Effects of Human Harvesting, Residences, and Forage Abundance on Deer Spatial Distribution
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Deer Spatial Distribution
2.3. Environmental Variables
2.4. Statistical Analyses
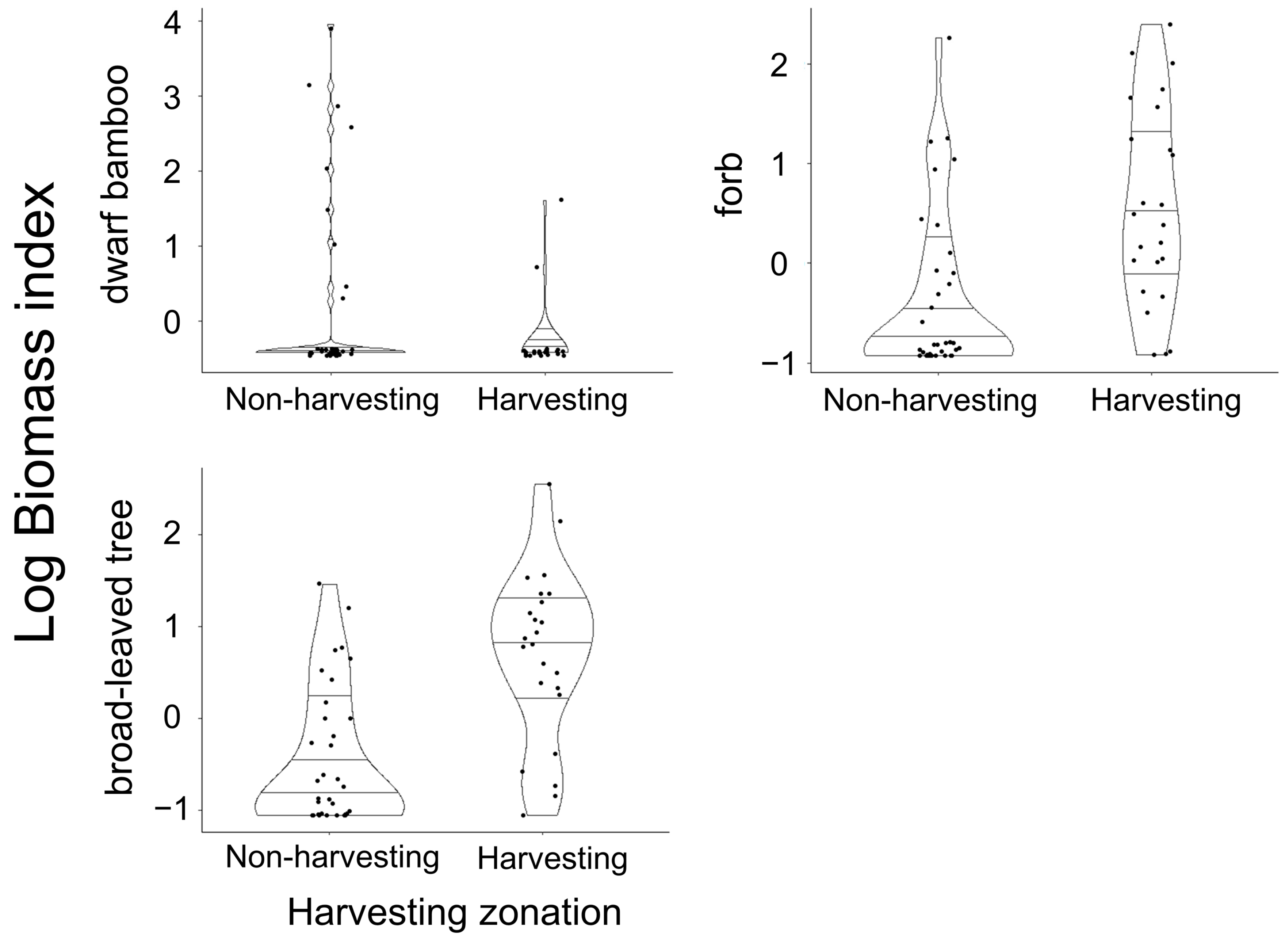
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mech, L.D. Wolf-pack buffer zones as prey reservoirs. Science 1977, 198, 320–321. [Google Scholar] [CrossRef]
- Edwards, J. Diet shifts in moose due to predator avoidance. Oecologia 1983, 60, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Altendorf, K.B.; Laundré, J.W.; López González, C.A.; Brown, J.S. Assessing effects of predation risk on foraging behavior of mule deer. J. Mammal. 2001, 82, 430–439. [Google Scholar] [CrossRef]
- Darlington, S.; Ladle, A.; Burton, A.C.; Volpe, J.P.; Fisher, J.T. Cumulative effects of human footprint, natural features and predation risk best predict seasonal resource selection by white-tailed deer. Sci. Rep. 2022, 12, 1072. [Google Scholar] [CrossRef] [PubMed]
- Edge, A.C.; Rosenberger, J.P.; Yates, C.J.; Little, A.R.; Killmaster, C.H.; Johannsen, K.L.; D’Angelo, G.J. White-tailed deer (Odocoileus virginianus) fawn survival and the influence of landscape characteristics on fawn predation risk in the Southern Appalachian Mountains, USA. PLoS ONE 2023, 18, e0288449. [Google Scholar] [CrossRef] [PubMed]
- Laundré, J.W.; Hernández, L.; Ripple, W.J. The landscape of fear: Ecological implications of being afraid. Open Ecol. J. 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Palmer, M.S.; Gaynor, K.M.; Becker, J.A.; Abraham, J.O.; Mumma, M.A.; Pringle, R.M. Dynamic landscapes of fear: Understanding spatiotemporal risk. Trends Ecol. Evol. 2022, 34, 911–925. [Google Scholar] [CrossRef]
- Hernández, L.; Laundré, J.W. Foraging in the ‘landscape of fear’ and its implications for habitat use and diet quality of elk Cervus elaphus and bison Bison bison. Wildl. Biol. 2005, 11, 215–220. [Google Scholar] [CrossRef]
- Tolon, V.; Dray, S.; Loison, A.; Zeileis, A.; Fischer, C.; Baubet, E. Responding to spatial and temporal variations in predation risk: Space use of a game species in a changing landscape of fear. Can. J. Zool. 2009, 87, 1129–1137. [Google Scholar] [CrossRef]
- Mao, J.S.; Boyce, M.S.; Smith, D.W.; Singer, F.J.; Vales, D.J.; Vore, J.M.; Merrill, E.H. Habitat selection by elk before and after wolf reintroduction in Yellowstone National Park. J. Wildl. Manag. 2005, 69, 1691–1707. [Google Scholar] [CrossRef]
- Hebblewhite, M.; Merrill, E.H. Trade-offs between predation risk and forage differ between migrant strategies in a migratory ungulate. Ecology 2009, 90, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, K.M.; Brown, J.S.; Middleton, A.D.; Power, M.E.; Brashares, J.S. Landscapes of fear: Spatial patterns of risk perception and response. Trends Ecolo. Evol. 2019, 34, 355–368. [Google Scholar] [CrossRef]
- Karns, G.R.; Gates, R.J.; Matthews, S.N.; Bruskotter, J.T.; McCoy, J.C.; Tonkovich, M.J. Factors influencing spatial heterogeneity of female white-tailed deer harvest dynamics. Wildl. Soc. Bull. 2016, 40, 758–763. [Google Scholar] [CrossRef]
- Latham, A.D.M.; Cecilia Latham, M.; Herries, D.; Barron, M.; Cruz, J.; Anderson, D.P. Assessing the efficacy of aerial culling of introduced wild deer in New Zealand with analytical decomposition of predation risk. Biol. Invasions 2018, 20, 251–266. [Google Scholar] [CrossRef]
- Henderson, C.J.; Gilby, B.L.; Schlacher, T.A.; Connolly, R.M.; Sheaves, M.; Maxwell, P.S.; Flint, N.; Borland, H.P.; Martin, T.S.H.; Gorissen, B.; et al. Landscape transformation alters functional diversity in coastal seascapes. Ecography 2020, 43, 138–148. [Google Scholar] [CrossRef]
- Bonnot, N.; Morellet, N.; Verheyden, H.; Cargnelutti, B.; Lourtet, B.; Klein, F.; Hewison, A.M. Habitat use under predation risk: Hunting, roads and human dwellings influence the spatial behaviour of roe deer. Eur. J. Wildl. Res. 2013, 59, 185–193. [Google Scholar] [CrossRef]
- Lone, K.; Loe, L.E.; Meisingset, E.L.; Stamnes, I.; Mysterud, A. An adaptive behavioural response to hunting: Surviving male red deer shift habitat at the onset of the hunting season. Anim. Behav. 2015, 102, 127–138. [Google Scholar] [CrossRef]
- Oka, T.; Iijima, H.; Kamata, A.; Ishida, A.; Eguchi, N.; Aikawa, T.; Takahashi, H.; Kondoh, H.; Yayota, C.; Hayamawa, M.; et al. The process of population expansion of sika deer. In Sika Deer: Life History Plasticity and Management; Kaji, K., Uno, H., Iijima, H., Eds.; Springer: Berlin, German, 2022; pp. 11–23. [Google Scholar]
- Takatsuki, S. Effects of sika deer on vegetation in Japan: A review. Biol. Conserv. 2009, 142, 1922–1929. [Google Scholar] [CrossRef]
- Ohashi, H. The impact of sika deer on vegetation in Japan. In Sika Deer: Life History Plasticity and Management; Kaji, K., Uno, H., Iijima, H., Eds.; Springer: Berlin, German, 2022; pp. 25–44. [Google Scholar]
- Nagaike, T.; Hayashi, A. Bark-stripping by sika deer (Cervus nippon) in Larix kaempferi plantations in central Japan. For. Ecol. Manag. 2003, 175, 563–572. [Google Scholar] [CrossRef]
- Nagaike, T. Effects of browsing by sika deer (Cervus nippon) on subalpine vegetation at Mt. Kita, central Japan. Ecol. Res. 2012, 27, 467–473. [Google Scholar] [CrossRef]
- Nagaike, T. Bark stripping by deer was more intensive on new recruits than on advanced regenerants in a subalpine forest. Forests 2020, 11, 490. [Google Scholar] [CrossRef]
- Nagaike, T. Effects of heavy, repeated bark stripping by Cervus nippon on survival of Abies veitchii in a subalpine coniferous forest in central Japan. J. For. Res. 2020, 31, 1139–1145. [Google Scholar] [CrossRef]
- Kaji, K.; Uno, H.; Iijima, H. Sika Deer: Life History Plasticity and Management; Springer: Berlin, German, 2022. [Google Scholar]
- Kaji, K.; Miyaki, M.; Saitoh, T.; Ono, S.; Kaneko, M. Spatial distribution of an expanding sika deer population on Hokkaido Island, Japan. Wildl. Soc. Bull. 2000, 28, 699–707. [Google Scholar] [CrossRef]
- Seki, Y.; Hayama, S.I. Habitat selection and activity patterns of Japanese serows and sika deer with currently sympatric distributions. Animals 2021, 11, 3398. [Google Scholar] [CrossRef] [PubMed]
- Kamei, T.; Takeda, K.I.; Izumiyama, S.; Ohshima, K. The effect of hunting on the behavior and habitat utilization of sika deer (Cervus nippon). Mammal Study 2010, 35, 235–241. [Google Scholar] [CrossRef]
- Laneng, L.A.; Tachiki, Y.; Akamatsu, R.; Kobayashi, K.; Takahata, C.; Nakamura, F. Seasonal home range and habitat selection patterns of sika deer Cervus nippon in southern Hokkaido, Japan. Wildl. Biol. 2023, 2023, e01060. [Google Scholar] [CrossRef]
- Borkowski, J. Influence of the density of a sika deer population on activity, habitat use, and group size. Can. J. Zool. 2000, 78, 1369–1374. [Google Scholar] [CrossRef]
- Borkowski, J.; Furubayashi, K. Home range size and habitat use in radio-collared female sika deer at high altitudes in the Tanzawa Mountains, Japan. Ann. Zool. Fenn. 1998, 35, 181–186. [Google Scholar]
- Borkowski, J.; Furubayashi, K. Seasonal changes in number and habitat use of foraging sika deer at the high altitude of Tanzawa Mountains, Japan. Acta Theriol. 1998, 43, 95–106. [Google Scholar] [CrossRef]
- Ito, T.Y.; Takatsuki, S. Home range, habitat selection, and food habits of the sika deer using the short-grass community on Kinkazan Island, northern Japan. In Sika Deer: Biology and Management of Native and Introduced Populations; McCullough, D.R., Takatsuki, S., Kaji, K., Eds.; Springer: Berlin, German, 2009; pp. 159–170. [Google Scholar]
- Takii, A.; Ozeki, M.; Takahata, C.; Izumiyama, S. Habitat selection of large herbivores evidenced as threats to alpine ecosystem. Acta Oecologica 2022, 114, 103812. [Google Scholar] [CrossRef]
- Takatsuki, S. Edge effects created by clear-cutting on habitat use by sika deer on Mt. Goyo, northern Honshu, Japan. Ecol. Res. 1989, 4, 287–295. [Google Scholar] [CrossRef]
- Takatsuki, S. A case study on the effects of a transmission-line corridor on Sika deer habitat use at the foothills of Mt Goyo, northern Honshu, Japan. Ecol. Res. 1992, 7, 141–146. [Google Scholar] [CrossRef]
- Takada, H.; Nakamura, K. Overlap in habitat use and activity patterns between sika deer (Cervus nippon) and Japanese serows (Capricornis crispus) in subalpine habitats: Exploitative competition rather than direct interference? Can. J. Zool. 2023, 101, 980–990. [Google Scholar] [CrossRef]
- Soga, A.; Hamasaki, S.I.; Yokoyama, N.; Sakai, T.; Kaji, K. Relationship between spatial distribution of sika deer–train collisions and sika deer movement in Japan. Hum. Wildl. Interact. 2015, 9, 198–210. [Google Scholar] [CrossRef]
- Takada, H.; Ohuchi, R.; Watanabe, H.; Yano, R.; Miyaoka, R.; Nakagawa, T.; Zenno, Y.; Minami, M. Habitat use and the coexistence of the sika deer and the Japanese serow, sympatric ungulates from Mt. Asama, central Japan. Mammalia 2020, 84, 503–511. [Google Scholar] [CrossRef]
- Sakuragi, M.; Igota, H.; Uno, H.; Kaji, K.; Kaneko, M.; Akamatsu, R.; Maekawa, K. Seasonal habitat selection of an expanding sika deer Cervus nippon population in eastern Hokkaido, Japan. Wildl. Biol. 2003, 9, 141–153. [Google Scholar] [CrossRef]
- Nowicki, P.; Koganezawa, M. Densities and habitat selection of the Sika deer and the Japanese serow in Nikko National Park, central Japan, as revealed by aerial censuses and GIS analysis. Biosph. Conserv. 2001, 3, 71–87. [Google Scholar] [CrossRef]
- Endo, H. Canis lupus (Linnaeus, 1758). In The Wild Mammals of Japan, 2nd ed.; Ohdachi, S.D., Ishibashi, Y., Iwasa, M.A., Fukui, D., Saitoh, T., Eds.; Shoukadoh Book Sellers and the Mammalogical Society of Japan: Kyoto, Japan, 2015; pp. 226–227. [Google Scholar]
- Yamazaki, K. 2015. Ursus thibetanus (G. Cuvier, 1823). In The Wild Mammals of Japan, 2nd ed.; Ohdachi, S.D., Ishibashi, Y., Iwasa, M.A., Fukui, D., Saitoh, T., Eds.; Shoukadoh Book Sellers and the Mammalogical Society of Japan: Kyoto, Japan, 2015; pp. 243–245. [Google Scholar]
- Takatsuki, S.; Hirabuki, Y. Effects of sika deer browsing on the structure and regeneration of the Abies firma forest on Kinkazan Island, northern Japan. J. Sustain. For. 1997, 6, 203–221. [Google Scholar] [CrossRef]
- Fujiki, D.; Kishimoto, Y.; Sakata, H. Assessing decline in physical structure of deciduous hardwood forest stands under sika deer grazing using shrub-layer vegetation cover. J. For. Res. 2010, 15, 140–144. [Google Scholar] [CrossRef]
- Deguchi, Y.; Sato, S.; Sugawara, K. Relationship between some chemical components of herbage, dietary preference and fresh herbage intake rate by the Japanese serow. Appl. Anim. Behav. Sci. 2001, 73, 69–79. [Google Scholar] [CrossRef]
- Codron, D.; Hofmann, R.R.; Clauss, M. Morphological and physiological adaptations for browsing and grazing. In The Ecology of Browsing and Grazing II; Gordon, I.J., Prins, H.H.T., Eds.; Springer: Berlin, German, 2019; pp. 81–125. [Google Scholar]
- Takatsuki, S. The Ecological History of Sika Deer; University of Tokyo Press: Tokyo, Japan, 2006. [Google Scholar]
- Furuya, Y.; Kuroda, N. Medium and large mammals in Mount Fuji. In Fujisan: Fujisan Sougou Gakujyutu Chosa Houkokusyo; Fujikyuko, Ed.; Fujikyuko: Fujikawaguchiko, Japan, 1971; pp. 807–816. (In Japanese) [Google Scholar]
- Torii, H. The Mammals of Sizuoka Prefecture; Daiichi Houki: Tokyo, Japan, 1989. [Google Scholar]
- Takada, H.; Hiruma, M.; Washida, A.; Katsumata, E. Present status of Japanese serow (Capricornis crispus) and sika deer (Cervus nippon) in the alpine habitat of Mt. Fuji. Mt. Fuji Res. 2020, 14, 1–10. (In Japanese) [Google Scholar]
- Takada, H. The summer Summer habitat use of the sika deer (Cervus nippon) at landscape scale in northern slope of Mount Fuji, central Japan. Mt. Fuji Res. 2022, 16, 11–22. (In Japanese) [Google Scholar]
- Takada, H.; Washida, A. Ecological drivers of group size variation in sika deer: Habitat structure, population density, or both? Mamm. Biol. 2020, 100, 445–452. [Google Scholar] [CrossRef]
- Hiruma, M.; Takada, H.; Washida, A.; Koike, S. Dietary partitioning and competition between sika deer and Japanese serows in high elevation habitats. Mamm. Res. 2023, 68, 305–315. [Google Scholar] [CrossRef]
- Takada, H. Behavioral ecology of sika deer in the Mount Fuji, Central Japan. Mt. Fuji Res. Unpublished data, in preparation.
- Takatsuki, S.; Kanomata, K.; Suzuki, K. Defecation rates of sika deer and Japanese serow. Jap. J. Ecol. 1981, 31, 435–439. (in Japanese). [Google Scholar] [CrossRef]
- Neff, D.J. The pellet-group count technique for big game trend, census, and distribution: A review. J. Wildl. Manag. 1968, 32, 597–614. [Google Scholar] [CrossRef]
- Putman, R. Facts from faeces. Mamm. Rev. 1984, 14, 79–97. [Google Scholar] [CrossRef]
- Heinze, E.; Boch, S.; Fischer, M.; Hessenmöller, D.; Klenk, B.; Müller, J.; Prati, D.; Schulze, E.; Seele, C.; Socher, S. Habitat use of large ungulates in northeastern Germany in relation to forest management. For. Ecol. Manag. 2011, 261, 288–296. [Google Scholar] [CrossRef]
- Loft, E.R.; Kie, J.G. Comparison of pellet-group and radio triangulation methods for assessing deer habitat use. J. Wildl. Manag. 1988, 52, 524–527. [Google Scholar] [CrossRef]
- Edge, W.D.; Marcum, C.L. Determining elk distribution with pellet-group and telemetry techniques. J. Wildl. Manag. 1989, 53, 621–624. [Google Scholar] [CrossRef]
- Månsson, J.; Andrén, H.; Sand, H. Can pellet counts be used to accurately describe winter habitat selection by moose Alces alces? Eur. J. Wildl. Res. 2011, 57, 1017–1023. [Google Scholar] [CrossRef]
- Takatsuki, S. On the “biomass index” for quantitative evaluation of plant communities in wildlife habitats: A proposal. J. Azabu Univ. 2010, 19, 1–24. (In Japanese) [Google Scholar]
- Takada, H. The summer spatial distribution of Japanese serows (Capricornis crispus) in an area without predation risk. Mamm. Biol. 2020, 100, 63–71. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Pebesma, E.; Bivand, R. Spatial Data Science with Applications in R; Chapman & Hall: Berlin, German, 2023. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.43.6. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 31 May 2024).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Sauzend Oaks, CA, USA, 2019; pp. 385–436. [Google Scholar]
- Moran, P.A. Notes on continuous stochastic phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Durbin, J.; Watson, G.S. Testing for Serial Correlation in Least Squares Regression I. Biometrika 1950, 37, 409–428. [Google Scholar] [CrossRef]
- Yamamura, K. Transformation using (x + 0.5) to stabilize the variance of populations. Popul. Ecol. 1999, 41, 229–234. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- Coppes, J.; Burghardt, F.; Hagen, R.; Suchant, R.; Braunisch, V. Human recreation affects spatio-temporal habitat use patterns in red deer (Cervus elaphus). PLoS ONE 2017, 12, e0175134. [Google Scholar] [CrossRef]
- Yokoyama, M.; Kaji, K.; Suzuki, M. Food habits of sika deer and nutritional value of sika deer diets in eastern Hokkaido, Japan. Ecol. Res. 2000, 15, 345–355. [Google Scholar] [CrossRef]
- Takahashi, K.; Uehara, A.; Takatsuki, S. Food habits of sika deer at Otome Highland, Yamanashi, with reference to Sasa nipponica. Mammal Study 2013, 38, 231–234. [Google Scholar] [CrossRef]
- Mount Fuji Research Institute. Fujisan Sakaime Zukan; Maruzen: Tokyo, Japan, 2020. [Google Scholar]
- Kaji, K. The Road to Wildlife Management; University of Tokyo Press: Tokyo, Japan, 2023. [Google Scholar]


| Model Number | Explanatory Variables | K | AICc | ΔAICc | wAICc |
|---|---|---|---|---|---|
| 45 | hv + hr + bb | 5 | 505.5 | 0.00 | 0.228 |
| 41 | hr + bb | 4 | 507.2 | 1.72 | 0.096 |
| 61 | hv + hr + bf + bb | 6 | 507.4 | 1..89 | 0.089 |
| 47 | hv + hr + vs + bb | 6 | 507.7 | 2.15 | 0.078 |
| 57 | hr + bf + bb | 5 | 507.7 | 2.16 | 0.078 |
| 46 | hv + hr + bd + bb | 6 | 507.9 | 2.35 | 0.070 |
| 43 | hr + vs + bb | 5 | 508.7 | 3.17 | 0.047 |
| 42 | hr + bd + bb | 5 | 509.0 | 3.53 | 0.039 |
| 62 | hv + hr + bd + bf + bb | 7 | 509.4 | 3.87 | 0.033 |
| 37 | hv + bb | 4 | 509.6 | 4.11 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, H.; Nakamura, K. Effects of Human Harvesting, Residences, and Forage Abundance on Deer Spatial Distribution. Animals 2024, 14, 1924. https://doi.org/10.3390/ani14131924
Takada H, Nakamura K. Effects of Human Harvesting, Residences, and Forage Abundance on Deer Spatial Distribution. Animals. 2024; 14(13):1924. https://doi.org/10.3390/ani14131924
Chicago/Turabian StyleTakada, Hayato, and Keita Nakamura. 2024. "Effects of Human Harvesting, Residences, and Forage Abundance on Deer Spatial Distribution" Animals 14, no. 13: 1924. https://doi.org/10.3390/ani14131924
APA StyleTakada, H., & Nakamura, K. (2024). Effects of Human Harvesting, Residences, and Forage Abundance on Deer Spatial Distribution. Animals, 14(13), 1924. https://doi.org/10.3390/ani14131924




