Dynamic and Postural Changes in Forelimb Amputee Dogs: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.1.1. Amputee Group
2.1.2. Control Group
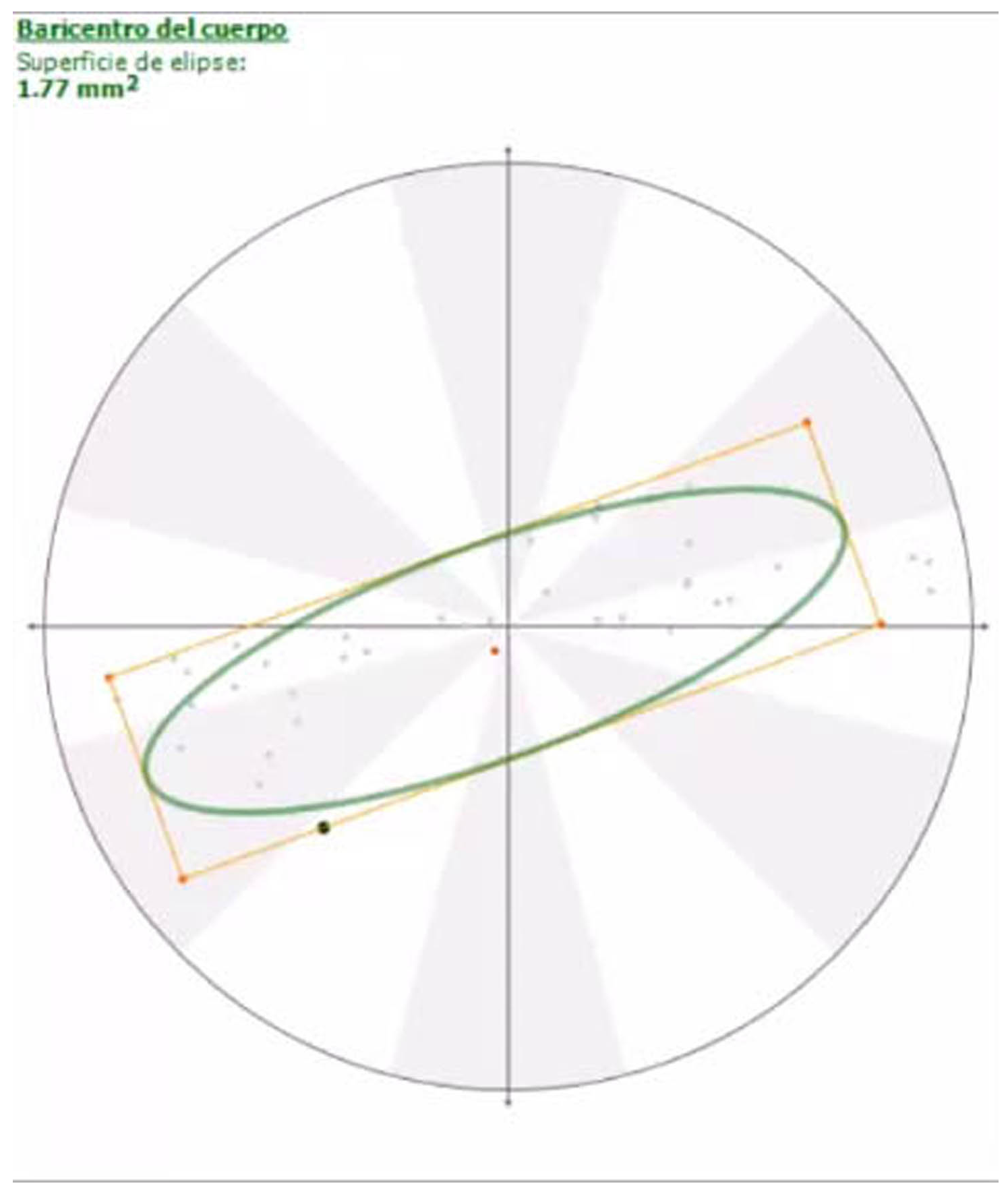
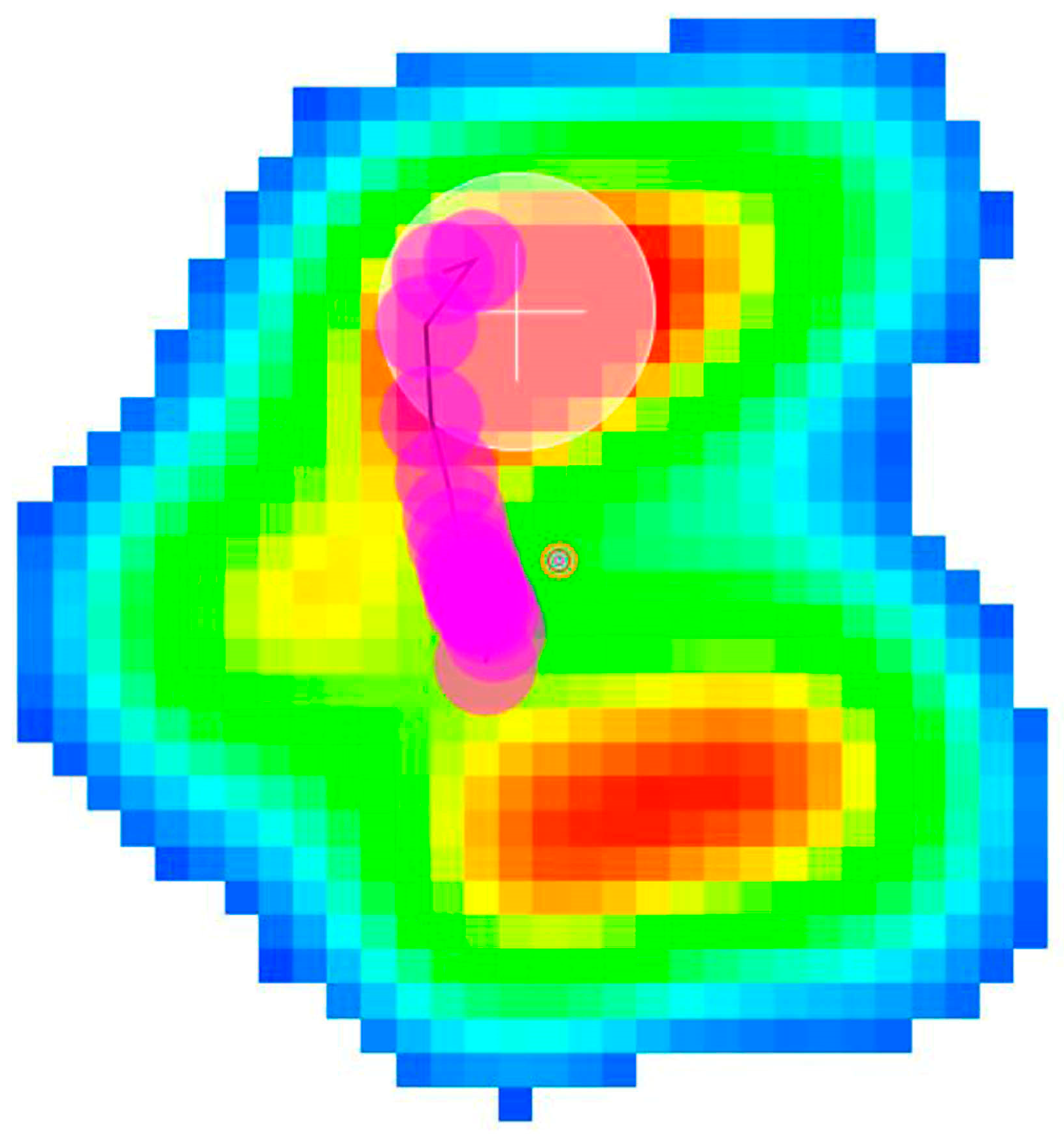
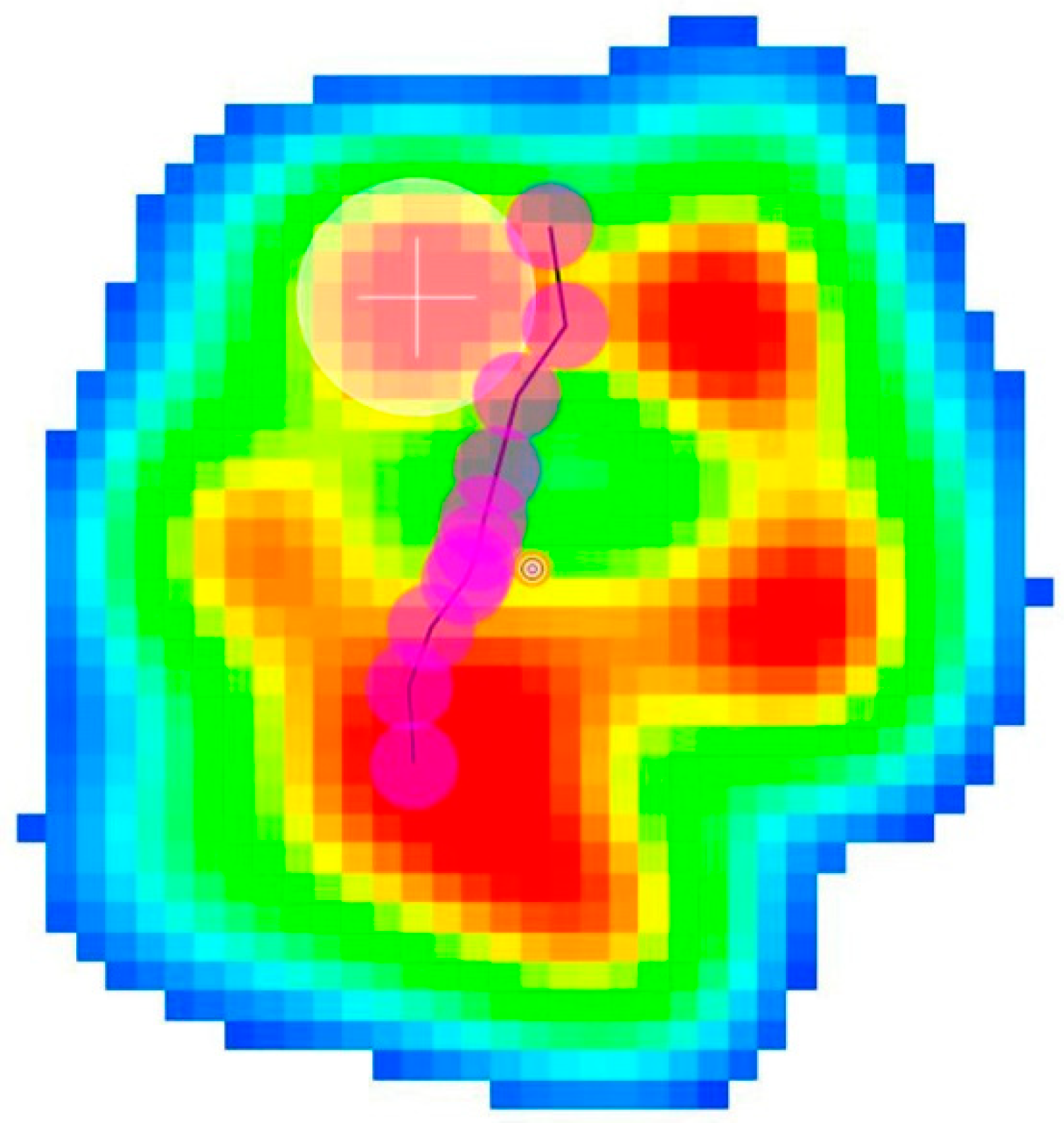
2.2. Force and Pressure Platform Analysis
2.2.1. Force Platform Analysis
2.2.2. Pressure Platform Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, L. Compensatory mechanisms in a dog after hind leg amputation. J. Small Anim. Pract. 1970, 11, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Anders, A.; Nolte, I.; Schilling, N. Limb and back muscle activity adaptations to tripedal locomotion in dogs. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2015, 323, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Zamora, V.; von Babo, V.; Eberle, N.; Betz, D.; Nolte, I.; Wefstaedt, P. Kinetic, kinematic, magnetic resonance and owner evaluation of dogs before and after the amputation of a hind limb. BMC Vet. Res. 2016, 12, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carberry, C.A.; Harvey, H.J. Owner satisfaction with limb amputation in dogs and cats. J. Am. Anim. Hosp. Assoc. 1987, 23, 227–232. [Google Scholar]
- Withrow, S.J.; Hirsch, V.M. Owner response to amputation of a pet’s leg. Vet. Med. Small Anim. Clin. 1979, 74, 332–334. [Google Scholar] [PubMed]
- Kirpensteijn, J.; Van den Bos, R.; Endenburg, N. Adaptation of dogs to the amputation of a limb and their owners’ satisfaction with the procedure. Vet. Rec. 1999, 144, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, R.; Dycus, D.; Levine, D.; Arruda, A.G.; Fagan, N.; Marcellin-Little, D. Stance and weight distribution after tibial plateau leveling osteotomy in fore limb and hind limb amputee dogs. BMC Vet. Res. 2020, 16, 188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raske, M.; McClaran, J.K.; Mariano, A. Short-term wound complications and predictive variables for complication after limb amput, ation in dogs and cats. J. Sm. Anim. Pract. 2015, 56, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Goldner, B.; Fuchs, A.; Nolte, I.; Schilling, N. Kinematic adaptations to tripedal locomotion in dogs. Vet. J. 2015, 204, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kirpensteijn, J.; van den Bos, R.; van den Brom, W.E.; Hazewinkel, H.A. Ground reaction force analysis of large breed dogs when walking after the amputation of a limb. Vet. Rec. 2000, 146, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.L.; Worley, D.R.; Hogy, S.M.; Hill, A.E.; Haussler, K.K.; Reiser, R.F., 2nd. Kinematic and kinetic analysis of dogs during trotting after amputation of a thoracic limb. Am. J. Vet. Res. 2013, 74, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Goldner, B.; Nolte, I.; Schilling, N. Ground reaction force adaptations to tripedal locomotion in dogs. Vet. J. 2014, 201, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Millis, D.L.; Levine, D.; Taylor, R.A. Canine Rehabilitation and Physical Therapy, 2nd ed.; Saunders: Philadelphia, PA, USA, 2004; pp. 201–210. [Google Scholar]
- Vail, D.M.; Hamm, D.H.; Liptak, J.M. Withrow and MacEwen’s Small Animal Clinical Oncology, 4th ed.; Saunders: Philadelphia, PA, USA, 2007; pp. 551–572. [Google Scholar]
- Johnston, S.A.; Tobias, K.M. Veterinary Surgery: Small Animal, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1210–1224. [Google Scholar]
- Bøaszczyk, J.W.; Lowe, D.L.; Hansen, P.D. Ranges of postural stability and their changes in the elderly. Gait Posture 1994, 2, 11–17. [Google Scholar] [CrossRef]
- Charalambous, D.; Lutonsky, C.; Keider, S.; Tichy, A.; Bockstahler, B. Vertical ground reaction forces, paw pressure distribution, and center of pressure during heelwork in working dogs competing in obedience. Front. Vet. Sci. 2023, 10, 1106170. [Google Scholar] [CrossRef] [PubMed]
- Virag, Y.; Gumpenberger, M.; Tichy, A.; Lutonsky, C.; Peham, C.; Bockstahler, B. Center of pressure and ground reaction forces in Labrador and Golden Retrievers with and without hip dysplasia at 4, 8, and 12 months of age. Vet. Sci. 2022, 9, 1087693. [Google Scholar] [CrossRef] [PubMed]
- Pitti, L.; Oosterlinck, M.; Díaz-Bertrana, M.L.; Carrillo, J.M.; Rubio, M.; Sopena, J.; Santana, A.; Vilar, J.M. Assessment of static posturography and pedobarography for the detection of unilateral forelimb lameness in ponies. BMC Vet. Res. 2018, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.M.; Manera, M.E.; Rubio, M.; Sopena, J.; Santana, A.; Vilar, J.M. Posturography and dynamic pedobarography in lame dogs with elbow dysplasia and cranial cruciate ligament rupture. Vet. Res. 2018, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 14 June 2024).
- Weigel, J.P.; Slatter, D. Textbook of Small Animal Surgery, 3rd ed.; Saunders: Philadelphia, PA, USA, 2003; pp. 2180–2190. [Google Scholar]
- Dernell, W.S.; Ehrhart, N.P.; Straw, R.C. Tumors of the skeletal system. In Withrow & MacEwen’s Small Animal Clinicial Oncology, 4th ed.; Withrow, S.J., Vail, D.M., Eds.; Saunders Elsevier: St Louis, MO, USA, 2007; pp. 540–582. [Google Scholar]
- Brodey, R.S.; Abt, D.A. Results of surgical treatment in 65 dogs with osteosarcoma. J. Am. Vet. Med. Assoc. 1976, 168, 1032–1035. [Google Scholar]
- Straw, R.C.; Withrow, S.J. Limb-sparing surgery versus amputation for dogs with bone tumors. Vet. Clin. N. Am. Small Anim. Pract. 1996, 26, 135–143. [Google Scholar]
- Budsberg, S.C. Amputations. In Small Animal Orthopedics; Olmstead, M.L., Ed.; Mosby: St Louis, MO, USA, 1995; pp. 531–548. [Google Scholar]
- Grabowski, A.M.; Kram, R. Effects of velocity and weight support on ground reaction forces and metabolic power during running. J. Appl. Biomech. 2008, 24, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Roush, J.K.; McLaughlin, R.M. Effects of subject stance time and velocity on ground reaction forces in clinically normal Greyhounds at the walk. Am. J. Vet. Res. 1994, 55, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, R.M.J.; Roush, J.K. Effects of subject stance time and velocity on ground reaction forces in clinically normal greyhounds at the trot. Am. J. Vet. Res. 1994, 55, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Budsberg, S.C.; Verstraete, M.C.; Soutas-Little, R.W. Force plate analysis of the walling gait in healthy dogs. Am. J. Vet. Res. 1987, 48, 915–918. [Google Scholar] [PubMed]
- Cole, G.L.; Millis, D. The effect of limb amputation on standing weight distribution in the remaining three limbs in dogs. Vet. Comp. Orthop. Traumatol. 2017, 30, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Hogy, S.M.; Worley, D.R.; Jarvis, S.L.; Hill, A.E.; Reiser, R.F., 2nd; Haussler, K.K. Kinematic and kinetic analysis of dogs during trotting after amputation of a pelvic limb. Am. J. Vet. Res. 2013, 74, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.E.; Carrillo, J.M.; Batista, M.; Rubio, M.; Sopena, J.; Santana, A. Static Posturography: A New Perspective in the Assessment of Lameness in a Canine Model. PLoS ONE 2017, 12, e0170692. [Google Scholar] [CrossRef]
- Della Valle, G.; Caterino, C.; Aragosa, F.; Balestriere, C.; Piscitelli, A.; Di Palma, C.; Pasolini, M.P.; Fatone, G. Relationship between ground reaction forces and morpho- metric measures in two different canine phenotypes using regression analysis. Vet. Sci. 2022, 9, 325. [Google Scholar] [CrossRef]



| Force Values | Sound | Amputee | p-Value |
|---|---|---|---|
| Fvmax | 99.35 ± 3.63 | 182.77 ± 10.59 | 0.0018 * |
| VIv | 15.86 ± 1.81 | 28.06 ± 0.58 | 0.0028 * |
| Fcrmax | 9.60 ± 1.68 | 25.31 ± 0.75 | 0.0004 * |
| VIcr | 0.74 ± 0.31 | 2.49 ± 0.15 | 0.0028 * |
| Fcamax | 5.21 ± 0.87 | 12.11 ± 2.21 | 0.0740 |
| VIca | 1.00 ± 0.20 | 1.16 ± 0.16 | 0.5672 |
| Stat | 0.23 ± 0.03 | 0.23 ± 0.05 | 0.9267 |
| PP | 215.8 ± 5.07 | 292.4 ± 10.16 | 0.0180 * |
| MP | 78 ± 0.71 | 82.50 ± 3.26 | 0.0740 |
| PA | 14.50 ± 2.38 | 20.00 ± 1.00 | 0.0632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, O.; Regueiro-Purriños, M.; Figueirinhas, P.; Gonzalo-Orden, J.M.; Prada, I.; Vilar, J.M.; Millán, L.; Rodríguez-Altónaga, J. Dynamic and Postural Changes in Forelimb Amputee Dogs: A Pilot Study. Animals 2024, 14, 1960. https://doi.org/10.3390/ani14131960
Rodriguez O, Regueiro-Purriños M, Figueirinhas P, Gonzalo-Orden JM, Prada I, Vilar JM, Millán L, Rodríguez-Altónaga J. Dynamic and Postural Changes in Forelimb Amputee Dogs: A Pilot Study. Animals. 2024; 14(13):1960. https://doi.org/10.3390/ani14131960
Chicago/Turabian StyleRodriguez, Oliver, Marta Regueiro-Purriños, Pedro Figueirinhas, José Manuel Gonzalo-Orden, Iván Prada, José Manuel Vilar, Lorena Millán, and José Rodríguez-Altónaga. 2024. "Dynamic and Postural Changes in Forelimb Amputee Dogs: A Pilot Study" Animals 14, no. 13: 1960. https://doi.org/10.3390/ani14131960





