Identification of Two Linear Epitopes on MGF_110-13L Protein of African Swine Fever Virus with Monoclonal Antibodies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Sera, and Viruses
2.2. Plasmids Construction and Transient Transfection
2.3. Expression and Purification of the MGF_110-13L Protein
2.4. Generation of Monoclonal Antibodies against MGF_110-13L
2.5. Immunofluorescence Assay
2.6. Expression of Short Peptide Fusion Proteins
2.7. SDS-PAGE and Western Blotting
2.8. ELISA
2.9. Dot Blot Assay
2.10. Immunization of Pigs
3. Results
3.1. MGF_110-13L Was Identified as an Antigenic Protein
3.2. Expression of MGF_110-13L Protein and Generation of Monoclonal Antibodies
3.3. Epitope Mapping of mAbs
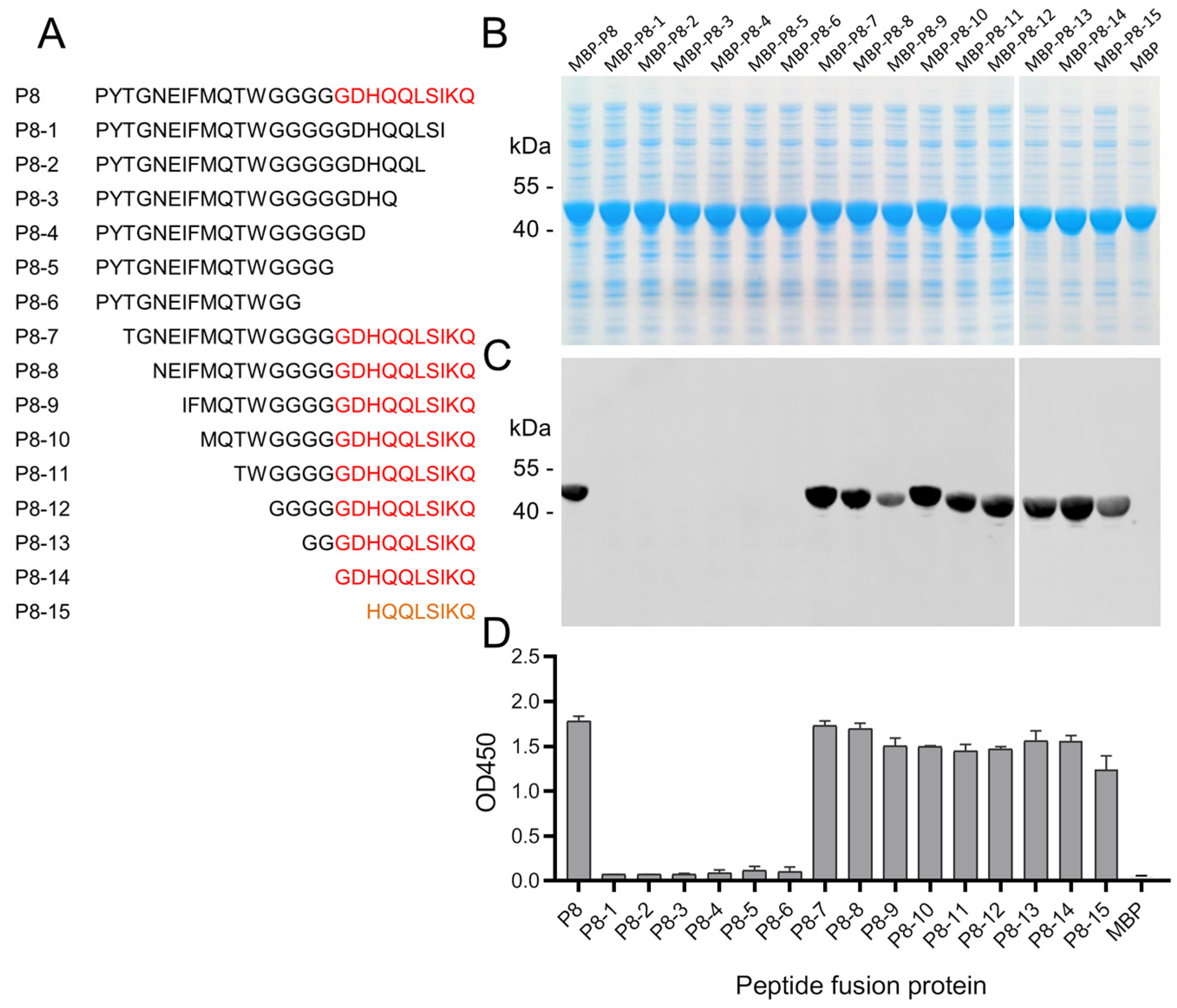
3.4. Fine-Mapping of the Epitope of 8C3
3.5. Fine-Mapping the Epitope of 10E4
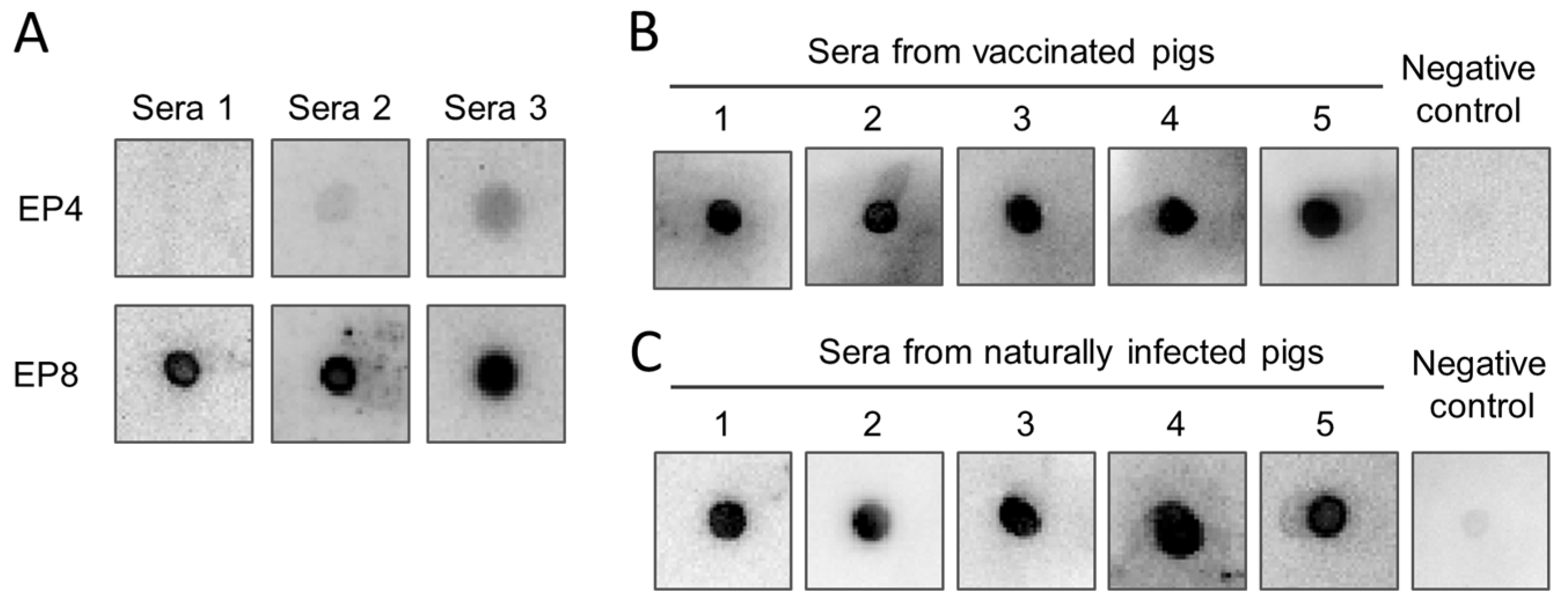
3.6. Immunoreactivity of the Epitope Peptides with Pig Sera
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penrith, M.-L. History of ‘swine fever’ in Southern Africa. J. S. Afr. Vet. Assoc. 2013, 84, a1106. [Google Scholar] [CrossRef]
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L.K. African Swine Fever Virus Isolate, Georgia, 2007. Emerg. Infect. Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African Swine Fever Epidemiology and Control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Chapman, D.A.G.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, E.P.; Gallardo, C.; Arias, M.; Da Silva, M.; Upton, C.; Martin, R.; Bishop, R.P. Phylogenomic analysis of 11 complete African swine fever virus genome sequences. Virology 2010, 400, 128–136. [Google Scholar] [CrossRef]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A Proteomic Atlas of the African Swine Fever Virus Particle. J. Virol. 2018, 92, e01293-18. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Puertas, P.; Rodríguez, F.; Oviedo, J.M.; Ramiro-Ibáñez, F.; Ruiz-Gonzalvo, F.; Alonso, C.; Escribano, J.M. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J. Virol. 1996, 70, 5689–5694. [Google Scholar] [CrossRef]
- Gómez-Puertas, P.; Rodríguez, F.; Oviedo, J.M.; Brun, A.; Alonso, C.; Escribano, J.M. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology 1998, 243, 461–471. [Google Scholar] [CrossRef]
- Giménez-Lirola, L.G.; Mur, L.; Rivera, B.; Mogler, M.; Sun, Y.; Lizano, S.; Goodell, C.; Harris, D.L.H.; Rowland, R.R.R.; Gallardo, C.; et al. Detection of African Swine Fever Virus Antibodies in Serum and Oral Fluid Specimens Using a Recombinant Protein 30 (p30) Dual Matrix Indirect ELISA. PLoS ONE 2016, 11, e0161230. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Richt, J.A. Subunit Vaccine Approaches for African Swine Fever Virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef]
- Muñoz, A.L.; Tabarés, E. Characteristics of the major structural proteins of African swine fever virus: Role as antigens in the induction of neutralizing antibodies. A review. Virology 2022, 571, 46–51. [Google Scholar] [CrossRef]
- Kollnberger, S.D.; Gutierrez-Castañeda, B.; Foster-Cuevas, M.; Corteyn, A.; Parkhouse, R.M.E. Identification of the principal serological immunodeterminants of African swine fever virus by screening a virus cDNA library with antibody. J. Gen. Virol. 2002, 83, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Castañeda, B.; Reis, A.L.; Corteyn, A.; Parkhouse, R.M.E.; Kollnberger, S. Expression, cellular localization and antibody responses of the African swine fever virus genes B602L and K205R. Arch. Virol. 2008, 153, 2303–2306. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Liu, J.; Niu, B.; Zhu, Y.-M.; Zhao, D.-M.; Chen, W.-Y.; Liu, R.-Q.; Bu, Z.-G.; Hua, R.-H. Comprehensive mapping of antigenic linear B-cell epitopes on K205R protein of African swine fever virus with monoclonal antibodies. Virus Res. 2023, 328, 199085. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiao, L.; Peng, B.; Wang, Y.; Yang, Z.; Yao, X.; Hu, L.; Lin, X. Prokaryotic expression, purification and antigenicity analysis of African swine fever virus pK205R protein. Pol. J. Vet. Sci. 2016, 19, 41–48. [Google Scholar] [CrossRef]
- Hua, R.-H.; Liu, J.; Zhang, S.-J.; Liu, R.-Q.; Zhang, X.-F.; He, X.-J.; Zhao, D.-M.; Bu, Z.-G. Mammalian Cell-Line-Expressed CD2v Protein of African Swine Fever Virus Provides Partial Protection against the HLJ/18 Strain in the Early Infection Stage. Viruses 2023, 15, 1467. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.L.; Andrés, G. African swine fever virus morphogenesis. Virus Res. 2013, 173, 29–41. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.-H.; Li, Y.-N.; Chen, Z.-S.; Liu, L.-K.; Huo, H.; Wang, X.-L.; Guo, L.-P.; Shen, N.; Wang, J.-F.; Bu, Z.-G. Generation and characterization of a new mammalian cell line continuously expressing virus-like particles of Japanese encephalitis virus for a subunit vaccine candidate. BMC Biotechnol. 2014, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.H.; Bu, Z.G. A monoclonal antibody against PrM/M protein of Japanese encephalitis virus. Hybridoma 2011, 30, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.-H.; Zhang, S.-J.; Niu, B.; Ge, J.-Y.; Lan, T.; Bu, Z.-G. A Novel Conserved Linear Neutralizing Epitope on the Receptor-Binding Domain of the SARS-CoV-2 Spike Protein. Microbiol. Spectr. 2023, 11, e01190-23. [Google Scholar] [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J. Virol. 2020, 94, e02017-19. [Google Scholar] [CrossRef]
- Brake, D.A. African Swine Fever Modified Live Vaccine Candidates: Transitioning from Discovery to Product Development through Harmonized Standards and Guidelines. Viruses 2022, 14, 2619. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Kang, L.; Huang, L.; Zhou, S.; Hu, L.; Zheng, J.; Li, C.; Zhang, X.; He, X.; et al. pMGF505-7R determines pathogenicity of African swine fever virus infection by inhibiting IL-1β and type I IFN production. PLoS Pathog. 2021, 17, e1009733. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Luo, R.; Zhang, J.; Lan, J.; Lu, Z.; Zhai, H.; Li, L.-F.; Sun, Y.; Qiu, H.-J. The African swine fever virus MGF300-4L protein is associated with viral pathogenicity by promoting the autophagic degradation of IKK β and increasing the stability of I κ B α. Emerg. Microbes Infect. 2024, 13, 2333381. [Google Scholar] [CrossRef]
- Wang, T.; Luo, R.; Zhang, J.; Lu, Z.; Li, L.-F.; Zheng, Y.-H.; Pan, L.; Lan, J.; Zhai, H.; Huang, S.; et al. The MGF300-2R protein of African swine fever virus is associated with viral pathogenicity by promoting the autophagic degradation of IKKα and IKKβ through the recruitment of TOLLIP. PLoS Pathog. 2023, 19, e1011580. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Feng, T.; Zhang, X.; Yang, F.; Cao, W.; Chen, H.; Liu, H.; Zhang, K.; Zhu, Z.; et al. African Swine Fever Virus F317L Protein Inhibits NF-κB Activation To Evade Host Immune Response and Promote Viral Replication. mSphere 2021, 6, e00658-21. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, B.; Shen, C.; Zhang, T.; Hao, Y.; Zhang, D.; Liu, H.; Shi, X.; Li, G.; Yang, J.; et al. MGF360-9L Is a Major Virulence Factor Associated with the African Swine Fever Virus by Antagonizing the JAK/STAT Signaling Pathway. mBio 2022, 13, e02330-21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Dai, J.; Zhang, K.; Wang, T.; Li, L.-F.; Luo, Y.; Sun, Y.; Qiu, H.-J.; Li, S. The H240R Protein of African Swine Fever Virus Inhibits Interleukin 1β Production by Inhibiting NEMO Expression and NLRP3 Oligomerization. J. Virol. 2022, 96, e00954-22. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, H.; Ye, G.; Liu, X.; Chen, W.; Wang, Z.; Zhao, D.; Zhang, Z.; Feng, C.; Hu, L.; et al. Deletion of African Swine Fever Virus (ASFV) H240R Gene Attenuates the Virulence of ASFV by Enhancing NLRP3-Mediated Inflammatory Responses. J. Virol. 2023, 97, e01227-22. [Google Scholar] [CrossRef] [PubMed]
- Galindo, I.; Almazán, F.; Bustos, M.J.; Viñuela, E.; Carrascosa, A.L. African Swine Fever Virus EP153R Open Reading Frame Encodes a Glycoprotein Involved in the Hemadsorption of Infected Cells. Virology 2000, 266, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Petrovan, V.; Rathakrishnan, A.; Islam, M.; Goatley, L.C.; Moffat, K.; Sanchez-Cordon, P.J.; Reis, A.L.; Dixon, L.K. Role of African Swine Fever Virus Proteins EP153R and EP402R in Reducing Viral Persistence in Blood and Virulence in Pigs Infected with BeninΔDP148R. J. Virol. 2022, 96, e0134021. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hu, Y.; Li, J.; Wang, A.; Meng, X.; Chen, L.; Wei, J.; Tong, W.; Kong, N.; Yu, L.; et al. Screening and identification of the dominant antigens of the African swine fever virus. Front. Vet. Sci. 2023, 10, 1175701. [Google Scholar] [CrossRef] [PubMed]
- Heimerman, M.E.; Murgia, M.V.; Wu, P.; Lowe, A.D.; Jia, W.; Rowland, R.R. Linear epitopes in African swine fever virus p72 recognized by monoclonal antibodies prepared against baculovirus-expressed antigen. J. Vet. Diagn. Investig. 2018, 30, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Tesfagaber, W.; Wang, W.; Wang, L.; Zhao, R.; Zhu, Y.; Li, F.; Sun, E.; Liu, R.; Bu, Z.; Meng, G.; et al. A highly efficient blocking ELISA based on p72 monoclonal antibody for the detection of African swine fever virus antibodies and identification of its linear B cell epitope. Int. J. Biol. Macromol. 2024, 268, 131695. [Google Scholar] [CrossRef]
- Cao, Y.; Han, D.; Zhang, Y.; Zhang, K.; Du, N.; Tong, W.; Li, G.; Zheng, H.; Liu, C.; Gao, F.; et al. Identification of one novel epitope targeting p54 protein of African swine fever virus using monoclonal antibody and development of a capable ELISA. Res. Vet. Sci. 2021, 141, 19–25. [Google Scholar] [CrossRef]
- Petrovan, V.; Murgia, M.V.; Wu, P.; Lowe, A.D.; Jia, W.; Rowland, R.R.R. Epitope mapping of African swine fever virus (ASFV) structural protein, p54. Virus Res. 2020, 279, 197871. [Google Scholar] [CrossRef]
- Zhou, G.; Shi, Z.; Luo, J.; Cao, L.; Yang, B.; Wan, Y.; Wang, L.; Song, R.; Ma, Y.; Tian, H.; et al. Preparation and epitope mapping of monoclonal antibodies against African swine fever virus P30 protein. Appl. Microbiol. Biotechnol. 2022, 106, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Zhang, G.; Bai, Y.; Liu, H.; Chen, Y.; Ding, P.; Zhou, J.; Feng, H.; Li, M.; Tian, Y.; et al. Identification of Linear B Cell Epitopes on CD2V Protein of African Swine Fever Virus by Monoclonal Antibodies. Microbiol. Spectr. 2022, 10, e01052-21. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Ding, P.; Liu, S.; Zhang, N.; Chen, Y.; Li, Q.; Fan, L.; Chang, Z.; Zhang, G. Development and characterization of recombinant ASFV CD2v protein nanoparticle-induced monoclonal antibody. Int. J. Biol. Macromol. 2022, 209, 533–541. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, H.; Liu, L.; Cao, Y.; Jiang, T.; Zou, Y.; Peng, Y. Classification and characterization of multigene family proteins of African swine fever viruses. Brief Bioinform. 2021, 22, bbaa380. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.-J.; Niu, B.; Liu, S.-M.; Zhu, Y.-M.; Zhao, D.-M.; Bu, Z.-G.; Hua, R.-H. Identification of Two Linear Epitopes on MGF_110-13L Protein of African Swine Fever Virus with Monoclonal Antibodies. Animals 2024, 14, 1951. https://doi.org/10.3390/ani14131951
Zhang S-J, Niu B, Liu S-M, Zhu Y-M, Zhao D-M, Bu Z-G, Hua R-H. Identification of Two Linear Epitopes on MGF_110-13L Protein of African Swine Fever Virus with Monoclonal Antibodies. Animals. 2024; 14(13):1951. https://doi.org/10.3390/ani14131951
Chicago/Turabian StyleZhang, Shu-Jian, Bei Niu, Shi-Meng Liu, Yuan-Mao Zhu, Dong-Ming Zhao, Zhi-Gao Bu, and Rong-Hong Hua. 2024. "Identification of Two Linear Epitopes on MGF_110-13L Protein of African Swine Fever Virus with Monoclonal Antibodies" Animals 14, no. 13: 1951. https://doi.org/10.3390/ani14131951




