Effects of Luteolin in an In Vitro Model of Porcine Intestinal Infections
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions
2.2. Treatment Scheme
2.3. Measurements
2.4. Statistics
3. Results
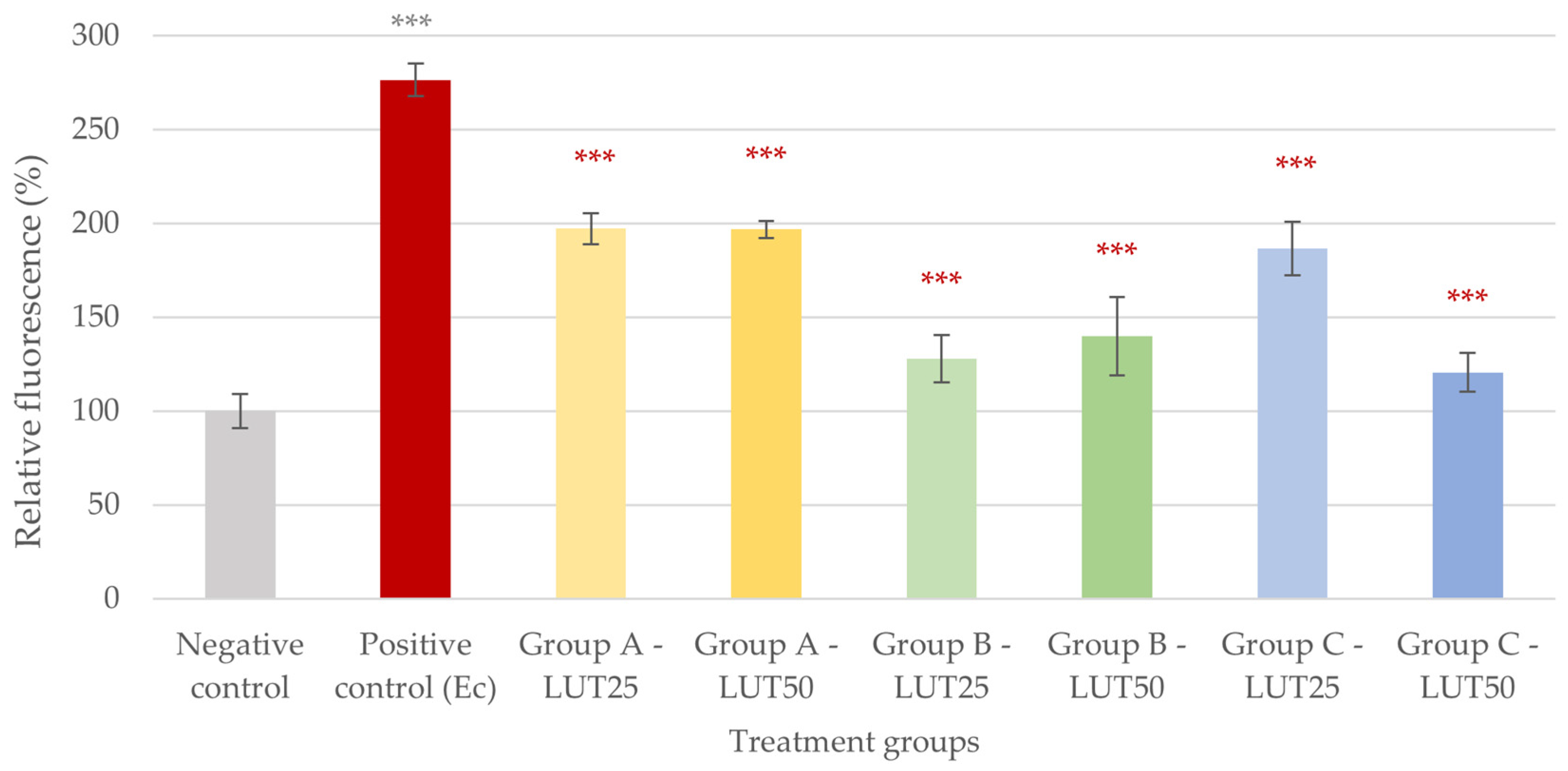
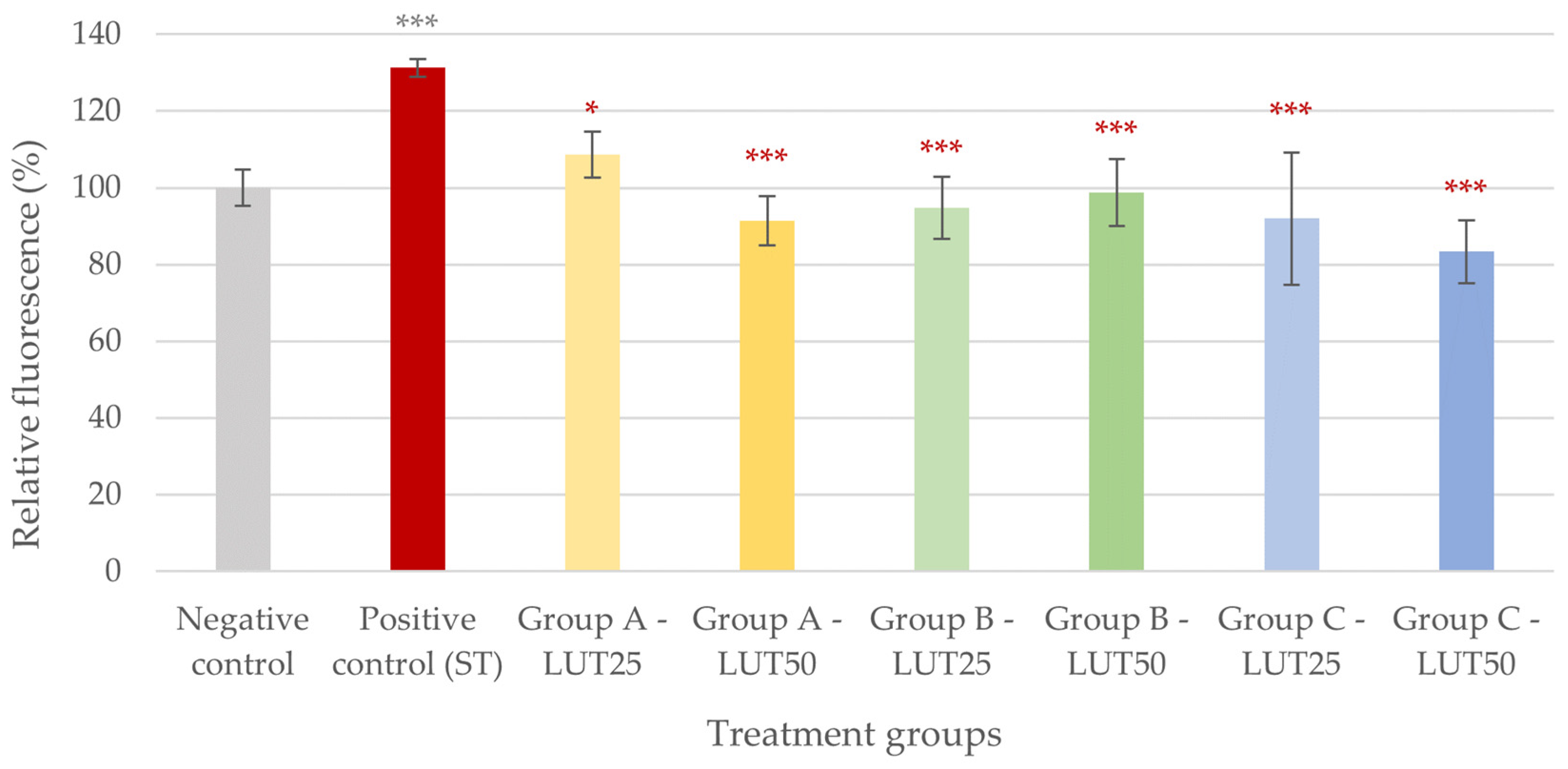
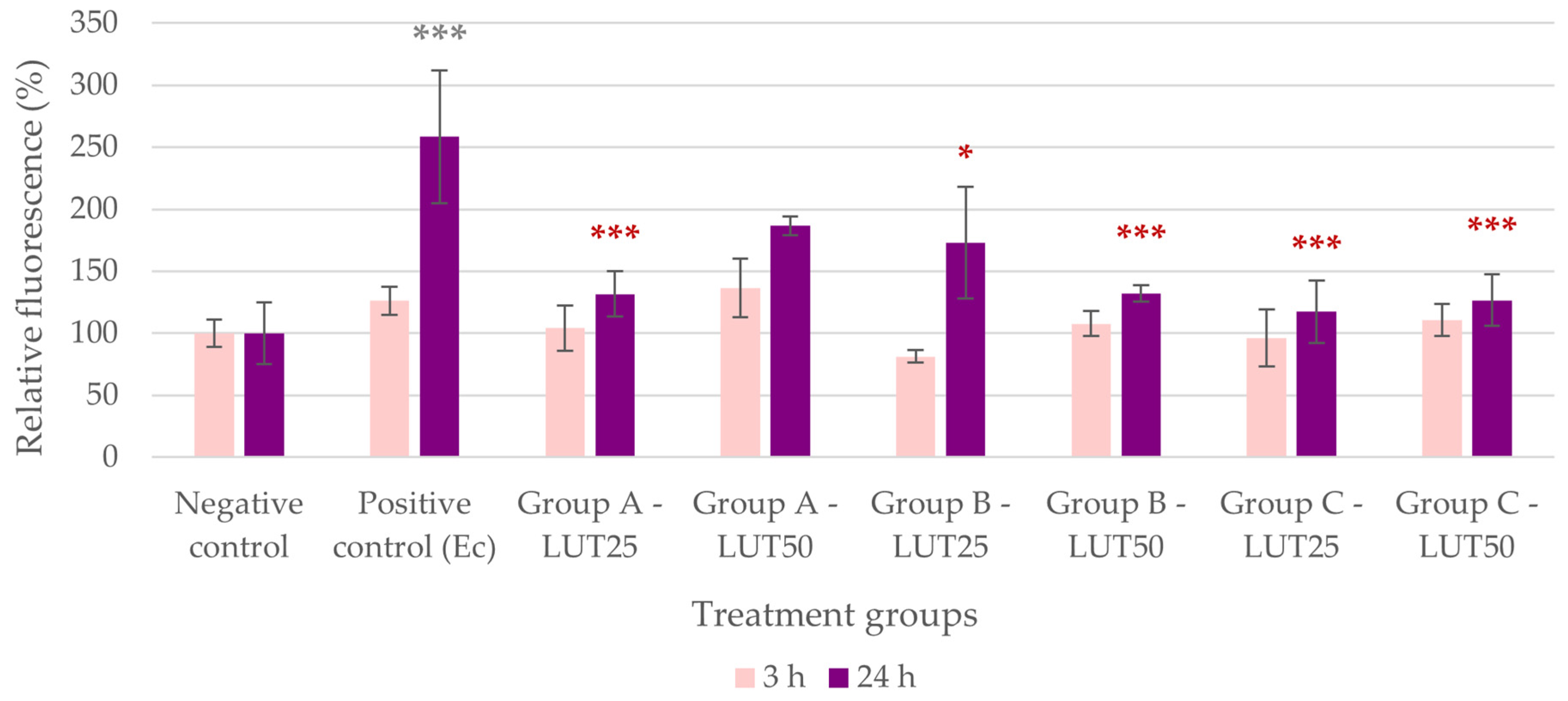
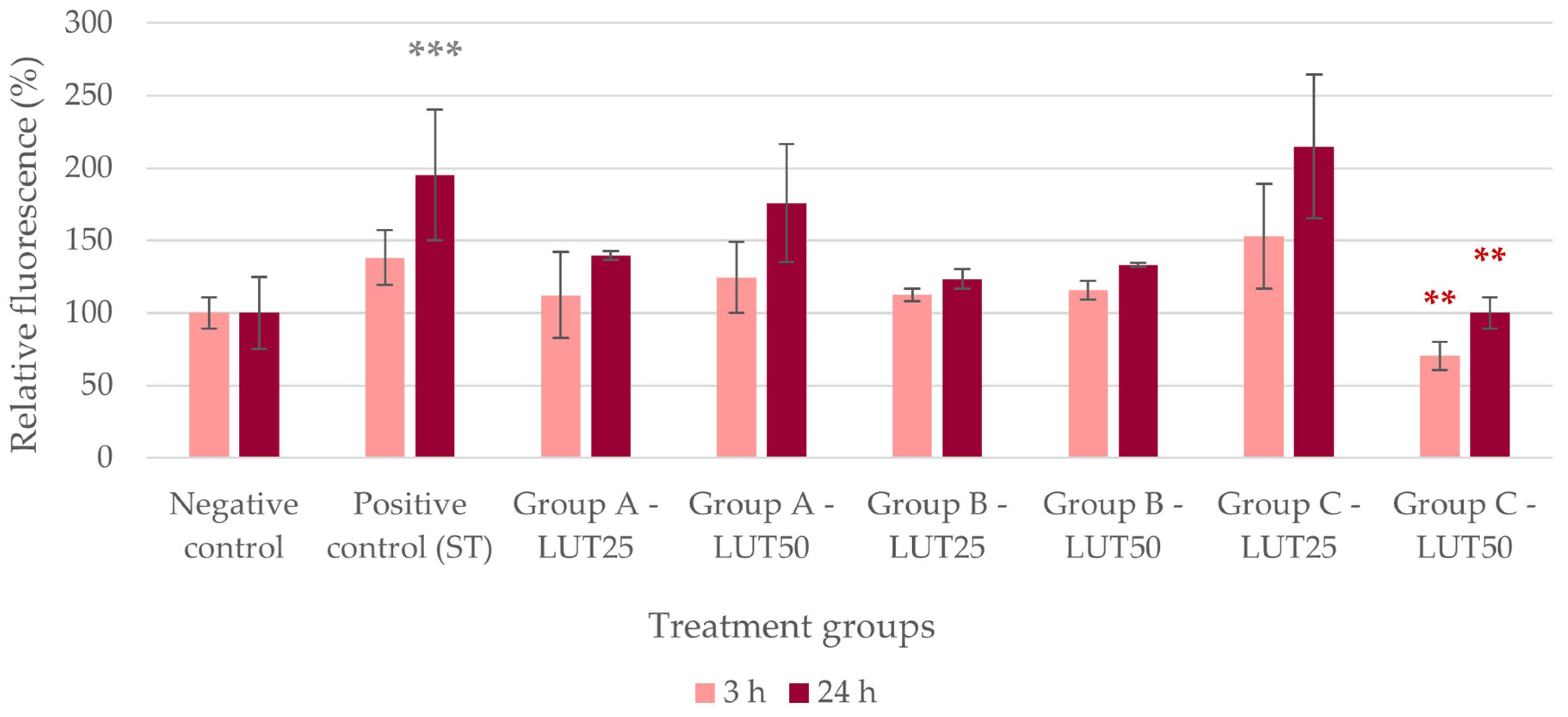
3.1. Antioxidant Activity
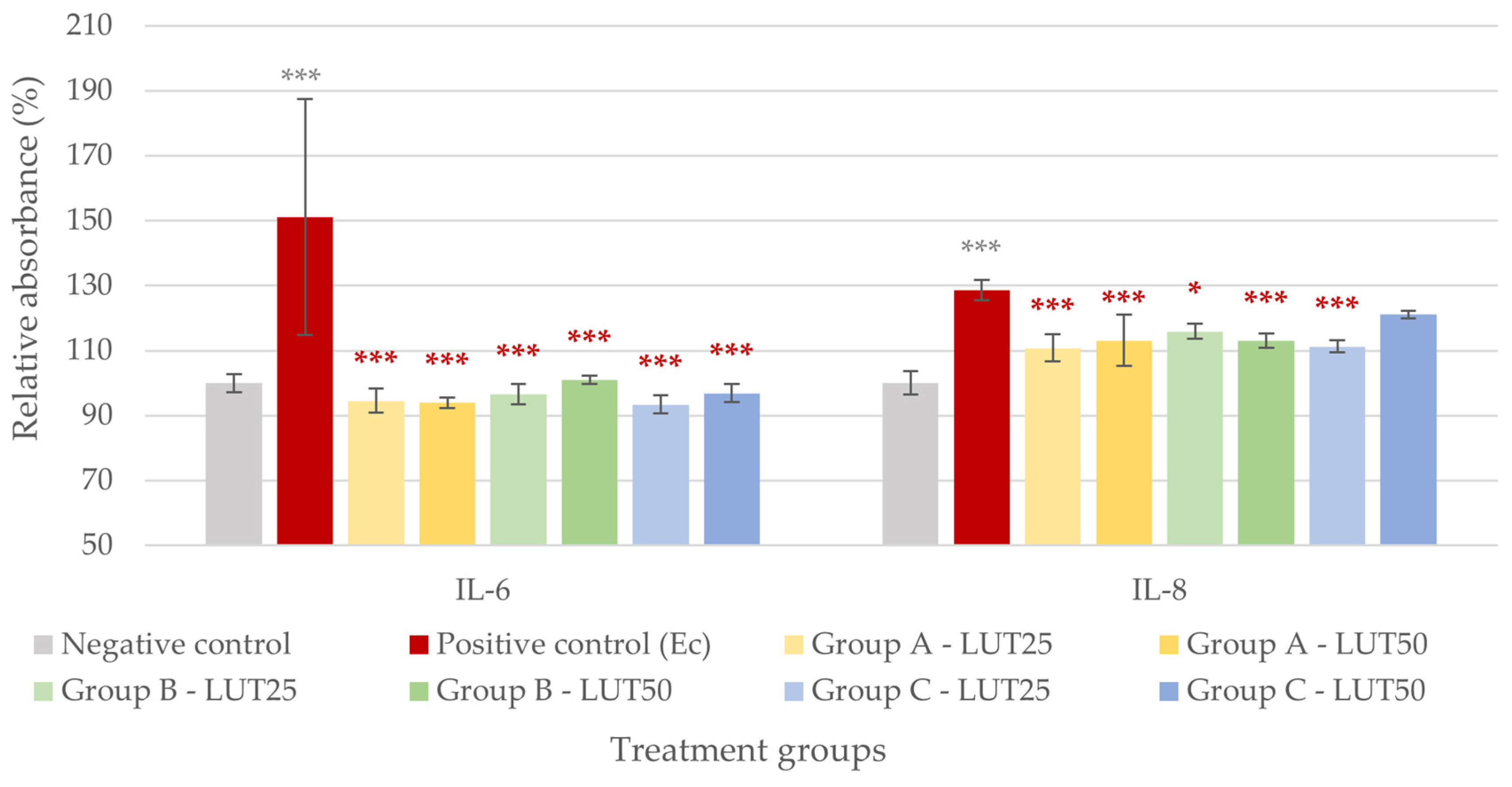
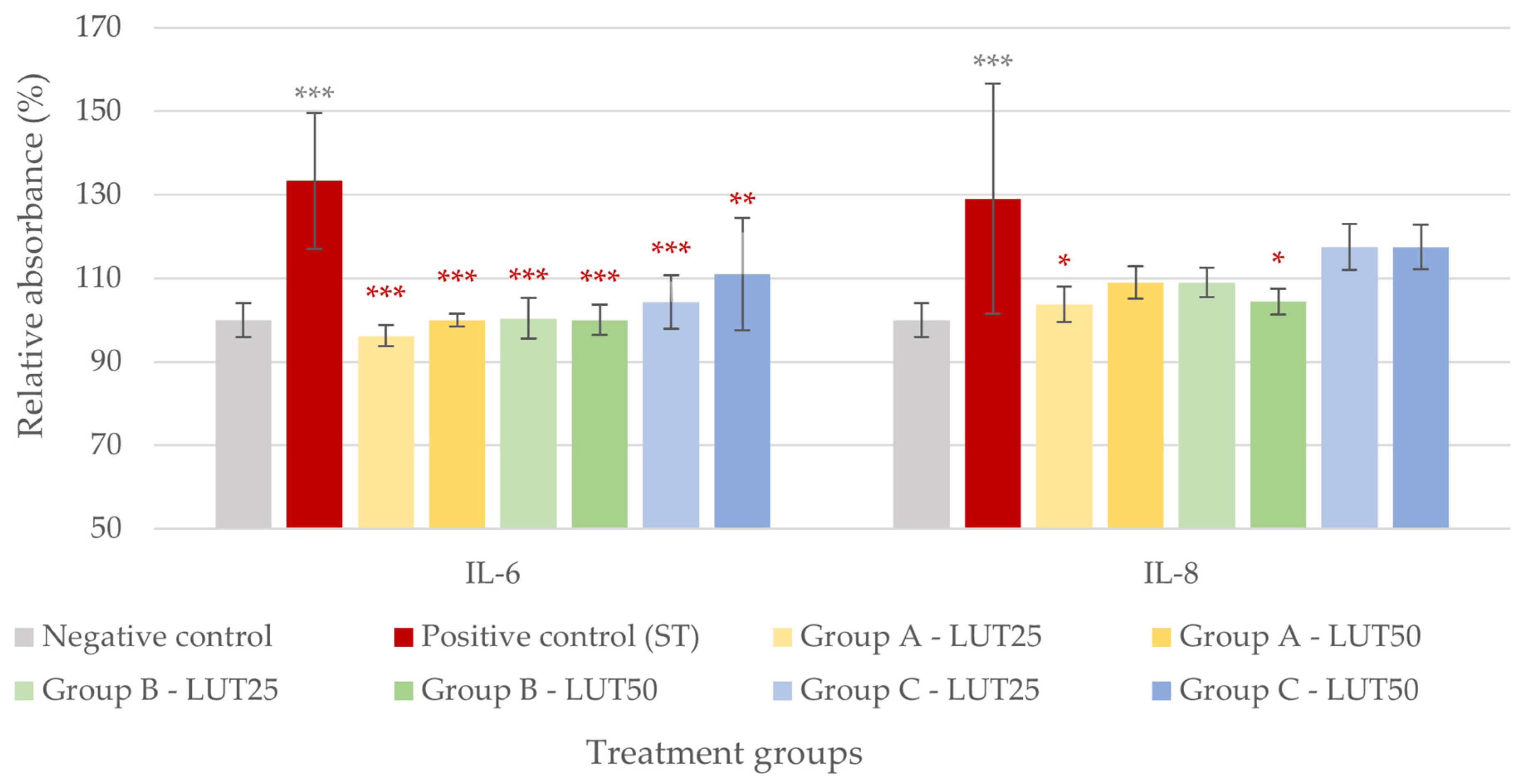
3.2. Anti-Inflammatory Activity
3.3. Barrier Protection
3.4. Adhesion Inhibition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Song, M.; Liu, Y.; Ji, P. Enterotoxigenic Escherichia coli infection of weaned pigs: Intestinal challenges and nutritional intervention to enhance disease resistance. Front. Immunol. 2022, 13, 885253. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Isaacson, R.E. Salmonella in Swine: Microbiota Interactions. Annu. Rev. Anim. Biosci. 2017, 5, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Gong, T.; Jiang, Z.; Lu, Z.; Wang, Y. The Role of Probiotics in Alleviating Postweaning Diarrhea in Piglets from the Perspective of Intestinal Barriers. Front. Cell Infect. Microbiol. 2022, 12, 883107. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.T.; Everaert, N.; Bindelle, J. Review on the effects of potential prebiotics on controlling intestinal enteropathogens Salmonella and Escherichia coli in pig production. J. Anim. Physiol. Anim. Nutr. 2018, 102, 17–32. [Google Scholar] [CrossRef]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Chen, Y.; Yu, C.; Azevedo, P.; Gong, J.; Yang, C. Bacillus licheniformis PF9 improves barrier function and alleviates inflammatory responses against enterotoxigenic Escherichia coli F4 infection in the porcine intestinal epithelial cells. J. Anim. Sci. Biotechnol. 2022, 13, 86. [Google Scholar] [CrossRef]
- Ter Kuile, B.H.; Kraupner, N.; Brul, S. The risk of low concentrations of antibiotics in agriculture for resistance in human health care. FEMS Microbiol. Lett. 2016, 363, fnw210. [Google Scholar] [CrossRef]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, 316–327. [Google Scholar] [CrossRef]
- Emes, D.; Naylor, N.; Waage, J.; Knight, G. Quantifying the Relationship between Antibiotic Use in Food-Producing Animals and Antibiotic Resistance in Humans. Antibiotics 2022, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Lin, Q.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Huang, Z.; Yu, J.; Luo, J.; et al. β-defensin 118 attenuates inflammation and injury of intestinal epithelial cells upon enterotoxigenic Escherichia coli challenge. BMC Vet. Res. 2022, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Gu, M.J.; Kye, Y.C.; Ju, Y.J.; Hong, R.; Ju, D.B.; Pyung, Y.J.; Han, S.H.; Park, B.C.; Yun, C.H. Bacteriophage EK99P-1 alleviates enterotoxigenic Escherichia coli K99-induced barrier dysfunction and inflammation. Sci. Rep. 2022, 12, 941. [Google Scholar] [CrossRef]
- Ponce de León-Rodríguez, M.D.C.; Guyot, J.P.; Laurent-Babot, C. Intestinal in vitro cell culture models and their potential to study the effect of food components on intestinal inflammation. Crit. Rev. Food Sci. Nutr. 2019, 59, 3648–3666. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, H. The IPEC-J2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Heidelberg, Germany, 2015; Chapter 12. [Google Scholar] [CrossRef]
- Berschneider, H.M. Development of normal cultured small intestinal epithelial cell lines which transport Na and Cl. Gastroenterology 1989, 96, A41. [Google Scholar]
- Cao, Z.; Xing, C.; Cheng, X.; Luo, J.; Hu, R.; Cao, H.; Guo, X.; Yang, F.; Zhuang, Y.; Hu, G. Luteolin Attenuates APEC-Induced Oxidative Stress and Inflammation via Inhibiting the HMGB1/TLR4/NF-κB Signal Axis in the Ileum of Chicks. Animals 2022, 13, 83. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential Anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Muruganathan, N.; Dhanapal, A.R.; Baskar, V.; Muthuramalingam, P.; Selvaraj, D.; Aara, H.; Shiek Abdullah, M.Z.; Sivanesan, I. Recent Updates on Source, Biosynthesis, and Therapeutic Potential of Natural Flavonoid Luteolin: A Review. Metabolites 2022, 12, 1145. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef]
- Ju, W.; Wang, X.; Shi, H.; Chen, W.; Belinsky, S.A.; Lin, Y. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol. Pharmacol. 2007, 71, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, H.; Jia, Y.; Pan, H.; Ding, H. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother. Pharmacol. 2017, 79, 1031–1041. [Google Scholar] [CrossRef]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Palkovicsné Pézsa, N.; Jerzsele, Á.; Süth, M.; Farkas, O. Protective Effects of Grape Seed Oligomeric Proanthocyanidins in IPEC-J2-Escherichia coli/Salmonella Typhimurium Co-Culture. Antibiotics 2022, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Karancsi, Z.; Farkas, O.; Jerzsele, Á. Antioxidant activity of flavonoids in LPS-treated IPEC-J2 porcine intestinal epithelial cells and their antibacterial effect against bacteria of swine origin. Antioxidants 2020, 9, 1267. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Loss, H.; Aschenbach, J.R.; Tedin, K.; Ebner, F.; Lodemann, U. The inflammatory response to enterotoxigenic E. coli and probiotic E. faecium in a coculture model of porcine intestinal epithelial and dendritic cells. Mediat. Inflamm. 2018, 2018, 9368295. [Google Scholar] [CrossRef]
- Loss, H.; Aschenbach, J.R.; Ebner, F.; Tedin, K.; Lodemann, U. Inflammatory Responses of Porcine MoDC and Intestinal Epithelial Cells in a Direct-Contact Co-culture System Following a Bacterial Challenge. Inflammation 2020, 43, 552–567. [Google Scholar] [CrossRef]
- Karancsi, Z.; Móritz, A.V.; Lewin, N.; Veres, A.M.; Jerzsele, Á.; Farkas, O. Beneficial Effect of a Fermented Wheat Germ Extract in Intestinal Epithelial Cells in case of Lipopolysaccharide-Evoked Inflammation. Oxid. Med. Cell Longev. 2020, 2020, 1482482. [Google Scholar] [CrossRef]
- Lückstädt, C.; Theobald, P. Control of E. coli and Salmonella in growing-finishing pigs through the use of potassium diformate (KDF)—European case studies. In Proceedings of the Ninth International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork, Maastricht, The Netherlands, 9–22 June 2011. [Google Scholar]
- Edfors-Lilja, I.; Wallgren, P.; Axford, R.F.E.; Bishop, S.C.; Nicholas, F.W.; Owen, J.B. Escherichia coli and Salmonella Diarrhoea in Pigs, Breeding for Disease Resistance in Farm Animals, 2nd ed.; CABI Publishing: Wallingford, UK, 2000; p. 253267. [Google Scholar]
- Souto, M.S.; Coura, F.M.; Dorneles, E.M.; Stynen, A.P.; Alves, T.M.; Santana, J.A.; Pauletti, R.B.; Guedes, R.M.; Viott, A.M.; Heinemann, M.B.; et al. Antimicrobial susceptibility and phylotyping profile of pathogenic Escherichia coli and Salmonella enterica isolates from calves and pigs in Minas Gerais, Brazil. Trop. Anim. Health Prod. 2017, 49, 13–23. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA J. 2021, 19, 6490. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Luppi, A. Swine enteric colibacillosis: Diagnosis, therapy and antimicrobial resistance. Porc. Health Manag. 2017, 8, 3–16. [Google Scholar] [CrossRef]
- Gao, J.; Cao, S.; Xiao, H.; Hu, S.; Yao, K.; Huang, K.; Jiang, Z.; Wang, L. Lactobacillus reuteri 1 Enhances Intestinal Epithelial Barrier Function and Alleviates the Inflammatory Response Induced by Enterotoxigenic Escherichia coli K88 via Suppressing the MLCK Signaling Pathway in IPEC-J2 Cells. Front. Immunol. 2022, 13, 897395. [Google Scholar] [CrossRef]
- Kiššová, Z.; Tkáčiková, L.; Mudroňová, D.; Bhide, M.R. Immunomodulatory Effect of Lactobacillus reuteri (Limosilactobacillus reuteri) and Its Exopolysaccharides Investigated on Epithelial Cell Line IPEC-J2 Challenged with Salmonella Typhimurium. Life 2022, 12, 1955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lian, S.; Shen, X.; Zhao, X.; Zhao, W.; Li, C. Recombinant porcine beta defensin 2 alleviates inflammatory responses induced by Escherichia coli in IPEC-J2 cells. Int. J. Biol. Macromol. 2022, 208, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Palkovicsné Pézsa, N.; Kovács, D.; Somogyi, F.; Karancsi, Z.; Móritz, A.V.; Jerzsele, Á.; Rácz, B.; Farkas, O. Effects of Lactobacillus rhamnosus DSM7133 on Intestinal Porcine Epithelial Cells. Animals 2023, 13, 3007. [Google Scholar] [CrossRef] [PubMed]
- Palkovicsné Pézsa, N.; Kovács, D.; Rácz, B.; Farkas, O. Effects of Bacillus licheniformis and Bacillus subtilis on Gut Barrier Function, Proinflammatory Response, ROS Production and Pathogen Inhibition Properties in IPEC-J2—Escherichia coli/Salmonella Typhimurium Co-Culture. Microorganisms 2022, 10, 936. [Google Scholar] [CrossRef]
- Palkovicsné Pézsa, N.; Kovács, D.; Gálfi, P.; Rácz, B.; Farkas, O. Effect of Enterococcus faecium NCIMB 10415 on Gut Barrier Function, Internal Redox State, Proinflammatory Response and Pathogen Inhibition Properties in Porcine Intestinal Epithelial Cells. Nutrients 2022, 14, 1486. [Google Scholar] [CrossRef]
- Tráj, P.; Sebők, C.; Mackei, M.; Kemény, Á.; Farkas, O.; Kákonyi, Á.; Kovács, L.; Neogrády, Z.; Jerzsele, Á.; Mátis, G. Luteolin: A Phytochemical to Mitigate S. Typhimurium Flagellin-Induced Inflammation in a Chicken In Vitro Hepatic Model. Animals 2023, 13, 1410. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Xu, N.N.; Sun, W.; Zhao, Y.; Li, C.Y.; Guo, M.Y. Luteolin reduces inflammation in Staphylococcus aureus-induced mastitis by inhibiting NF-kB activation and MMPs expression. Oncotarget 2017, 8, 28481–28493. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Gao, Y.; Cai, L.; Qin, R. Luteolin attenuates Staphylococcus aureus-induced endometritis through inhibiting ferroptosis and inflammation via activating the Nrf2/GPX4 signaling pathway. Microbiol. Spectr. 2024, 12, e0327923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.C.; Li, Z.; Xu, W.; Xiang, C.H.; Ma, Y.F. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am. J. Transl. Res. 2018, 10, 265–273. [Google Scholar] [PubMed]
- Nishitani, Y.; Yamamoto, K.; Yoshida, M.; Azuma, T.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. Intestinal anti-inflammatory activity of luteolin: Role of the aglycone in NF-κB inactivation in macrophages co-cultured with intestinal epithelial cells. Biofactors 2013, 39, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Jobin, C. The flavonoid luteolin prevents lipopolysaccharide-induced NF-kappaB signalling and gene expression by blocking IkappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology 2005, 115, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, R.; Li, Z.; Xiao, R.; Lv, P.; Sun, X.; Olson, M.A.; Gong, Y. Luteolin alleviates non-alcoholic fatty liver disease in rats via restoration of intestinal mucosal barrier damage and microbiota imbalance involving in gut-liver axis. Arch. Biochem. Biophys. 2021, 711, 109019. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.L.; Yang, J.W.; Dou, H.Y.; Li, G.Q.; Li, X.Y.; Shen, L.; Ji, H.F. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorg. Chem. 2021, 112, 104966. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Che, S.; Zhang, L.; Ruan, Z. Reparative Effects of Ethanol-Induced Intestinal Barrier Injury by Flavonoid Luteolin via MAPK/NF-κB/MLCK and Nrf2 Signaling Pathway. J. Agric. Food Chem. 2021, 69, 4101–4110. [Google Scholar] [CrossRef]
- Yuan, J.; Che, S.; Ruan, Z.; Song, L.; Tang, R.; Zhang, L. Regulatory effects of flavonoids luteolin on BDE-209-induced intestinal epithelial barrier damage in Caco-2 cell monolayer model. Food Chem. Toxicol. 2021, 150, 112098. [Google Scholar] [CrossRef]
- Li, B.L.; Zhao, D.Y.; Du, P.L.; Wang, X.T.; Yang, Q.; Cai, Y.R. Luteolin alleviates ulcerative colitis through SHP-1/STAT3 pathway. Inflamm. Res. 2021, 70, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, W.; Zeng, Q.; Wang, T.; Qian, W. Antibiofilm Efficacy of Luteolin Against Single and Dual Species of Candida albicans and Enterococcus faecalis. Front. Microbiol. 2021, 12, 715156. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.F.; Ren, L.B.; Teng, Y.; Zheng, S.; Yang, X.L.; Guo, X.J.; Wang, X.Y.; Sha, K.H.; Li, N.; Xu, G.Y.; et al. Luteolin decreases the attachment, invasion and cytotoxicity of UPEC in bladder epithelial cells and inhibits UPEC biofilm formation. Food Chem. Toxicol. 2014, 72, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Šikić Pogačar, M.; Klančnik, A.; Bucar, F.; Langerholc, T.; Smole Možina, S. Anti-adhesion activity of thyme (Thymus vulgaris L.) extract, thyme post-distillation waste, and olive (Olea europea L.) leaf extract against Campylobacter jejuni on polystyrene and intestine epithelial cells. J. Sci. Food Agric. 2016, 96, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Gao, X.; Sun, T.T.; Yu, L.; Guo, Y.; Hong, W.; Zhang, D.; Liu, M. The antimicrobial activity of luteolin against four bacteria in vitro. Chin. J. Vet. Sci. 2017, 37, 1558–1561. [Google Scholar]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef]
| Step 1 | Step 2 | Step 3 | ||
|---|---|---|---|---|
| Positive control | Addition of bacteria |  | Measurements
| |
| Group A LUT treatment before challenge with bacteria | Addition of LUT | Addition of bacteria | ||
| Group B LUT treatment concurrently with challenge with bacteria | Addition of LUT and bacteria | |||
| Group C LUT treatment after challenge with bacteria | Addition of bacteria | Addition of LUT | ||
| Negative control | Addition of plain medium | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, D.; Palkovicsné Pézsa, N.; Móritz, A.V.; Jerzsele, Á.; Farkas, O. Effects of Luteolin in an In Vitro Model of Porcine Intestinal Infections. Animals 2024, 14, 1952. https://doi.org/10.3390/ani14131952
Kovács D, Palkovicsné Pézsa N, Móritz AV, Jerzsele Á, Farkas O. Effects of Luteolin in an In Vitro Model of Porcine Intestinal Infections. Animals. 2024; 14(13):1952. https://doi.org/10.3390/ani14131952
Chicago/Turabian StyleKovács, Dóra, Nikolett Palkovicsné Pézsa, Alma Virág Móritz, Ákos Jerzsele, and Orsolya Farkas. 2024. "Effects of Luteolin in an In Vitro Model of Porcine Intestinal Infections" Animals 14, no. 13: 1952. https://doi.org/10.3390/ani14131952







