Genome-Wide Association Study Reveals Novel Candidate Genes Influencing Semen Traits in Landrace Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Phenotypic and Pedigree Data
2.2. Genotypic Data
2.3. Statistical Model
2.4. Annotation of Candidate Genes
3. Results
3.1. Phenotypic Data Analysis and Heritability Estimates
3.2. The Genetic and Phenotypic Correlation Coefficients of Semen Traits in Boars
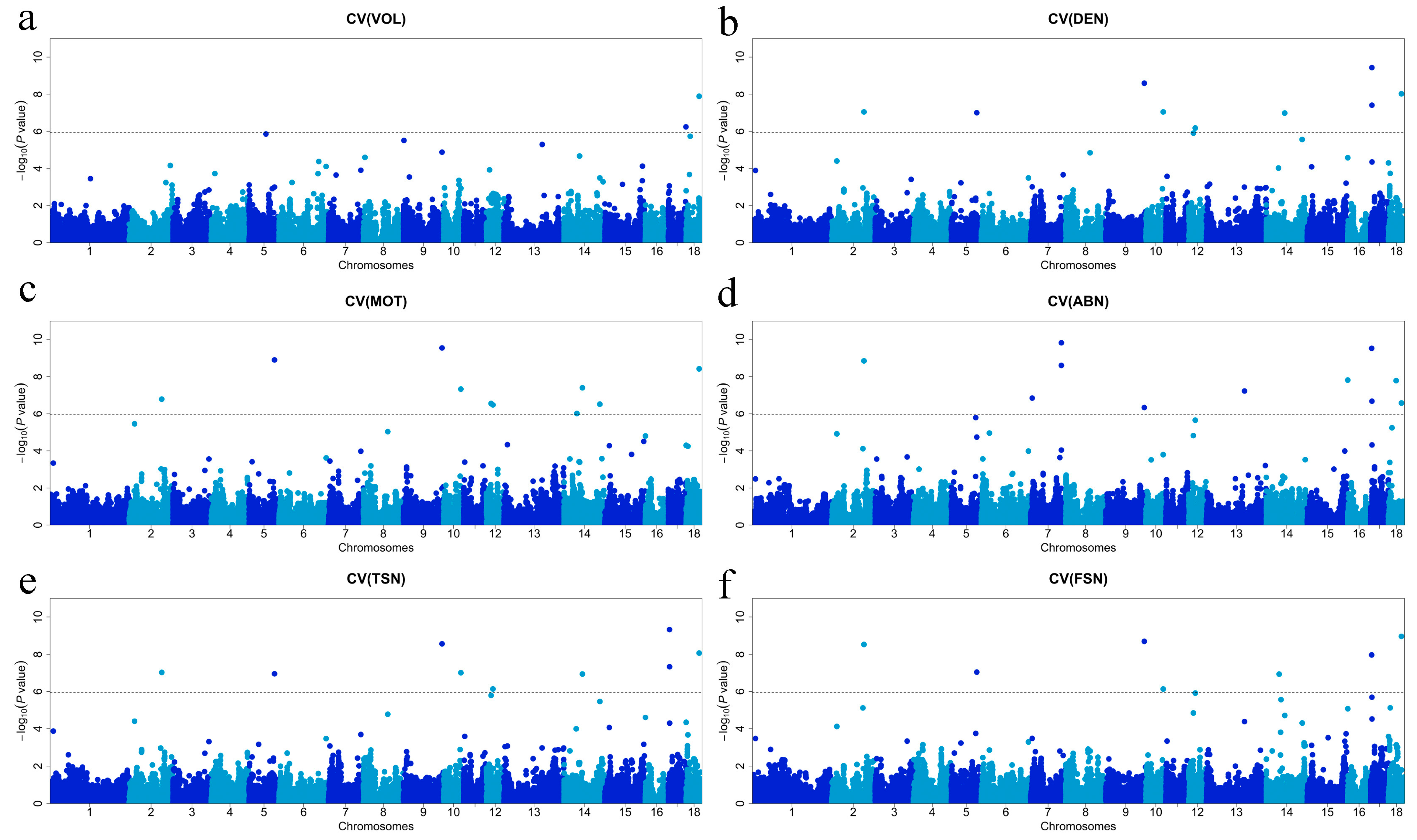
3.3. GWAS for Semen Traits and Candidate Gene Searching
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez Rodriguez, A.; Van Soom, A.; Arsenakis, I.; Maes, D. Boar management and semen handling factors affect the quality of boar extended semen. Porc. Health Manag. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, B.; Wang, X.; Liu, X.; Zhang, Q.; Chen, Y. Estimation of genetic parameters and season effects for semen traits in three pig breeds of South China. J. Anim. Breed. Genet. 2019, 136, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.L.; Wei, H.K.; Zhou, Y.F.; Jiang, S.W.; Peng, J. Linear model analysis of the influencing factors of boar longevity in Southern China. Theriogenology 2017, 93, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Sutovsky, P. New Approaches to Boar Semen Evaluation, Processing and Improvement. Reprod Domest Anim 2015, 50 (Suppl. S2), 11–19. [Google Scholar] [CrossRef] [PubMed]
- Diniz, D.B.; Lopes, M.S.; Broekhuijse, M.L.; Lopes, P.S.; Harlizius, B.; Guimaraes, S.E.; Duijvesteijn, N.; Knol, E.F.; Silva, F.F. A genome-wide association study reveals a novel candidate gene for sperm motility in pigs. Anim. Reprod. Sci. 2014, 151, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Ren, J.; Ren, D.; Guo, Y.; Wu, Y.; Yang, G.; Mao, H.; Brenig, B.; Huang, L. A whole genome scanning for quantitative trait loci on traits related to sperm quality and ejaculation in pigs. Anim. Reprod. Sci. 2009, 114, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Wellcome Trust Case Control, C. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 2007, 447, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Fu, C.; Sahana, G.; Chen, Y.; Yin, L.; Miao, Y.; Zhao, S.; Xiang, T. Identification of new semen trait-related candidate genes in Duroc boars through genome-wide association and weighted gene co-expression network analyses. J. Anim. Sci. 2021, 99, skab188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, N.; Li, X.; El-Ashram, S.; Wang, Z.; Zhu, L.; Jiang, W.; Peng, X.; Zhang, C.; Chen, Y.; et al. Identifying candidate genes associated with sperm morphology abnormalities using weighted single-step GWAS in a Duroc boar population. Theriogenology 2020, 141, 9–15. [Google Scholar] [CrossRef]
- Gao, N.; Chen, Y.; Liu, X.; Zhao, Y.; Zhu, L.; Liu, A.; Jiang, W.; Peng, X.; Zhang, C.; Tang, Z.; et al. Weighted single-step GWAS identified candidate genes associated with semen traits in a Duroc boar population. BMC Genom. 2019, 20, 797. [Google Scholar] [CrossRef]
- Ogawa, S.; Kimata, M.; Tomiyama, M.; Satoh, M. Heritability and genetic correlation estimates of semen production traits with litter traits and pork production traits in purebred Duroc pigs. J. Anim. Sci. 2022, 100, skac055. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Browning, B.L.; Browning, S.R. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am. J. Hum. Genet. 2009, 84, 210–223. [Google Scholar] [CrossRef] [PubMed]
- VanRaden, P.M.; Wiggans, G.R. Derivation, calculation, and use of national animal model information. J. Dairy Sci. 1991, 74, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, S.; Sargolzaei, M.; Abo-Ismail, M.K.; May, N.; Miller, S.P.; Schenkel, F.; Moore, S.S.; Stothard, P. Genome-wide association for milk production and female fertility traits in Canadian dairy Holstein cattle. BMC Genet. 2016, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, M.; Fan, B.; Buckler, E.S.; Zhang, Z. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLoS Genet. 2016, 12, e1005767. [Google Scholar] [CrossRef] [PubMed]
- Tasi, Y.C.; Chao, H.C.; Chung, C.L.; Liu, X.Y.; Lin, Y.M.; Liao, P.C.; Pan, H.A.; Chiang, H.S.; Kuo, P.L.; Lin, Y.H. Characterization of 3-hydroxyisobutyrate dehydrogenase, HIBADH, as a sperm-motility marker. J. Assist. Reprod. Genet. 2013, 30, 505–512. [Google Scholar] [CrossRef]
- Hering, D.M.; Olenski, K.; Kaminski, S. Genome-wide association study for poor sperm motility in Holstein-Friesian bulls. Anim. Reprod. Sci. 2014, 146, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Montiel, W.; Martinez-Nunez, M.A.; Ramon-Ugalde, J.P.; Roman-Ponce, S.I.; Calderon-Chagoya, R.; Zamora-Bustillos, R. Genome-Wide Association Study Reveals Candidate Genes for Litter Size Traits in Pelibuey Sheep. Animals 2020, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Marziali, F.; Dizanzo, M.P.; Cavatorta, A.L.; Gardiol, D. Differential expression of DLG1 as a common trait in different human diseases: An encouraging issue in molecular pathology. Biol. Chem. 2019, 400, 699–710. [Google Scholar] [CrossRef]
- Huang, J.H.; Rajkovic, A.; Szafranski, P.; Ochsner, S.; Richards, J.; Goode, S. Expression of Drosophila neoplastic tumor suppressor genes discslarge, scribble, and lethal giant larvae in the mammalian ovary. Gene. Expr. Patterns 2003, 3, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Cavatorta, A.L.; Di Gregorio, A.; Bugnon Valdano, M.; Marziali, F.; Cabral, M.; Bottai, H.; Cittadini, J.; Nocito, A.L.; Gardiol, D. DLG1 polarity protein expression associates with the disease progress of low-grade cervical intraepithelial lesions. Exp. Mol. Pathol. 2017, 102, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Viswakarma, N.; Jia, Y.; Bai, L.; Gao, Q.; Lin, B.; Zhang, X.; Misra, P.; Rana, A.; Jain, S.; Gonzalez, F.J.; et al. The Med1 subunit of the mediator complex induces liver cell proliferation and is phosphorylated by AMP kinase. J. Biol. Chem. 2013, 288, 27898–27911. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; You, J.; Mao, C.; Zhou, E.; Han, Z.; Zhang, J.; Zhang, T.; Wang, C. Circular RNA Fbxl5 Regulates Cardiomyocyte Apoptosis During Ischemia Reperfusion Injury via Sponging microRNA-146a. J. Inflamm. Res. 2022, 15, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Bovo, S.; Schiavo, G.; Mazzoni, G.; Dall’Olio, S.; Galimberti, G.; Calo, D.G.; Scotti, E.; Bertolini, F.; Buttazzoni, L.; Samore, A.B.; et al. Genome-wide association study for the level of serum electrolytes in Italian Large White pigs. Anim. Genet. 2016, 47, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Ohno, A.; Ohya, S.; Yamamura, H.; Imaizumi, Y. Regulation of ryanodine receptor-mediated Ca(2+) release in vas deferens smooth muscle cells. J. Pharmacol. Sci. 2009, 110, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.J.; Navarro, B.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat. Commun. 2011, 2, 153. [Google Scholar] [CrossRef] [PubMed]
- Sancho, M.; Gortat, A.; Herrera, A.E.; Andreu-Fernandez, V.; Ferraro, E.; Cecconi, F.; Orzaez, M.; Perez-Paya, E. Altered mitochondria morphology and cell metabolism in Apaf1-deficient cells. PLoS ONE 2014, 9, e84666. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.E.; Nishibori, M.; Isobe, N.; Hisaeda, K. Detection of APAF1 mutation in Holstein cows and mummified foetuses in Japanese dairy herds. Reprod. Domest. Anim. 2018, 53, 137–142. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Schröder, O.; Jakobsson, P.J.; Samuelsson, B.; Haeggström, J.Z.; Hökfelt, T. Expression of microsomal glutathione S-transferase type 3 mRNA in the rat nervous system. Gene. Expr. Patterns 2002, 115, 891–897. [Google Scholar] [CrossRef]
- Kashuba, V.I.; Stakhovsky, E.A.; Gryzodub, O.P.; Vitruk, Y.V.; Lytvynenko, R.A.; Nekrasov, K.A.; Mevs, L.V.; Hryshchenko, N.V.; Gerashchenko, G.V.; Rosenberg, E.E. Expression of Cancer-Associated Genes in Prostate Tumors. Exp. Oncol. 2017, 39, 131–137. [Google Scholar]
- Verma, A.; Rajput, S.; De, S.; Kumar, R.; Chakravarty, A.K.; Datta, T.K. Genome-wide profiling of sperm DNA methylation in relation to buffalo (Bubalus bubalis) bull fertility. Theriogenology 2014, 82, 750–759e751. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, C.; Chen, H.; Li, R.; Chong, Q.; Xiao, H.; Chen, S. Genome-wide scan of selection signatures in Dehong humped cattle for heat tolerance and disease resistance. Anim. Genet. 2020, 51, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Girouard, J.; Frenette, G.; Sullivan, R. Seminal plasma proteins regulate the association of lipids and proteins within detergent-resistant membrane domains of bovine spermatozoa. Biol. Reprod. 2008, 78, 921–931. [Google Scholar] [CrossRef]
- Girouard, J.; Frenette, G.; Sullivan, R. Compartmentalization of proteins in epididymosomes coordinates the association of epididymal proteins with the different functional structures of bovine spermatozoa. Biol. Reprod. 2009, 80, 965–972. [Google Scholar] [CrossRef]
- Eickhoff, R.; Wilhelm, B.; Renneberg, H.; Wennemuth, G.; Bacher, M.; Linder, D.; Bucala, R.; Seitz, J.; Meinhardt, A. Purification and characterization of macrophage migration inhibitory factor as a secretory protein from rat epididymis: Evidences for alternative release and transfer to spermatozoa. Mol. Med. 2001, 7, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, R.; Baldauf, C.; Koyro, H.W.; Wennemuth, G.; Suga, Y.; Seitz, J.; Henkel, R.; Meinhardt, A. Influence of macrophage migration inhibitory factor (MIF) on the zinc content and redox state of protein-bound sulphydryl groups in rat sperm: Indications for a new role of MIF in sperm maturation. Mol. Hum. Reprod. 2004, 10, 605–611. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Ribas-Maynou, J.; Delgado-Bermudez, A.; Llavanera, M.; Recuero, S.; Barranco, I.; Yeste, M. Aldose Reductase B1 in Pig Sperm Is Related to Their Function and Fertilizing Ability. Front. Endocrinol. 2022, 13, 773249. [Google Scholar] [CrossRef]
- Cebrian-Serrano, A.; Salvador, I.; Garcia-Rosello, E.; Pericuesta, E.; Perez-Cerezales, S.; Gutierrez-Adan, A.; Coy, P.; Silvestre, M.A. Effect of the bovine oviductal fluid on in vitro fertilization, development and gene expression of in vitro-produced bovine blastocysts. Reprod. Domest. Anim. 2013, 48, 331–338. [Google Scholar] [CrossRef]
- Gupta, S.K.; Bansal, P.; Ganguly, A.; Bhandari, B.; Chakrabarti, K. Human zona pellucida glycoproteins: Functional relevance during fertilization. J. Reprod. Immunol. 2009, 83, 50–55. [Google Scholar] [CrossRef]
- Yauger, B.; Boggs, N.A.; Dean, J. Human ZP4 is not sufficient for taxon-specific sperm recognition of the zona pellucida in transgenic mice. Reproduction 2011, 141, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Rico, M.J.; Moros-Nicolas, C.; Perez-Crespo, M.; Laguna-Barraza, R.; Gutierrez-Adan, A.; Veyrunes, F.; Ballesta, J.; Laudet, V.; Chevret, P.; Aviles, M. ZP4 Is Present in Murine Zona Pellucida and Is Not Responsible for the Specific Gamete Interaction. Front. Cell Dev. Biol. 2020, 8, 626679. [Google Scholar] [CrossRef] [PubMed]
- Julieta, G.H.; María, J.-M.; Raquel, R. Sperm binding to ZP2-coated beads improve the efficiency of porcine in vitro fertilisation. Reproduction 2020, 160, 725–735. [Google Scholar]
- Zeng, M.H.; Wang, Y.; Huang, H.L.; Quan, R.P.; Yang, J.T.; Guo, D.; Sun, Y.; Lv, C.; Li, T.Y.; Wang, L.; et al. Zp4 is completely dispensable for fertility in female ratsdagger. Biol. Reprod. 2021, 104, 1282–1291. [Google Scholar] [CrossRef] [PubMed]

| Trait | N1 | N2 | Average | Mean | S.D | Min | Mam | C.V (%) |
|---|---|---|---|---|---|---|---|---|
| VOL, mL | 2059 | 225,468 | 109.50 | 207.18 | 78.26 | 31.00 | 650.00 | 37.77 |
| DEN, 108/mL | 2059 | 225,468 | 109.50 | 3.73 | 1.72 | 0.03 | 10 | 46.11 |
| MOT, % | 2059 | 225,468 | 109.50 | 88.72 | 7.16 | 11.00 | 99.80 | 8.07 |
| ABN, % | 2059 | 225,468 | 109.50 | 7.28 | 6.48 | 0.00 | 96.00 | 89.01 |
| TSN, 108 | 2059 | 225,468 | 109.50 | 636.45 | 274.63 | 0.80 | 2313.90 | 43.15 |
| FSN, 108 | 2059 | 225,468 | 109.50 | 590.36 | 255.73 | 0.80 | 2198.21 | 43.32 |
| CVVOL, % | 2059 | 15,992 | 7.76 | 29.00 | 7.27 | 3.06 | 82.03 | 25.07 |
| CVDEN, % | 2059 | 15,992 | 7.76 | 36.42 | 10.32 | 10.00 | 209.44 | 28.34 |
| CVMOT, % | 2059 | 15,992 | 7.76 | 7.03 | 5.52 | 0.55 | 62.08 | 78.52 |
| CVABN, % | 2059 | 15,992 | 7.76 | 55.82 | 18.86 | 11.53 | 264.58 | 33.79 |
| CVTSN, % | 2059 | 15,992 | 7.76 | 37.00 | 12.62 | 8.36 | 210.39 | 34.11 |
| CVFSN, % | 2059 | 15,992 | 7.76 | 37.66 | 13.44 | 10.69 | 220.22 | 35.69 |
| VOL | DEN | MOT | ABN | TSN | FSN | |
|---|---|---|---|---|---|---|
| VOL | −0.43 | −0.02 | −0.02 | 0.38 | 0.38 | |
| DEN | −0.62 (0.07) | 0.16 | 0.08 | 0.57 | 0.55 | |
| MOT | 0.06 (0.11) | 0.38 (0.10) | −0.47 | 0.31 | 0.36 | |
| ABN | −0.12 (0.09) | −0.29 (0.09) | −0.87 (0.03) | −0.02 | −0.16 | |
| TSN | 0.27 (0.11) | 0.53 (0.08) | 0.73 (0.07) | −0.62 (0.08) | 0.99 | |
| FSN | 0.26 (0.10) | 0.53 (0.08) | 0.81 (0.05) | −0.76 (0.05) | 0.98 (0.01) |
| SNP | Traits | Chromosome | Position | Located Gene | Flanking Genes |
|---|---|---|---|---|---|
| ALGA0107690 | CVMOT, CVABN | 2 | 18,451,516 | - | ALKBH3/HSD17B12 |
| MARC0067122 | CVDEN, CVMOT, CVABN, CVTSN, CVFSN | 2 | 112,667,955 | EFNA5 | -/FBXL17 |
| MARC0085656 | DEN | 4 | 12,149,395 | - | -/MYC |
| ALGA0026338 | MOT, ABN, FSN | 4 | 85,073,958 | MGST3 | TMCO1/LRRC52 |
| MARC0066013 | CVVOL | 5 | 59,098,986 | - | GRIN2B/EMP1 |
| WU_10.2_5_89558832 | CVABN | 5 | 85,236,461 | APAF1 | ANKS1B/IKBIP |
| ASGA0094241 | CVDEN, CVMOT, CVABN, CVTSN, CVFSN | 5 | 88,898,348 | CEP83 | TMCC3/PLXNC1 |
| WU_10.2_6_25519992 | CVABN | 6 | 28,217,706 | CTCF | AGRP/CARMIL2 |
| ASGA0030861 | CVABN | 7 | 5,812,005 | - | SLC35B3/- |
| WU_10.2_7_113446920 | CVABN | 7 | 106,992,267 | - | -/- |
| MARC0029691 | CVABN, CVFSN | 7 | 107,017,794 | - | -/- |
| H3GA0024811 | MOT | 8 | 39,096,198 | - | DCUN1D4/SGCB |
| MARC0054558 | CVDEN, CVMOT, CVTSN | 8 | 84,708,131 | - | GAB1/- |
| ALGA0048589 | DEN | 8 | 88,060,364 | - | NOCT/- |
| ALGA0118936 | CVVOL | 9 | 1,823,736 | - | -/OVCH2 |
| WU_10.2_9_120884225 | TSN, FSN | 9 | 109,865,752 | - | CUL1/- |
| ALGA0055832 | CVVOL, CVDEN, CVMOT, CVABN, CVTSN, CVFSN | 9 | 133,977,561 | - | U6/- |
| H3GA0029253 | FSN | 10 | 11,009,092 | - | DUSP10/HHIPL2 |
| ASGA0101355 | MOT | 10 | 15,906,379 | - | PLD5/HHIPL2 |
| WU_10.2_10_65681460 | CVDEN, CVMOT, CVTSN, CVFSN | 10 | 59,980,174 | - | UPF2/PROSER2 |
| MARC0097207 | CVDEN, CVMOT, CVABN, CVTSN, CVFSN | 12 | 16,290,215 | - | TLK2/EFCAB3 |
| MARC0113309 | CVDEN, CVMOT, CVABN, CVTSN, CVFSN | 12 | 22,708,807 | - | PPP1R1B/NEUROD2 |
| ASGA0058857 | CVVOL, CVMOT | 13 | 132,659,741 | - | BDH1/DLG1 |
| ALGA0077301 | CVMOT, CVFSN | 14 | 44,439,785 | - | CRYBA4/- |
| ASGA0063383 | CVFSN | 14 | 50,930,578 | DGCR2 | ZNF74/TSSK1B |
| ASGA0063433 | CVVOL | 14 | 53,990,384 | RYR2 | ZP4/- |
| DRGA0013964 | CVDEN, CVMOT, CVTSN, CVFSN | 14 | 63,781,108 | - | U6/- |
| WU_10.2_14_135493445 | CVDEN, CVMOT, CVTSN | 14 | 124,371,607 | - | NHLRC2/ADRB1 |
| WU_10.2_16_238186 | CVMOT, CVABN, CVFSN | 16 | 514,821 | CTNND2 | DAP/U6 |
| WU_10.2_17_4199456 | CVABN, CVFSN | 17 | 3,947,416 | - | MSR1/- |
| WU_10.2_17_5093088 | CVDEN, CVTSN | 17 | 4,699,290 | FGF20 | U2/MICU3 |
| ALGA0110656 | CVDEN, CVABN, CVTSN, CVFSN | 17 | 4,872,377 | ZDHHC2 | MICU3/CNOT7 |
| WU_10.2_17_69108942 | CVVOL | 17 | 61,588,040 | SS18L1 | PSMA7/MTG2 |
| MARC0056921 | CVFSN | 18 | 6,400,611 | - | GIMAP2/GIMAP4 |
| WU_10.2_18_13174146 | CVABN | 18 | 12,468,287 | CHRM2 | Metazoa_SRP/ssc-mir-490-1 |
| WU_10.2_18_15516325 | CVVOL | 18 | 14,653,756 | - | BPGM/AKR1B1 |
| Affx-115137014 | CVABN | 18 | 26,996,757 | - | U2/- |
| ASGA0080065 | CVVOL, CVDEN, CVMOT, CVABN, CVTSN, CVFSN | 18 | 45,424,614 | HOXA5 | HOXA7/HOXA3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Z.; Li, K.; Yang, K.; Gao, G.; Li, Z.; Zhu, X.; Zhao, Y. Genome-Wide Association Study Reveals Novel Candidate Genes Influencing Semen Traits in Landrace Pigs. Animals 2024, 14, 1839. https://doi.org/10.3390/ani14131839
Zhuang Z, Li K, Yang K, Gao G, Li Z, Zhu X, Zhao Y. Genome-Wide Association Study Reveals Novel Candidate Genes Influencing Semen Traits in Landrace Pigs. Animals. 2024; 14(13):1839. https://doi.org/10.3390/ani14131839
Chicago/Turabian StyleZhuang, Zhanwei, Kebiao Li, Kai Yang, Guangxiong Gao, Zhili Li, Xiaoping Zhu, and Yunxiang Zhao. 2024. "Genome-Wide Association Study Reveals Novel Candidate Genes Influencing Semen Traits in Landrace Pigs" Animals 14, no. 13: 1839. https://doi.org/10.3390/ani14131839




