Infrared Thermography of the Blowhole as a Potential Diagnostic Tool for Health Assessment in Killer Whales (Orcinus orca)
Abstract
:Simple Summary
Abstract
1. Introduction
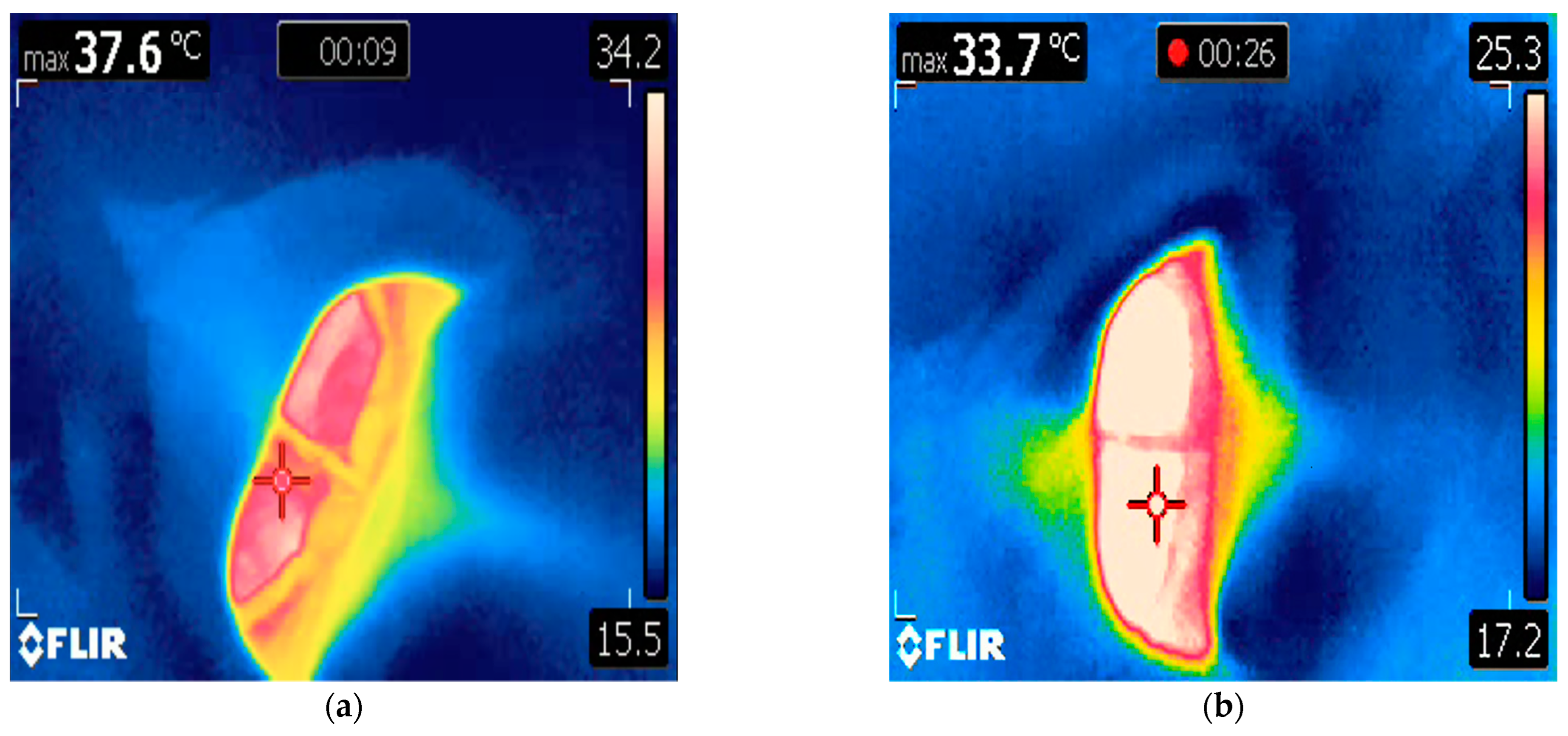
2. Case 1
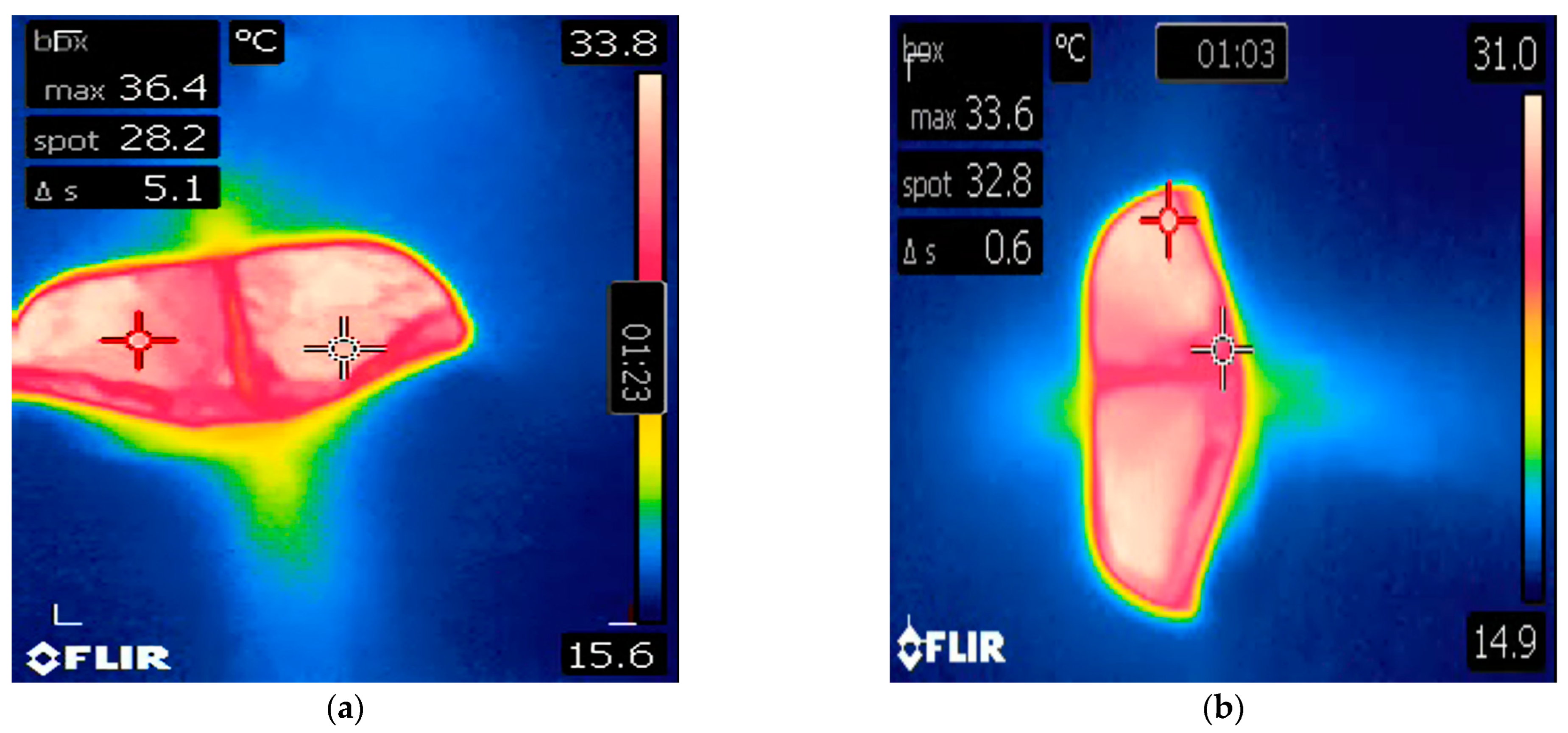
3. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, R.; Lacy, R.C.; Ashe, E.; Barrett-Lennard, L.; Brown, T.M.; Gaydos, J.K.; Gulland, F.; MacDuffee, M.; Nelson, B.W.; Nielsen, K.A.; et al. Warning sign of an accelerating decline in critically endangered killer whales (Orcinus orca). Commun. Earth Environ. 2024, 5, 173. [Google Scholar] [CrossRef]
- Andvik, C.; Jourdain, E.; Lyche, J.L.; Karoliussen, R.; Borgå, K. High Levels of Legacy and Emerging Contaminants in Killer Whales (Orcinus orca) from Norway, 2015 to 2017. Environ. Toxicol. Chem. 2021, 40, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.-P.; Hall, A.; McConnell, B.; Rosing-Asvid, A.; Barber, J.L.; Brownlow, A.; De Guise, S.; Eulaers, I.; Jepson, P.D.; Letcher, R.J.; et al. Predicting global killer whale population collapse from PCB pollution. Science 2018, 361, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, J.K.; St. Leger, J.; Raverty, S.; Nollens, H.H.; Haulena, M.; Ward, E.J.; Emmons, C.K.; Hanson, M.B.; Balcomb, K.C.; Ellifrit, D.; et al. Epidemiology of skin changes in endangered Southern Resident killer whales (Orcinus orca). PLoS ONE 2023, 18, e0286551. [Google Scholar] [CrossRef] [PubMed]
- Raverty, S.; St. Leger, J.; Noren, D.P.; Burek Huntington, K.; Rotstein, D.S.; Gulland, F.M.D.; Ford, J.K.B.; Hanson, M.B.; Lambourn, D.M.; Huggins, J.; et al. Pathology findings and correlation with body condition index in stranded killer whales (Orcinus orca) in the northeastern Pacific and Hawaii from 2004 to 2013. PLoS ONE 2020, 15, e0242505. [Google Scholar] [CrossRef]
- Hunt, K.E.; Moore, M.J.; Rolland, R.M.; Kellar, N.M.; Hall, A.J.; Kershaw, J.; Raverty, S.A.; Davis, C.E.; Yeates, L.C.; Fauquier, D.A.; et al. Overcoming the challenges of studying conservation physiology in large whales: A review of available methods. Conserv. Physiol. 2013, 1, cot006. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.D.; Emmons, C.K.; Wisswaesser, G.S.; Wells, A.H.; Hanson, M.B. Bacterial microbiomes from mucus and breath of southern resident killer whales (Orcinus orca). Conserv. Physiol. 2022, 10, coac014. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.; Dierauf, L.A. Stress and Marine Mammals. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F.M.D., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 153–168. [Google Scholar]
- Karaer, M.C.; Čebulj-Kadunc, N.; Snoj, T. Stress in wildlife: Comparison of the stress response among domestic, captive, and free-ranging animals. Front. Vet. Sci. 2023, 10, 1167016. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-J.; Zhao, Q.-Z.; Wu, H.-P.; Chen, D.-Q.; Gong, C.; Li, L.; Wang, D. Physiological responses to capture and handling of free-ranging male Yangtze finless porpoises (Neophocaena phocaenoides asiaeorientalis): Marine & Freshwater Behaviour & Physiology. Mar. Freshw. Behav. Physiol. 2009, 42, 315–327. [Google Scholar] [CrossRef]
- Herráez, P.; Sierra, E.; Arbelo, M.; Jaber, J.R.; de Los Monteros, A.E.; Fernández, A. Rhabdomyolysis and myoglobinuric nephrosis (capture myopathy) in a striped dolphin. J. Wildl. Dis. 2007, 43, 770–774. [Google Scholar] [CrossRef]
- Sierra, E.; Fernández, A.; Espinosa de Los Monteros, A.; Jaber, J.R.; Andrada, M.; Herráez, P. Complex polysaccharide inclusions in the skeletal muscle of stranded cetaceans. Vet. J. 2012, 193, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Roe, W.; Spraker, T.R. Capture-related myopathy in marine mammals and exertional rhabdomyolysis in horses: A possible link? Vet. J. 2012, 193, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Dold, C.; Ridgway, S. Cetaceans. In Zoo Animal and Wildlife Immobilization and Anesthesia; John Wiley & Sons, Ltd.: Oxford, UK, 2014; pp. 679–691. [Google Scholar] [CrossRef]
- McBain, J.F. Cetacean Medicine. In CRC Handbook of Marine Mammal Medicine, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 895–906. [Google Scholar]
- Sweeney, J.C.; Ridgway, S.H. Procedures for the clinical management of small cetaceans. J. Am. Vet. Med. Assoc. 1975, 167, 540–545. [Google Scholar] [PubMed]
- Russell, J.P.; St. Germain, M.; Osborn, S.D.; Schmitt, T.L.; Herrick, K.E.S.; Robeck, T. Comparison of infrared thermography of blowhole mucosa with rectal temperatures in killer whales (Orcinus orca). Front. Mar. Sci. 2024, 11, 1369287. [Google Scholar] [CrossRef]
- McCafferty, D.J. The value of infrared thermography for research on mammals: Previous applications and future directions. Mammal. Rev. 2007, 37, 207–223. [Google Scholar] [CrossRef]
- McCafferty, D.J.; Gallon, S.; Nord, A. Challenges of measuring body temperatures of free-ranging birds and mammals. Anim. Biotelemetry 2015, 3, 33. [Google Scholar] [CrossRef]
- Andrade, D.V. Thermal windows and heat exchange. Temperature 2015, 2, 451. [Google Scholar] [CrossRef]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef]
- Rekant, S.I.; Lyons, M.A.; Pacheco, J.M.; Arzt, J.; Rodriguez, L.L. Veterinary applications of infrared thermography. Am. J. Vet. Res. 2016, 77, 98–107. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Domínguez-Oliva, A.; Mora-Medina, P.; Casas-Alvarado, A.; Rios-Sandoval, J.; de Mira Geraldo, A.; Wang, D. Thermal Imaging to Assess the Health Status in Wildlife Animals under Human Care: Limitations and Perspectives. Animals 2022, 12, 3558. [Google Scholar] [CrossRef]
- Cuyler, L.C.; Wiulsrød, R.; ØRitsland, N.A. Thermal Infrared Radiation from Free Living Whales. Mar. Mammal. Sci. 1992, 8, 120–134. [Google Scholar] [CrossRef]
- Costidis, A.; Rommel, S.A. Vascularization of Air Sinuses and Fat Bodies in the Head of the Bottlenose Dolphin (Tursiops truncatus): Morphological Implications on Physiology. Front. Physiol. 2012, 3, 243. [Google Scholar] [CrossRef] [PubMed]
- Melero, M.; Rodríguez-Prieto, V.; Rubio-García, A.; García-Párraga, D.; Sánchez-Vizcaíno, J.M. Thermal reference points as an index for monitoring body temperature in marine mammals. BMC Res. Notes 2015, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Lonati, G.; Zitterbart, D.; Miller, C.; Corkeron, P.; Murphy, C.; Moore, M. Investigating the thermal physiology of Critically Endangered North Atlantic right whales Eubalaena glacialis via aerial infrared thermography. Endanger. Species Res. 2022, 48, 139–154. [Google Scholar] [CrossRef]
- Bossart, G. Marine Mammals as Sentinel Species for Oceans and Human Health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Barratclough, A.; Wells, R.S.; Schwacke, L.H.; Rowles, T.K.; Gomez, F.M.; Fauquier, D.A.; Sweeney, J.C.; Townsend, F.I.; Hansen, L.J.; Zolman, E.S.; et al. Health Assessments of Common Bottlenose Dolphins (Tursiops truncatus): Past, Present, and Potential Conservation Applications. Front. Vet. Sci. 2019, 6, 444. [Google Scholar] [CrossRef] [PubMed]
- Nollens, H.H.; Robeck, T.R.; Schmitt, T.L.; Croft, L.L.; Osborn, S.; McBain, J.F. Effect of age, sex, and season on the variation in blood analytes of a clinically normal ex situ population of killer whales (Orcinus orca). Vet. Clin. Pathol. 2019, 48, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Gulland, F.M.D.; Dierauf, L.; Whitman, K.L. Appendix 1: Normal Hematology and Serum Chemistry Ranges. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2018; p. 1008. [Google Scholar]
- Simeone, C.A.; Stoskopf, M.K. Pharmaceuticals and Formularies. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 607–673. [Google Scholar]
- Usamentiaga, R.; Venegas, P.; Guerediaga, J.; Vega, L.; Molleda, J.; Bulnes, F. Infrared Thermography for Temperature Measurement and Non-Destructive Testing. Sensors 2014, 14, 12305–12348. [Google Scholar] [CrossRef] [PubMed]
- Black, V.L.; Whitworth, F.J.S.; Adamantos, S. Pyrexia in juvenile dogs: A review of 140 referred cases. J. Small Anim. Pract. 2019, 60, 116–120. [Google Scholar] [CrossRef]
- Balli, S.; Shumway, K.R.; Sharan, S. Physiology, Fever. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chow, A.W. Complications, Diagnosis, and Treatment of Odontogenic Infections. UpToDate 2024. Available online: https://www.uptodate.com/contents/complications-diagnosis-and-treatment-of-odontogenic-infections (accessed on 6 June 2024).
- Flood, J. The Diagnostic Approach to Fever of Unknown Origin in Dogs. In Compendium: Continuing Education for Veterinarians; CompendiumVet.com: Irvine, CA, USA, 2009; pp. 14–21. [Google Scholar]
- Burke, C.; Rashman, M.; Wich, S.; Symons, A.; Theron, C.; Longmore, S. Optimizing observing strategies for monitoring animals using drone-mounted thermal infrared cameras. Int. J. Remote Sens. 2019, 40, 439–467. [Google Scholar] [CrossRef]
- Horton, T.W.; Hauser, N.; Cassel, S.; Klaus, K.F.; Fettermann, T.; Key, N. Doctor Drone: Non-invasive Measurement of Humpback Whale Vital Signs Using Unoccupied Aerial System Infrared Thermography. Front. Mar. Sci. 2019, 6, 466. [Google Scholar] [CrossRef]
- Pirotta, V.; Smith, A.; Ostrowski, M.; Russell, D.; Jonsen, I.D.; Grech, A.; Harcourt, R. An Economical Custom-Built Drone for Assessing Whale Health. Front. Mar. Sci. 2017, 4, 425. [Google Scholar] [CrossRef]
- Dixon, O.F.L.; Gallagher, A.J.; Towner, A.V. Novel aerial observations of a group of killer whales Orcinus orca in The Bahamas. Front. Mar. Sci. 2023, 10, 1265064. [Google Scholar] [CrossRef]
- Cilulko, J.; Janiszewski, P.; Bogdaszewski, M.; Szczygielska, E. Infrared thermal imaging in studies of wild animals. Eur. J. Wildl. Res. 2013, 59, 17–23. [Google Scholar] [CrossRef]
- Pabst, D.A.; Rommel, S.A.; McLellan, W.A.; Williams, T.M.; Rowles, T.K. Thermoregulation of the Intra-Abdominal Testes of the Bottlenose Dolphin (Tursiops Truncatus) During Exercise. J. Exp. Biol. 1995, 198, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, E.; Jaroenporn, S.; Katsumata, H.; Konno, S.; Maeda, Y.; Watanabe, G.; Taya, K. Body Temperature and Circulating Progesterone Levels before and after Parturition in Killer Whales (Orcinus orca). J. Reprod. Dev. 2006, 52, 65–71. [Google Scholar] [CrossRef]
- Tersawa, F.; Yokoyama, Y.; Kitamura, M. Rectal temperatures before and after parturition in bottlenose dolphins. Zoo. Biol. 1999, 18, 153–156. [Google Scholar] [CrossRef]
- Shore, R. Ailing Orca Successfully Dosed with Antibiotics in Emergency Bid to Save Her Life. The Vancouver Sun. Available online: https://vancouversun.com/news/local-news/international-team-gives-medication-to-sick-killer-whale-at-sea (accessed on 10 August 2018).
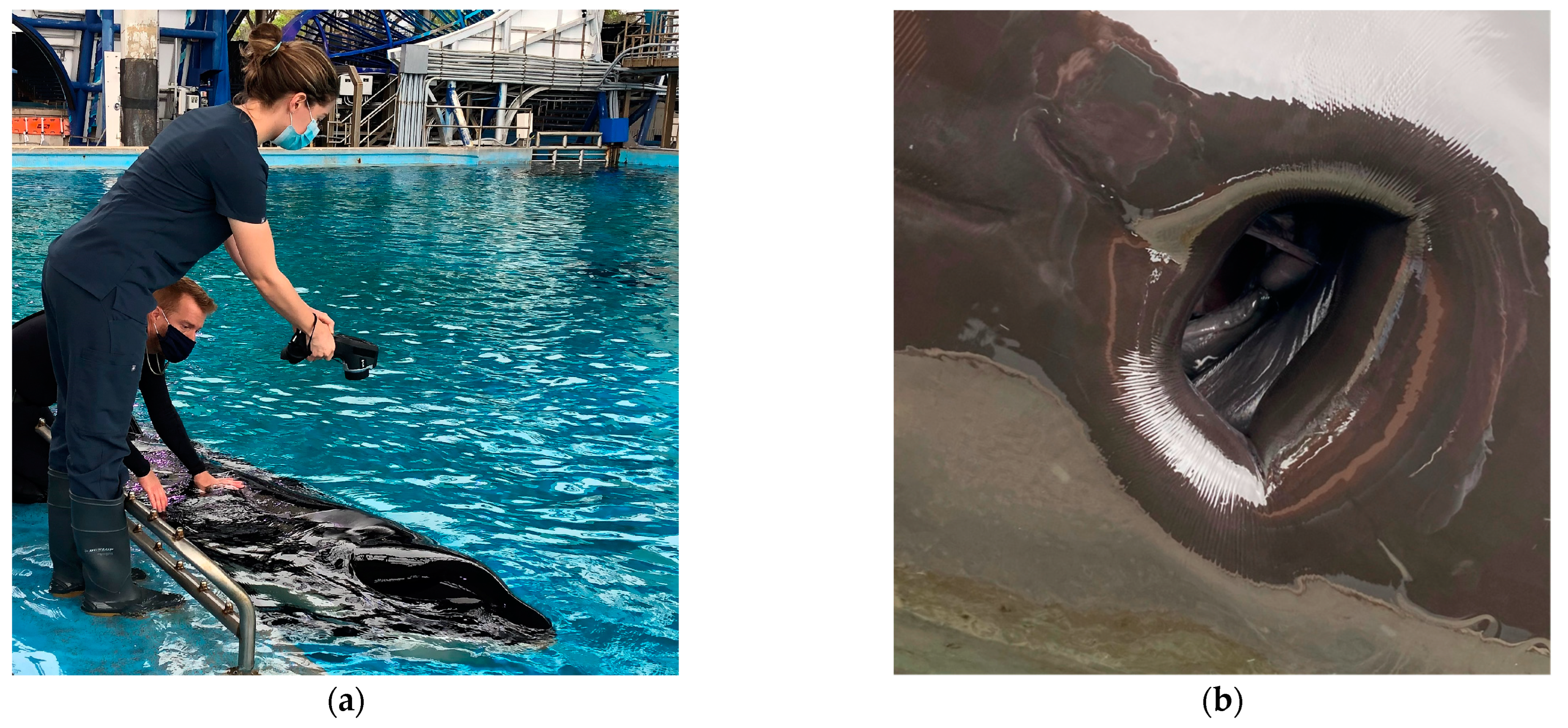
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, J.P.; Osborn, S.D.; Herrick, K.E.S.; Schmitt, T.L.; Robeck, T. Infrared Thermography of the Blowhole as a Potential Diagnostic Tool for Health Assessment in Killer Whales (Orcinus orca). Animals 2024, 14, 1867. https://doi.org/10.3390/ani14131867
Russell JP, Osborn SD, Herrick KES, Schmitt TL, Robeck T. Infrared Thermography of the Blowhole as a Potential Diagnostic Tool for Health Assessment in Killer Whales (Orcinus orca). Animals. 2024; 14(13):1867. https://doi.org/10.3390/ani14131867
Chicago/Turabian StyleRussell, Jennifer P., Steve D. Osborn, Kelsey E. S. Herrick, Todd L. Schmitt, and Todd Robeck. 2024. "Infrared Thermography of the Blowhole as a Potential Diagnostic Tool for Health Assessment in Killer Whales (Orcinus orca)" Animals 14, no. 13: 1867. https://doi.org/10.3390/ani14131867





