffin, L.R.; Nolan, M.W.; Selmic, L.E.; Randall, E.; Custis, J.; LaRue, S. Stereotactic radiation therapy for treatment of canine intracranial meningiomas. Vet. Comp. Oncol. 2016, 14, e158–e170. [Google Scholar] [CrossRef] [PubMed]
- Gieger, T.L.; Nolan, M.W. Linac-based stereotactic radiation therapy for canine non-lymphomatous nasal tumours: 29 cases (2013–2016). Vet. Comp. Oncol. 2018, 16, E68–E75. [Google Scholar] [CrossRef] [PubMed]
- Hau, H.; Khanal, D.; Rogers, L.; Ali, K.; Ward, B.; Yakisich, J.S.; de la Rosa, J.M. Dose enhancement and cytotoxicity of gold nanoparticles in colon cancer cells when irradiated with kilo- and mega-voltage radiation. Bioeng. Transl. Med. 2016, 1, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Szumiel, I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: The pivotal role of mitochondria. Int. J. Radiat. Biol. 2015, 91, 1–12. [Google Scholar] [CrossRef]
- Dulić, V.; Kaufmann, W.K.; Wilson, S.J.; Tisty, T.D.; Lees, E.; Harper, J.W.; Elledge, S.J.; Reed, S.I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 1994, 76, 1013–1023. [Google Scholar] [CrossRef]
- Coleman, M.A.; Yin, E.; Peterson, L.E.; Nelson, D.; Sorensen, K.; Pegg, G.; Wyatt, R.; Kodell, R.; Lingerfelt, B.; Dynan, W.S.; et al. Low-dose irradiation alters the transcript profiles of human lymphoblastoid cells including genes associated with cytogenetic radioadaptive response. Radiat. Res. 2005, 164, 369–382. [Google Scholar] [CrossRef]
- Albrecht, H.; Durbin-Johnson, B.; Yunis, R.; Witz, P.; Lynch, C.; Yokoyama, N.; Coleman, M.A.; Joiner, E.; von Eyben, R.; Marquez, H.; et al. Transcriptional response of ex vivo human skin to ionizing radiation: Comparison between low- and high-dose effects. Radiat. Res. 2012, 177, 69–83. [Google Scholar] [CrossRef]
- Kadhim, M.A.; Hill, M.A. Non-targeted effects of radiation exposure: Recent advances and implications. Radiat. Prot. Dosim. 2015, 166, 118–124. [Google Scholar] [CrossRef]
- Sokolov, M.; Neumann, R. Global gene expression alterations as a crucial constituent of human cell response to low doses of ionizing radiation exposure. Int. J. Mol. Sci. 2016, 17, 55. [Google Scholar] [CrossRef]
- Chan, R.J.; Webster, J.; Chung, B.; Marquart, L.; Ahmed, M.; Garantziotis, S. Prevention and treatment of acute radiation-induced skin reactions: A systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2014, 14, 53. [Google Scholar] [CrossRef]
- Wei, J.; Meng, L.; Hou, X.; Yu, W.; Wang, H.; Dong, X.; Wang, B.; Zhao, H. Radiation-induced skin reactions: Mechanism and treatment. Cancer Manag. Res. 2019, 11, 167–177. [Google Scholar] [CrossRef]
- Yang, H.J.; Youn, H.S.; Seong, K.M.; Jin, Y.W.; Kim, J. Psoralidin, a dual inhibitor of COX–2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation. Biochem. Pharmacol. 2011, 82, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Lee, H.J.; Jung, Y.M.; Jung, Y.J. Regulated Necrotic Cell Death in Alternative Tumor Therapeutic Strategies. Cells 2020, 9, 2709. [Google Scholar] [CrossRef]
- Verneri, P.; Vazquez Echegaray, C.; Oses, C.; Stortz, M.; Guberman, A.; Levi, V. Dynamical reorganization of the pluripotency transcription factors Oct4 and Sox2 during early differentiation of embryonic stem cells. Sci. Rep. 2020, 10, 5195. [Google Scholar] [CrossRef]
- Moschetta, M.G.; Maschio, L.B.; Jardim-Perassi, B.V.; Ferreira, L.C.; Damasceno, K.A.; Rodini, C.O.; Vassallo, J.; Pires de Campos Zuccari, D.A. Prognostic value of vascular endothelial growth factor and hypoxia-inducible factor 1α in canine malignant mammary tumors. Oncol. Rep. 2015, 33, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, F.L.; Pires, I.; Parente, M.; Gregório, H.; Lopes, C.S. COX–2 over-expression correlates with VEGF and tumour angiogenesis in canine mammary cancer. Vet. J. 2011, 189, 77–82. [Google Scholar] [CrossRef]
- Lavalle, G.E.; Bertagnolli, A.C.; Tavares, W.L.F.; Cassali, G.D. COX–2 expression in canine mammary carcinomas: Correlation with angiogenesis and overall survival. Vet. Pathol. 2009, 46, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Ito, K.; Ishikawa, T.; Nakano, M.; Nakamura, T. Damage of hair follicle stem cells and alteration of keratin expression in external radiation-induced acute alopecia. Int. J. Mol. Med. 2012, 30, 579–584. [Google Scholar] [CrossRef]
- Patel, G.K.; Wilson, C.H.; Harding, K.G.; Finlay, A.Y.; Bowden, P.E. Numerous keratinocyte subtypes involved in wound re-epithelialization. J. Investig. Dermatol. 2006, 126, 497–502. [Google Scholar] [CrossRef]
- Cheng, F.; Eriksson, J.E. Intermediate filaments and the regulation of cell motility during regeneration and wound healing. Cold Spring Harb. Perspect. Biol. 2017, 9, a022046. [Google Scholar] [CrossRef]
- Bloomfield, R. Stereotactic radiation therapy in veterinary medicine. Can. Vet. J. 2015, 56, 95–97. [Google Scholar] [PubMed]
- Bray, F.N.; Simmons, B.J.; Wolfson, A.H.; Nouri, K. Acute and Chronic Cutaneous Reactions to Ionizing Radiation Therapy. Dermatol. Ther. 2016, 6, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Asaad, N.; Held, K.D. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene 2005, 24, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kang, J.W.; Lee, D.W.; Hur, S.Y.; Park, H.S.; Cho, C.K.; Lee, Y.J.; Soh, J.W.; Lee, S.J.; Lee, Y.S. Pyruvate metabolism: A therapeutic opportunity in radiation-induced skin injury. Biochem. Biophys. Res. Commun. 2015, 460, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choe, Y.H.; Han, J.H.; Shin, M.Y.; Park, H.S.; Lee, Y.J.; Soh, J.W.; Lee, S.J.; Lee, Y.S. HPRT1 Most Suitable Reference Gene for Accurate Normalization of mRNA Expression in Canine Dermal Tissues with Radiation Therapy. Genes 2022, 13, 1928. [Google Scholar] [CrossRef]
- Ben-Porath, I.; Thomson, M.W.; Carey, V.J.; Ge, R.; Bell, G.W.; Regev, A.; Weinberg, R.A. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008, 40, 499–507. [Google Scholar] [CrossRef]
- Kreso, A.; Dick, J.E. Evolution of the cancer stem cell model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef]
- El-Benhawy, S.A.; El-Sheredy, H.G.; Ghanem, H.B.; Abo El-Soud, A.A. Berberine can amplify cytotoxic effect of radiotherapy by targeting cancer stem cells. Breast Cancer Manag. 2020, 9, BMT41. [Google Scholar] [CrossRef]
- Saigusa, S.; Tanaka, K.; Toiyama, Y.; Yokoe, T.; Okugawa, Y.; Kawamoto, A.; Yasuda, H.; Morimoto, Y.; Fujikawa, H.; Inoue, Y.; et al. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann. Surg. Oncol. 2009, 16, 3488–3498. [Google Scholar] [CrossRef]
- Wang, L.K.; Wu, T.J.; Hong, J.H.; Chen, F.H.; Yu, J.; Wang, C.C. Radiation Induces Pulmonary Fibrosis by Promoting the Fibrogenic Differentiation of Alveolar Stem Cells. Stem Cells Int. 2020, 2020, 6312053. [Google Scholar] [CrossRef]
- Lee, C.G.; Moon, S.R.; Cho, M.Y.; Park, K.R. Mast cell degranulation and vascular endothelial growth factor expression in mouse skin following ionizing irradiation. J. Radiat. Res. 2021, 62, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Nojima, K.; Majima, H.; Yamaguchi, Y.; Yamada, M.; Mori, S.; Maeda, M.; Noda, S. Evidence for mRNA expression of vascular endothelial growth factor by X-ray irradiation in a lung squamous carcinoma cell line. Cancer Lett. 1998, 132, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Bartholdi, D.; Rubin, B.P.; Schwab, M.E. VEGF mRNA Induction Correlates With Changes in the Vascular Architecture Upon Spinal Cord Damage in the Rat. Eur. J. Neurosci. 1997, 9, 2549–2560. [Google Scholar] [CrossRef]
- Wise, L.M.; Stuart, G.S.; Real, N.C.; Fleming, S.B.; Mercer, A.A. VEGF Receptor-2 Activation Mediated by VEGF-E Limits Scar Tissue Formation Following Cutaneous Injury. Adv. Wound Care 2018, 7, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L. High COX–2 expression is associated with increased angiogenesis, proliferation and tumoural inflammatory infiltrate in canine malignant mammary tumours: A multivariate survival study. Vet. Comp. Oncol. 2017, 15, 619–631. [Google Scholar] [CrossRef]
- Greenhough, A.; Smartt, H.J.M.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX–2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef]
- Kirkpatrick, K.; Ogunkolade, W.; Elkak, A.; Bustin, S.A.; Jenkins, P.J.; Ghilchik, M.W.; Mokbel, K. The mRNA expression of cyclo-oxygenase-2 (COX–2) and vascular endothelial growth factor (VEGF) in human breast cancer. Curr. Med. Res. Opin. 2002, 18, 237–241. [Google Scholar] [CrossRef]
- Lim, S.C. Role of COX–2, VEGF and cyclin D1 in mammary infiltrating duct carcinoma. Oncol. Rep. 2003, 10, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Santini, D.; Vincenzi, B.; Tonini, G.; Vincenzi, B.; Vannoni, C.; Martinelli, E.; Sabbatini, R.; Lai, R.; Angeletti, S.; et al. COX–2 expression in DCIS: Correlation with VEGF, HER-2/neu, prognostic molecular markers and clinicopathological features. Histopathology 2005, 46, 561–568. [Google Scholar] [CrossRef]
- Buckman, S.Y.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX–2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef]
- Athar, M.; An, K.P.; Morel, K.D.; Kim, A.L.; Mukhtar, H.; Aszterbaum, M.; Kopelovich, L. Ultraviolet B (UVB)-induced COX–2 expression in murine skin: An immunohistochemical study. Biochem. Biophys. Res. Commun. 2001, 280, 1042–1047. [Google Scholar] [CrossRef]
- Chen, W.; Tang, Q.; Gonzales, M.S.; Bowden, G.T. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene 2001, 20, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Tripp, C.S.; Blomme, E.A.G.; Chinn, K.S.; Hardy, M.M.; LaCelle, P.; Pentland, A.P. Epidermal COX–2 induction following ultraviolet irradiation: Suggested mechanism for the role of COX–2 inhibition in photoprotection. J. Investig. Dermatol. 2003, 121, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Laube, M.; Kniess, T.; Pietzsch, J. Development of Antioxidant COX–2 Inhibitors as Radioprotective Agents for Radiation Therapy—A Hypothesis-Driven Review. Antioxidants 2016, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Cheki, M.; Yahyapour, R.; Farhood, B.; Rezaeyan, A.; Shabeeb, D.; Amini, P.; Rezapoor, S. COX–2 in Radiotherapy: A Potential Target for Radioprotection and Radiosensitization. Curr. Mol. Pharmacol. 2018, 11, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, M.; Zhang, L.J. Keratin 6, 16 and 17—Critical barrier alarmin molecules in skin wounds and psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef]
- Santos, M.; Paramio, J.S.; Bravo, A.; Ramirez, A.; Jorcano, J.L. The expression of keratin K10 in the basal layer of the epidermis inhibits cell proliferation and prevents skin tumorigenesis. J. Biol. Chem. 2002, 277, 19122–19130. [Google Scholar] [CrossRef]
- Sheng, X.; Zhou, Y.; Wang, H.; Hu, W.; Zhang, W.; Zhou, Q.; Chen, H.; Qian, H.; Xu, W. Establishment and characterization of a radiation-induced dermatitis rat model. J. Cell. Mol. Med. 2019, 23, 3178–3189. [Google Scholar] [CrossRef]
- Hubenak, J.R.; Zhang, Q.; Branch, C.D.; Kronowitz, S.J. Mechanisms of injury to normal tissue after radiotherapy: A review. Plast. Reconstr. Surg. 2014, 133, 49–56. [Google Scholar] [CrossRef]
- Yao, C.; Zhou, Y.; Wang, H.; Zhang, W.; Hu, W.; Chen, H.; Qian, H.; Yang, H.; Wang, L.; Xu, W.; et al. Adipose-derived stem cells alleviate radiation-induced dermatitis by suppressing apoptosis and downregulating cathepsin F expression. Stem Cell Res. Ther. 2021, 12, 90. [Google Scholar] [CrossRef]



| Gene Name (Symbol) | Primer Sequences | Amplicon Size (bp) | Accession Number |
|---|---|---|---|
| POU domain class 5 transcription factor 1 (Oct4) | F: AACGATCAAGCAGTGACTATTCG R: AGTAGAGCGTAGTGAAGTGAGG | 147 | NM_001003142.2 |
| Sex determining region Y box 2 (Sox2) | F: AGTCTCCAAGCGACGAAAAA R: CCACGTTTGCAACTGTCCTA | 189 | DR105272 |
| Homeobox protein NANOG (Nanog) | F: GACCGTCTCTCCTCTTCCTTCC R: CGTCCTCATCTTCTGTTTCTTGC | 157 | XM_014108418.1 |
| Vascular endothelial growth factor 188 (VEGF 188) | F: CGAGTACATCTTCAAGCCATCC R: GTGATGTTGAACTCCTCAGTGG | 102 | AF133250.1 |
| Prostaglandin–endoperoxide synthase–2 (COX–2) | F: TGTTCACCTGACTACTGGAAGC R: GACAGCCCTTCACGTTATTGC | 109 | NM_001003354.1 |
| Cytokeratin 10 (Keratin 10) | F: CTCGTGACTACAGCAAATACTACC R: TGGCATTGTCGATCTGAAGC | 105 | NM_001013425.1 |
| Hypoxanthine phosphoribosyltransferase 1 (HPRT1) | F: GACTGAAGAGCTACTGTAATGACC R: TCTTTGGATTATGCTCCTTGACC | 168 | XM538830.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; Hwang, G.; Choi, M.; Jo, C.-H.; Oh, S.-J.; Jin, Y.B.; Lee, W.-J.; Rho, G.-J.; Lee, H.C.; Lee, S.-L.; et al. Histological and Molecular Biological Changes in Canine Skin Following Acute Radiation Therapy-Induced Skin Injury. Animals 2024, 14, 2505. https://doi.org/10.3390/ani14172505
Lee S-Y, Hwang G, Choi M, Jo C-H, Oh S-J, Jin YB, Lee W-J, Rho G-J, Lee HC, Lee S-L, et al. Histological and Molecular Biological Changes in Canine Skin Following Acute Radiation Therapy-Induced Skin Injury. Animals. 2024; 14(17):2505. https://doi.org/10.3390/ani14172505
Chicago/Turabian StyleLee, Sang-Yun, Gunha Hwang, Moonyeong Choi, Chan-Hee Jo, Seong-Ju Oh, Yeung Bae Jin, Won-Jae Lee, Gyu-Jin Rho, Hee Chun Lee, Sung-Lim Lee, and et al. 2024. "Histological and Molecular Biological Changes in Canine Skin Following Acute Radiation Therapy-Induced Skin Injury" Animals 14, no. 17: 2505. https://doi.org/10.3390/ani14172505
APA StyleLee, S.-Y., Hwang, G., Choi, M., Jo, C.-H., Oh, S.-J., Jin, Y. B., Lee, W.-J., Rho, G.-J., Lee, H. C., Lee, S.-L., & Hwang, T. S. (2024). Histological and Molecular Biological Changes in Canine Skin Following Acute Radiation Therapy-Induced Skin Injury. Animals, 14(17), 2505. https://doi.org/10.3390/ani14172505







