Bacterial Contamination of Environmental Surfaces of Veterinary Rehabilitation Clinics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stull, J.W.; Weese, J.S. Hospital-Associated Infections in small animal practice. Vet. Clin. N. Am. Small Anim. 2015, 45, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Brigando, G.; Sutton, C.; Uebelhor, O.; Pitsoulakis, N.; Pytynia, M.; Dillon, T.; Elliott-Burke, T.; Hubert, N.; Martinez-Guryn, K.; Bolch, C.; et al. The microbiome of an outpatient rehabilitation clinic and predictors of contamination: A pilot study. PLoS ONE 2023, 18, e0281299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kirkby, S.K.; Alvarez, L.; Foster, S.A.; Tomlinson, J.E.; Shaw, A.J.; Pozzi, A. Fundamental principles of rehabilitation and musculoskeletal tissue healing. Vet. Surg. 2020, 49, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.; Millis, N.; Jones, R.; Johnson, B.; Kania, S.; Odoi, A. Patterns of antimicrobial, multidrug and methicillin resistance among Staphylococcus spp. isolated from canine specimens submitted to a diagnostic laboratory in Tennessee, USA: A descriptive study. BMC Vet. Res. 2022, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Spratt, H., Jr.; Levine, D.; McDonald, S.; Drake, S.; Duke, K.; Kluttz, C.; Noonan, K. Survival of Staphylococcus aureus on therapeutic ultrasound heads. Am. J. Infect. Control 2019, 47, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, S.; Blanco, J.; Harmanus, C.; Kuijper, E.; García, M. Data from a survey of Clostridium perfringens and Clostridium difficile shedding by dogs and cats in the Madrid region (Spain), including phenotypic and genetic characteristics of recovered isolates. Data Brief 2017, 14, 88–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanks, J.; Levine, D.; Bockstahler, B. Physical agent modalities in physical therapy and rehabilitation of small animals. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Dycus, D.L.; Levine, D.; Ratsch, B.E.; Marcellin-Little, D.J. Physical Rehabilitation for the Management of Canine Hip Dysplasia: 2021 Update. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 719–747. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Knight, D.; Riley, T. Clostridium difficile and One Health. Clin. Microbiol. Infect. 2019, 26, 857–863. [Google Scholar] [CrossRef]
- Alalawi, M.; Aljahdali, S.; Alharbi, B.; Fagih, L.; Fatani, R.; Aljuhani, O. Clostridium difficile infection in an academic medical center in Saudi Arabia: Prevalence and risk factors. Ann. Saudi Med. 2020, 40, 305–309. [Google Scholar] [CrossRef]
- Orden, C.; Blanco, J.; Álvarez-Pérez, S.; Garcia, M.; Blanco, J.; Garcia-Sancho, M.; Rodriguez-Franco, F.; Sainz, A.; Villaescusa, A.; Harmanus, C.; et al. Isolation of Clostridium difficile from dogs with digestive disorders, including stable metronidazole-resistant strains. Anaerobe 2017, 43, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Rabold, D.; Espelage, W.; Sin, M.; Eckmanns, T.; Schneeberg, A.; Neubauer, H.; Mobius, N.; Hille, K.; Wieler, L.; Seyboldt, C.; et al. The zoonotic potential of Clostridium difficile from small companion animals and their owners. PLoS ONE 2018, 13, e0193411. [Google Scholar] [CrossRef] [PubMed]
- Weese, J. Methicllin-Resistant Staphylococcus aureus in Animals. ILAR J. 2010, 51, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Lautenbach, E.; Zaoutis, T.; Leckerman, K.; Edelstein, P.; Rankin, S. Potential for pet animals to harbour methicillin-resistant Staphylococcus aureus when residing with human MRSA patients. Zoonoses Public Health 2012, 59, 286–293. [Google Scholar] [CrossRef]
- Worthing, K.; Brown, J.; Gerber, L.; Trott, D.; Abraham, S.; Norris, J. Methicillin-resistant staphylococci amongst veterinary personnel, personnel-owned pets, patients and the hospital environment of two small animal veterinary hospitals. Vet. Microbiol. 2018, 223, 79–85. [Google Scholar] [CrossRef]
- Tong, S.; Davis, J.; Eichenberger, E.; Holland, T.; Fowler, V. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Gómez-Sanz, E.; Torres, C.; Lozano, C.; Zarazaga, M. High diversity of Staphylococcus aureus and Staphylococcus pseudintermedius lineages and toxigenic traits in healthy pet-owning household members. Underestimating normal household contact? Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; de Oliveira Júnior, C.; Blanc, D.; Pereira, S.; de Araujo, M.; Vasconcelos, A.; Lobato, F. Clostridioides difficile infection in dogs with chronic-recurring diarrhea responsive to dietary changes. Anaerobe 2018, 51, 50–53. [Google Scholar] [CrossRef]
- Tomo, Y.; Sobashima, E.; Eto, H.; Yamazaki, A.; Tanegashima, K.; Edamura, K. Treatment of methicillin-resistant Staphylococcus aureus infection following tibial plateau leveling osteotomy in a dog. Open Vet. J. 2021, 11, 728–733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mork, R.; Hogan, P.; Muenks, C.; Boyle, M.; Thompson, R.; Sullivan, M.; Morelli, J.; Seigel, J.; Orschein, R.; Wardenburg, J.; et al. Longitudinal, strain-specific Staphylococcus aureus introduction and transmission events in households of children with community-associated meticillin-resistant S. aureus skin and soft tissue infection: A prospective cohort study. Lancet Infect. Dis. 2020, 20, 188–198. [Google Scholar] [CrossRef]
- Weese, J. Bacterial enteritis in dogs and cats: Diagnosis, therapy, and zoonotic potential. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, E.; Kurt, T.; Hyatt, D.; Lappin, M.; Ruch-Gallie, R. Prevalence of methicillin-resistant staphylococci in northern Colorado shelter animals. J. Vet. Diagn. Investig. 2011, 23, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Nienhoff, U.; Kadlec, K.; Chaberny, I.; Verspohl, J.; Gerlach, G.-F.; Kreienbrock, L.; Schwarz, S.; Simon, D.; Nolte, I. Methicillin-resistant Staphylococcus pseudintermedius among dogs admitted to a small animal hospital. Vet. Microbiol. 2011, 150, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zur, G.; Gurevich, B.; Elad, D. Prior antimicrobial use as a risk factor for resistance in selected Staphylococcus pseudintermedius isolates from the skin and ears of dogs. Vet. Dermatol. 2016, 27, 468-e125. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, R.; Rubin, J.; Priyantha, M.; Church, D. Exploring Staphylococcus pseudintermedius: An emerging zoonotic pathogen? Future Microbiol. 2016, 11, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.; Loeffler, A. What’s happened to Staphylococcus intermedius? Taxonomic revision and emergence of multi-drug resistance. J. Small Anim. Pract. 2012, 53, 147–154. [Google Scholar] [CrossRef]
- Sasaki, T.; Kikuchi, K.; Tanaka, Y.; Takahashi, N.; Kamata, S.; Hiramatsu, K. Methicillin-resistant Staphylococcus pseudintermedius in a veterinary teaching hospital. J. Clin. Microbiol. 2007, 45, 1118–1125. [Google Scholar] [CrossRef]
- Bannoehr, J.; Franco, A.; Iurescia, M.; Battisti, A.; Fitzgerald, J. Molecular diagnostic identification of Staphylococcus pseudintermedius. J. Clin. Microbiol. 2009, 47, 469–471. [Google Scholar] [CrossRef]
- Bannoehr, J.; Guardabassi, L. Staphylococcus pseudintermedius in the dog: Taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet. Dermatol. 2012, 23, 253-e52. [Google Scholar] [CrossRef]
- Abouelkhair, M.; Frank, L.; Bemis, D.; Giannone, R.; Kania, S. Staphylococcus pseudintermedius 5′-nucleotidase suppresses canine phagocytic activity. Vet. Microbiol. 2020, 246, 108720. [Google Scholar] [CrossRef]
- Iverson, S.; Brazil, A.; Ferguson, J.; Nelson, K.; Lautenbach, E.; Rankin, S.; Morris, D.; Davis, M. Anatomical patterns of colonization of pets with staphylococcal species in homes of people with methicillin-resistant Staphylococcus aureus (MRSA) skin or soft tissue infection (SSTI). Vet. Microbiol. 2015, 176, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kmieciak, W.; Szewczyk, E. Are zoonotic Staphylococcus pseudintermedius strains a growing threat for humans? Folia Microbiol. 2018, 63, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Keilman, R.; Harding, S.; Rowin, M.; Reade, E.; Klingborg, P.; Levine, D.; Spratt, H., Jr. Investigations of Staphylococcal contamination on environmental surfaces of a neonatal intensive care unit of a children’s hospital. Am. J. Infect. Control 2021, 49, 1450–1453. [Google Scholar] [CrossRef] [PubMed]
- Flayhart, D.; Hindler, J.; Bruckner, D.; Hall, G.; Shrestha, R.; Vogel, S.; Richter, S.; Howard, W.; Walther, R.; Carroll, K. Multicenter evaluation of BBL CHROMagar MRSA medium for direct detection of methicillin-resistant Staphylococcus aureus from surveillance cultures of the anterior nares. J. Clin. Microbiol. 2005, 43, 5536–5540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelesidis, T.; Tsiodras, S. Staphylococcus intermedius is not only a zoonotic pathogen, but may also cause skin abscesses in humans after exposure to saliva. Int. J. Infect. Dis. 2010, 14, e838–e841. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, S.; Blanco, J.; Harmanus, C.; Kuijper, E.; García, M. Prevalence and characteristics of Clostridium perfringens and Clostridium difficile in dogs and cats attended in diverse veterinary clinics from the Madrid region. Anaerobe 2017, 48, 47–55. [Google Scholar] [CrossRef]
- Bonadonna, L.; Briancesco, R.; Coccia, A.; Meloni, P.; Rosa, G.; Moscato, U. Microbial Air Quality in Healthcare Facilities. Int. J. Environ. Res. Public Health 2021, 18, 6226. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Reid-Smith, R.; Boerlin, P.; Weese, J.; Prescott, J.; Janecko, N.; Hassard, L.; McEwen, S. Escherichia coli and selected veterinary and zoonotic pathogens isolated from environmental sites in companion animal veterinary hospitals in southern Ontario. Can. Vet. J. 2010, 51, 963–972. [Google Scholar] [PubMed] [PubMed Central]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huynh, T.Q.; Tran, V.N.; Thai, V.C.; Nguyen, H.A.; Nguyen, N.T.G.; Tran, M.K.; Nguyen, T.P.T.; Le, C.A.; Ho, L.T.N.; Surian, N.U.; et al. Genomic alterations involved in fluoroquinolone resistance development in Staphylococcus aureus. PLoS ONE 2023, 18, e0287973. [Google Scholar] [CrossRef]
- Souza-Silva, T.; Rossi, C.; Andrade-Oliveira, A.; Vilar, L.; Pereira, M.; de Araujo Penna, B.; Giambiagi-deMarval, M. Interspecies transfer of plasmid-borne gentamicin resistance between Staphylococcus isolated from Staphylococcus aureus. Infect. Genet. Evol. 2022, 98, 105230. [Google Scholar] [CrossRef] [PubMed]
- Lax, S.; Sangwan, N.; Smith, D.; Larsen, P.; Handley, K.; Richardson, M.; Guyton, K.; Krezalek, M.; Shogan, B.; Defazio, J.; et al. Bacterial colonization and succession in a newly opened hospital. Sci. Transl. Med. 2017, 9, eaah6500. [Google Scholar] [CrossRef] [PubMed]
- Beckton, Dickinson Co. Quality Control Procedures. BBL Mannitol Salt Agar, L997389, Rev. 9 June 2017. Available online: https://static.bd.com/documents/eifu/L007389_EN.pdf (accessed on 15 June 2024).
- Lu, Z.; Guo, W.; Liu, C. Isolation, identification and characterization of novel Bacillus subtilis. J. Vet. Med. Sci. 2018, 80, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Spratt, H., Jr.; Levine, D.; Tillman, L. Physical therapy clinic therapeutic ultrasound equipment as a source for bacterial contamination. Physiother. Theory Pract. 2014, 30, 507–511. [Google Scholar] [CrossRef] [PubMed]
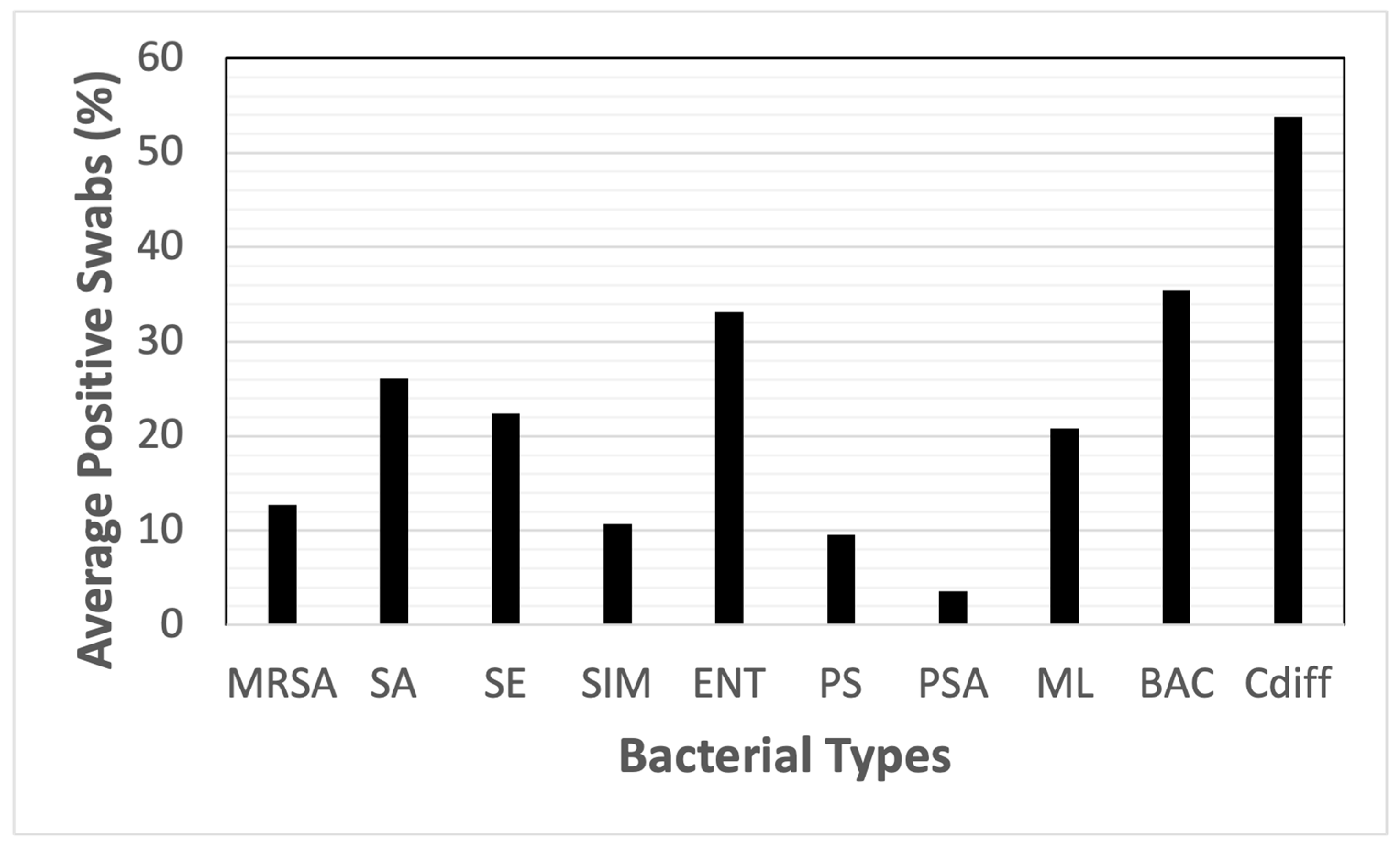
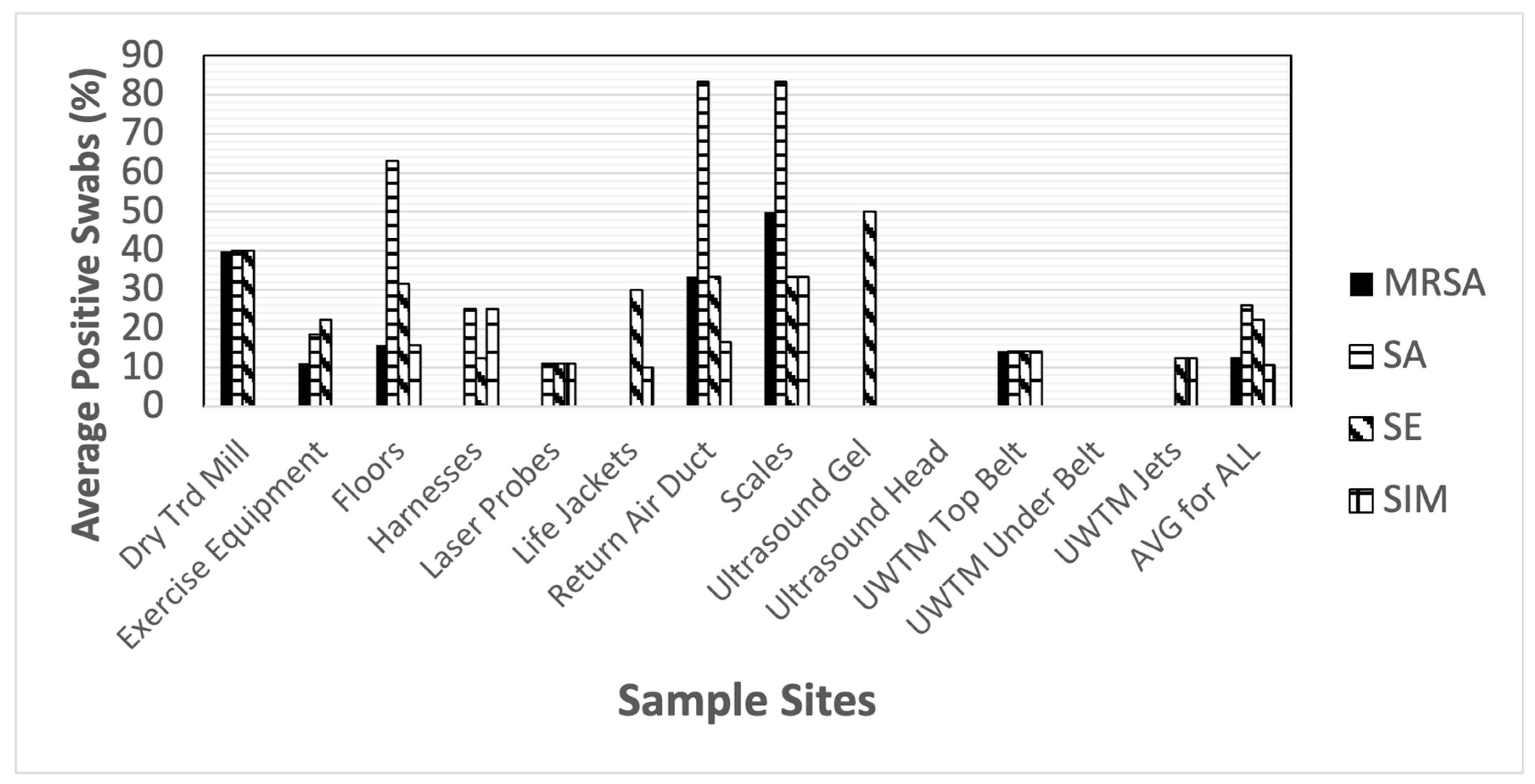
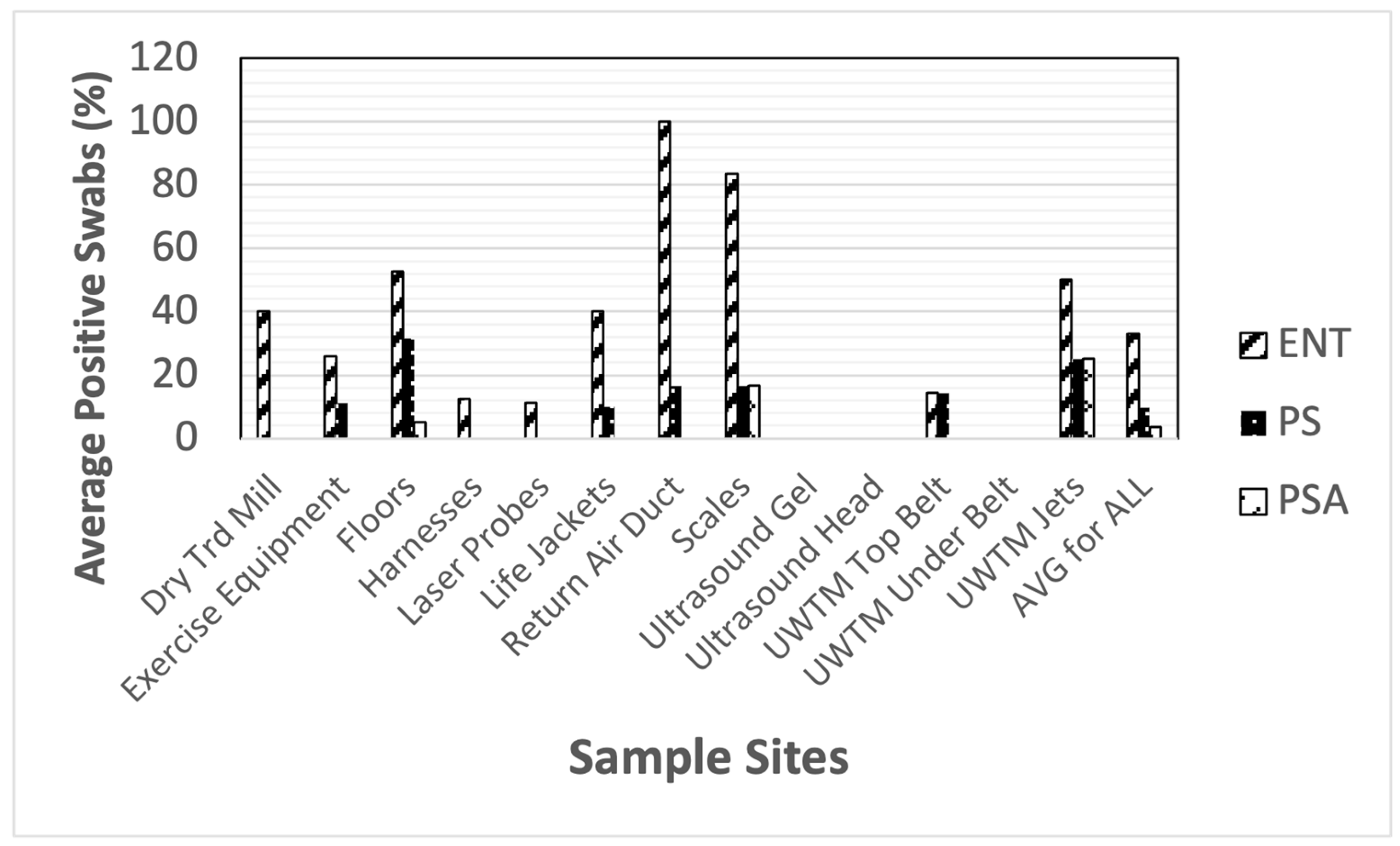


| MRSA | SA | SE | SIM | ENT | PS | PSA | ML | BAC | Cdiff | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dry Treadmill (belt, n = 5) | 40% | 40% | 40% | 0% | 40% | 0% | 0% | 100% | 60% | 80% |
| Exercise Equipment (n = 27) | 11.1% | 18.5% | 22.2% | 0% | 25.9% | 11.1% | 0% | 22.2% | 44.4% | 74.1% |
| Floors (n = 19) | 15.8% | 52.6% | 31.6% | 15.8% | 52.6% | 31.6% | 5.3% | 21.1% | 63.2% | 94.7% |
| Harnesses (n = 8) | 0% | 25% | 12.5% | 25% | 12.5% | 0% | 0% | 12.5% | 12.5% | 75% |
| Laser Probes (tip of probe, n = 9) | 0% | 11.1% | 11.1% | 11.1% | 11.1% | 0% | 0% | 0% | 0% | 33.3% |
| Life Jackets (n = 10) | 0% | 0% | 30% | 10% | 40% | 10% | 0% | 40% | 10% | 60% |
| Return Air Ducts (n = 6) | 15.8% | 83.3% | 33.3% | 16.7% | 100% | 16.7% | 0% | 16.7% | 100% | 16.7% |
| Scales (n = 6) | 50% | 83.3% | 33.3% | 33.3% | 83.3% | 16.7% | 16.7% | 16.7% | 100% | 66.7% |
| Ultrasound Gel (bottle tip, n = 4) | 0% | 0% | 50% | 0% | 0% | 0% | 0% | 0% | 0% | 25% |
| Ultrasound Heads (n = 6) | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 16.7% | 16.7% |
| UWTM Top Belt (n = 7) | 14.3% | 14.3% | 14.3% | 14.3% | 14.3% | 14.3% | 0% | 28.6% | 28.6% | 28.6% |
| UWTM Bottom Surface of Belt (n = 7) | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 28.6% |
| UWTM Jets (inside surface) (n = 8) | 0% | 0% | 12.5% | 12.5% | 50% | 25% | 25% | 12.5% | 25% | 12.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spratt, H.G.; Millis, N.; Levine, D.; Brackett, J.; Millis, D. Bacterial Contamination of Environmental Surfaces of Veterinary Rehabilitation Clinics. Animals 2024, 14, 1896. https://doi.org/10.3390/ani14131896
Spratt HG, Millis N, Levine D, Brackett J, Millis D. Bacterial Contamination of Environmental Surfaces of Veterinary Rehabilitation Clinics. Animals. 2024; 14(13):1896. https://doi.org/10.3390/ani14131896
Chicago/Turabian StyleSpratt, Henry G., Nicholas Millis, David Levine, Jenna Brackett, and Darryl Millis. 2024. "Bacterial Contamination of Environmental Surfaces of Veterinary Rehabilitation Clinics" Animals 14, no. 13: 1896. https://doi.org/10.3390/ani14131896





