Feline Cognition and the Role of Nutrition: An Evolutionary Perspective and Historical Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Evolutionary History of Cats
2.1. Overview of the Feline Lineage
2.2. Social and Behavioral Characteristics
2.3. Nutritional Considerations
3. Cat Domestication
3.1. History of Domestication
3.2. Sociality in Domesticated Cats Versus Other Cat Species
4. Cognitive Function in Cats: An Overview
4.1. The Cat Brain
4.2. Perception
4.3. Physical Cognition and Working Memory
4.4. Socialization and Early Cat–Human Interactions
4.5. Cooperative–Communicative Cue Following
4.6. Trainability
4.7. Vocal Communication
4.8. Social Referencing and Sensitivity to Human Emotion
4.9. Attachment to Humans
4.10. Personality
4.11. Emotion and Mental Experiences
4.12. Stress
4.13. Aging
5. Nutrition, Behavior, and Cognition
5.1. Food Preferences
5.2. Impact of Diet on Behavior
5.3. Dietary Management of CDS: An Overview
5.4. Role of Antioxidants
5.5. Role of Omega-3 Fatty Acids
5.6. Specific Diets Evaluated in Aging Cats
5.7. Other Nutraceuticals and Functional Ingredients for CDS
5.8. Drug Therapy for CDS
5.9. Opportunities in Feline Cognition and Nutrition
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merola, I.; Lazzaroni, M.; Marshall-Pescini, S.; Prato-Previde, E. Social referencing and cat-human communication. Anim. Cogn. 2015, 18, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Udell, M.A.; Dorey, N.R.; Wynne, C.D. What did domestication do to dogs? A new account of dogs’ sensitivity to human actions. Biol. Rev. Camb. Philos. Soc. 2010, 85, 327–345. [Google Scholar] [PubMed]
- Vitale Shreve, K.R.; Udell, M.A. What’s inside your cat’s head? A review of cat (Felis silvestris catus) cognition research past, present and future. Anim. Cogn. 2015, 18, 1195–1206. [Google Scholar] [PubMed]
- Vitale Shreve, K.R.; Mehrkam, L.R.; Udell, M.A.R. Social interaction, food, scent or toys? A formal assessment of domestic pet and shelter cat (Felis silvestris catus) preferences. Behav. Process. 2017, 141 Pt 3, 322–328. [Google Scholar]
- Galvan, M.; Vonk, J. Man’s other best friend: Domestic cats (F. silvestris catus) and their discrimination of human emotion cues. Anim. Cogn. 2016, 19, 193–205. [Google Scholar] [PubMed]
- Gow, E.A.; Burant, J.B.; Sutton, A.O.; Freeman, N.E.; Grahame, E.R.; Fuirst, M.; Sorensen, M.C.; Knight, S.M.; Clyde, H.E.; Quarrell, N.J.; et al. Popular press portrayal of issues surrounding free-roaming domestic cats Felis catus. People Nat. 2022, 4, 143–154. [Google Scholar] [CrossRef]
- Lawrence, E.A. Feline Fortunes: Contrasting Views of Cats in Popular Culture. J. Pop. Cult. 2003, 36, 623–635. [Google Scholar] [CrossRef]
- Serpell, J.A. Domestication and history of the cat. In The Domestic Cat: The Biology of Its Behaviour; Bateson, P., Turner, D.C., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 83–100. [Google Scholar]
- Stromberg, J. What Research Says About Cats: They’re Selfish, Unfeeling, Environmentally Harmful Creatures. Vox 2014. Available online: http://www.vox.com/2014/10/16/6982177/the-case-against-owningcats (accessed on 8 July 2023).
- McComb, K.; Taylor, A.M.; Wilson, C.; Charlton, B.D. The cry embedded within the purr. Curr. Biol. 2009, 19, R507–R508. [Google Scholar] [CrossRef] [PubMed]
- Heath, S. Feline behavioral medicine--an important veterinary discipline. Adv. Small Anim. Care 2022, 3, 13–22. [Google Scholar] [CrossRef]
- Shettleworth, S.J. Animal cognition and animal behaviour. Anim. Behav. 2001, 61, 277–286. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Johnson, W.E. Big cat genomics. Annu. Rev. Genom. Hum. Genet. 2005, 6, 407–429. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.J.; Eizirik, E.; Johnson, W.E.; Zhang, Y.P.; Ryder, O.A.; O’Brien, S.J. Molecular phylogenetics and the origins of placental mammals. Nature 2001, 409, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.J.; Eizirik, E.; O’Brien, S.J.; Madsen, O.; Scally, M.; Douady, C.J.; Teeling, E.; Ryder, O.A.; Stanhope, M.J.; de Jong, W.W.; et al. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science 2001, 294, 2348–2351. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.; Dunbar, R. Encephalization is not a universal macroevolutionary phenomenon in mammals but is associated with sociality. Proc. Natl. Acad. Sci. USA 2010, 107, 21582–21586. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.J.; Johnson, W.; Driscoll, C.; Pontius, J.; Pecon-Slattery, J.; Menotti-Raymond, M. State of cat genomics. Trends Genet. 2008, 24, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.A.; Menotti-Raymond, M.; Roca, A.L.; Hupe, K.; Johnson, W.E.; Geffen, E.; Harley, E.H.; Delibes, M.; Pontier, D.; Kitchener, A.C.; et al. The Near Eastern origin of cat domestication. Science 2007, 317, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Kitchener, A.C.; Breitenmoser-Wursten, C.; Eizirik, E.; Gentry, A.; Werdelin, L.; Wilting, A.; Yamaguchi, N.; Abramov, A.V.; Christiansen, P.; Driscoll, C.A.; et al. A revised taxonomy of the Felidae: The final report of the Cat Classification Task Force of the IUCN Cat Specialist Group. In Cat News; Cat Specialist Group: Muri, Switzerland, 2017; Volume 11, p. 80. [Google Scholar]
- Macdonald, D.; Yamaguchi, N.; Kerby, G. Group-living in the Domestic Cat: Its Sociobiology and Epidemiology. Domest. Cat Biol. Its Behav. 2000, 2, 95–118. [Google Scholar]
- Bradshaw, J.W.S. Sociality in cats: A comparative review. J. Vet. Behav. 2016, 11, 113–124. [Google Scholar] [CrossRef]
- Gartner, M.C.; Powell, D.M.; Weiss, A. Personality structure in the domestic cat (Felis silvestris catus), Scottish wildcat (Felis silvestris grampia), clouded leopard (Neofelis nebulosa), snow leopard (Panthera uncia), and African lion (Panthera leo): A comparative study. J. Comp. Psychol. 2014, 128, 414–426. [Google Scholar] [CrossRef]
- Caro, T. Determinants of asociality in felids. In Comparative Socioecology: The Behavioural Ecology of Humans and Other Mammals; Standen, V., Foley, R., Eds.; Blackwell Scientific Publications: Oxford, UK, 1989; p. 519. [Google Scholar]
- Morris, J.G. Idiosyncratic nutrient requirements of cats appear to be diet-induced evolutionary adaptations. Nutr. Res. Rev. 2002, 15, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.W.; Goodwin, D.; Legrand-Defretin, V.; Nott, H.M. Food selection by the domestic cat, an obligate carnivore. Comp. Biochem. Physiol. A Physiol. 1996, 114, 205–209. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Fantle-Lepczyk, J.E.; Dunham, K.D.; Bonnaud, E.; Lindner, J.; Doherty, T.S.; Woinarski, J.C. A global synthesis and assessment of free-ranging domestic cat diet. Nat. Commun. 2023, 14, 7809. [Google Scholar] [CrossRef]
- Zoran, D.L. The carnivore connection to nutrition in cats. J. Am. Vet. Med. Assoc. 2002, 221, 1559–1567. [Google Scholar] [CrossRef]
- Armstrong, P.J.; Gross, K.L.; Becvarova, I.; Debraekeleer, J. Introduction to feeding normal cats. In Small Animal Clinical Nutrition; Hand, M.S., Thatcher, C.D., Remillard, R.L., Roudebush, P., Novotny, B.J., Eds.; Mark Morris Institute: Topeka, KS, USA, 2010; Volume 5, pp. 361–372. [Google Scholar]
- Knopf, K.; Sturman, J.A.; Armstrong, M.; Hayes, K.C. Taurine: An essential nutrient for the cat. J. Nutr. 1978, 108, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.G.; Rogers, Q.R. Ammonia intoxication in the near-adult cat as a result of a dietary deficiency of arginine. Science 1978, 199, 431–432. [Google Scholar] [CrossRef]
- Morris, J.G.; Rogers, Q.R.; Winterrowd, D.L.; Kamikawa, E.M. The utilization of ornithine and citrulline by the growing kitten. J. Nutr. 1979, 109, 724–729. [Google Scholar] [CrossRef]
- Boudreau, J.C. Neurophysiology and human taste sensations. J. Sens. Stud. 1986, 1, 185–202. [Google Scholar] [CrossRef]
- Bradshaw, J.W. The evolutionary basis for the feeding behavior of domestic dogs (Canis familiaris) and cats (Felis catus). J. Nutr. 2006, 136 (Suppl. 7), 1927s–1931s. [Google Scholar] [CrossRef]
- Green, A.S.; Tang, G.; Lango, J.; Klasing, K.C.; Fascetti, A.J. Domestic cats convert [2H8]-β-carotene to [2H4]-retinol following a single oral dose. J. Anim. Physiol. Anim. Nutr. 2012, 96, 681–692. [Google Scholar] [CrossRef]
- Hurst, E.A.; Homer, N.Z.; Mellanby, R.J. Vitamin D Metabolism and Profiling in Veterinary Species. Metabolites 2020, 10, 371. [Google Scholar] [CrossRef]
- Kienzle, E. Carbohydrate metabolism of the cat 1. Activity of amylase in the gastrointestinal tract of the cat1. J. Anim. Physiol. Anim. Nutr. 1993, 69, 92–101. [Google Scholar] [CrossRef]
- Verbrugghe, A.; Bakovic, M. Peculiarities of one-carbon metabolism in the strict carnivorous cat and the role in feline hepatic lipidosis. Nutrients 2013, 5, 2811–2835. [Google Scholar] [CrossRef] [PubMed]
- Verbrugghe, A.; Hesta, M. Cats and Carbohydrates: The Carnivore Fantasy? Vet. Sci. 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D.P.; Backus, R.C.; Forrester, S.D.; Hoenig, M. Evidence does not support the controversy regarding carbohydrates in feline diets. J. Am. Vet. Med. Assoc. 2022, 260, 506–513. [Google Scholar] [CrossRef]
- Cucchi, T.; Vigne, J.D. Origin and diffusion of the house mouse in the Mediterranean. Hum. Evol. 2006, 21, 95–106. [Google Scholar] [CrossRef]
- Bradshaw, J. Normal feline behaviour: … and why problem behaviours develop. J. Feline Med. Surg. 2018, 20, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Rodan, I.; Heath, S. Feline behavior and welfare. In Feline Behavioral Health and Welfare; Elsevier: St.Louis, MO, USA, 2015. [Google Scholar]
- Vigne, J.D.; Guilaine, J.; Debue, K.; Haye, L.; Gérard, P. Early taming of the cat in Cyprus. Science 2004, 304, 259. [Google Scholar] [CrossRef]
- Málek, J. The Cat in Ancient Egypt; British Museum Press: London, UK, 2006. [Google Scholar]
- Bradshaw, J. Behaviour of cats. In The Ethology of Domestic Animals—An Introductory Text; CABI: Wallingford, UK, 2017; pp. 241–254. [Google Scholar]
- Smits, M.; Joonsten, H.; Faye, B.; Burger, P. Domestication of the Dromedary Revisited and Its Consequences for Legislation as to Keeping Livestock or Pet Animals. Animals 2023, 13, 2050. [Google Scholar] [CrossRef]
- Crowell-Davis, S.L.; Barry, K.; Wolfe, R. Social behavior and aggressive problems of cats. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 549–568. [Google Scholar] [CrossRef]
- Vitale, K.R. The Social Lives of Free-Ranging Cats. Animals 2022, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Crowell-Davis, S.L. Understanding cats. Compend. Contin. Educ. Vet. 2007, 29, 241–243. [Google Scholar] [PubMed]
- Curtis, T.M.; Knowles, R.J.; Crowell-Davis, S.L. Influence of familiarity and relatedness on proximity and allogrooming in domestic cats (Felis catus). Am. J. Vet. Res. 2003, 64, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.W.; Apps, P.J.; Carr, G.M.; Kerby, G. Social dynamics, nursing coalitions and infanticide among farm cats (Felis catus). Ethology 1987, 28, 66. [Google Scholar]
- Denny, E.A.; Yakovlevich, P.; Eldridge, M.D.; Dickman, C. Social and genetic analysis of a population of free-living cats (Felis catus L.) exploiting a resource-rich habitat. Wildl. Res. 2002, 29, 405–413. [Google Scholar]
- Bradshaw, J.; Casey, R.; Brown, S. The Behaviour of the Domestic Cat, 2nd ed.; CABI: Wallingford, UK, 2012. [Google Scholar]
- Bradshaw, J.; Cameron-Beaumont, C. The signaling repertoire of the domestic cat and its undomesticated relatives. In The Domestic Cat: The Biology of Its Behaviour; Turner, D.C., Bateson, P.P.G., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2000. [Google Scholar]
- Clutton-Brock, J. The process of domestication. Mammal Rev. 1992, 22, 79–85. [Google Scholar] [CrossRef]
- Wilkins, A.S.; Wrangham, R.W.; Fitch, W.T. The “Domestication Syndrome” in Mammals: A Unified Explanation Based on Neural Crest Cell Behavior and Genetics. Genetics 2014, 197, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Coppinger, R.; Schneider, R. Evolution of working dogs. In The Domestic Dog-Its Evolution, Behaviour and Interactions with People; Serpell, J., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 22–47. [Google Scholar]
- Trut, L.; Oskina, I.; Kharlamova, A. Animal evolution during domestication: The domesticated fox as a model. Bioessays 2009, 31, 349–360. [Google Scholar] [CrossRef]
- Lord, K.A.; Larson, G.; Coppinger, R.P.; Karlsson, E.K. The History of Farm Foxes Undermines the Animal Domestication Syndrome. Trends Ecol. Evol. 2020, 35, 125–136. [Google Scholar] [CrossRef]
- Hare, B. Survival of the Friendliest: Homo sapiens Evolved via Selection for Prosociality. Annu. Rev. Psychol. 2017, 68, 155–186. [Google Scholar] [CrossRef]
- Berns, G.S.; Brooks, A.M.; Spivak, M. Functional MRI in awake unrestrained dogs. PLoS ONE 2012, 7, e38027. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Qian, C.; Li, Y.; Zuo, Z.; Liu, Z. Setup and data analysis for functional magnetic resonance imaging of awake cat visual cortex. Neurosci. Bull. 2013, 29, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Herculano-Houzel, S.; Collins, C.E.; Wong, P.; Kaas, J.H. Cellular scaling rules for primate brains. Proc. Natl. Acad. Sci. USA 2007, 104, 3562–3567. [Google Scholar] [CrossRef] [PubMed]
- Herculano-Houzel, S. Neuronal scaling rules for primate brains: The primate advantage. Prog. Brain. Res. 2012, 195, 325–340. [Google Scholar]
- Jardim-Messeder, D.; Lambert, K.; Noctor, S.; Pestana, F.M.; de Castro Leal, M.E.; Bertelsen, M.F.; Alagaili, A.N.; Mohammad, O.B.; Manger, P.R.; Herculano-Houzel, S. Dogs Have the Most Neurons, Though Not the Largest Brain: Trade-Off between Body Mass and Number of Neurons in the Cerebral Cortex of Large Carnivoran Species. Front. Neuroanat. 2017, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, J.; Hanus, D.; Pika, S.; Gray, R.; Uomini, N. Old and New Approaches to Animal Cognition: There Is Not “One Cognition”. J. Intell. 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, L.; Bautista, A.; González, D.; Hudson, R. Smell, Suck, Survive: Chemical Signals and Suckling in the Rabbit, Cat, and Dog. Chem. Signals Vertebr. 2013, 12, 51–59. [Google Scholar]
- Raihani, G.; González, D.; Arteaga, L.; Hudson, R. Olfactory guidance of nipple attachment and suckling in kittens of the domestic cat: Inborn and learned responses. Dev. Psychobiol. 2009, 51, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Natoli, E. Behavioural responses of urban feral cats to different types of urine marks. Behaviour 1985, 94, 234–243. [Google Scholar] [CrossRef]
- Verberne, G.; de Boer, J. Chemocommunication among domestic cats, mediated by the olfactory and vomeronasal senses: I. Chemocommunication. Z Tierpsychol. 1976, 42, 86–109. [Google Scholar] [CrossRef]
- Feldman, H.N. Methods of scent marking in the domestic cat. Can. J. Zool. 1994, 72, 1093–1099. [Google Scholar] [CrossRef]
- Mills, D.S.; Redgate, S.E.; Landsberg, G.M. A meta-analysis of studies of treatments for feline urine spraying. PLoS ONE 2011, 6, e18448. [Google Scholar] [CrossRef]
- Pageat, P.; Gaultier, E. Current research in canine and feline pheromones. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Montague, M.J.; Li, G.; Gandolfi, B.; Khan, R.; Aken, B.L.; Searle, S.M.J.; Minx, P.; Hillier, L.W.; Koboldt, D.C.; Davis, B.W.; et al. Comparative analysis of the domestic cat genome reveals genetic signatures underlying feline biology and domestication. Proc. Natl. Acad. Sci. USA 2014, 111, 17230–17235. [Google Scholar] [CrossRef] [PubMed]
- Vitale Shreve, K.R.; Udell, M.A.R. Stress, security, and scent: The influence of chemical signals on the social lives of domestic cats and implications for applied settings. Appl. Anim. Behav. Sci. 2017, 187, 69–76. [Google Scholar] [CrossRef]
- Tirindelli, R. Coding of pheromones by vomeronasal receptors. Cell Tissue Res. 2021, 383, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Dumas, C.; Doré, F.Y. Cognitive development in kittens (Felis catus): An observational study of object permanence and sensorimotor intelligence. J. Comp. Psychol. 1991, 105, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Fiset, S.; Doré, F.Y. Duration of cats’ (Felis catus) working memory for disappearing objects. Anim. Cogn. 2006, 9, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Shajid Pyari, M.; Vékony, K.; Uccheddu, S.; Pongrácz, P. Companion Cats Show No Effect of Trial-and-Error Learning Compared to Dogs in a Transparent-Obstacle Detour Task. Animals 2022, 13, 32. [Google Scholar] [CrossRef]
- Doré, F.Y. Search behaviour of cats (Felis catus) in an invisible displacement test: Cognition and experience. Can J. Psychol. 1990, 44, 359–370. [Google Scholar] [CrossRef]
- Dumas, C. Object permanence in cats (Felis catus): An ecological approach to the study of invisible displacements. J. Comp. Psychol. 1992, 106, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Goulet, S.; Doré, F.Y.; Lehotkay, R. Activation of locations in working memory in cats. Q. J. Exp. Psychol. B 1996, 49, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Okujava, V.; Natishvili, T.; Mishkin, M.; Gurashvili, T.; Chipashvili, S.; Bagashvili, T.; Andronikashvili, G.; Kvernadze, G. One-trial visual recognition in cats. Acta Neurobiol. Exp. 2005, 65, 205–211. [Google Scholar] [CrossRef]
- Pisa, P.E.; Agrillo, C. Quantity discrimination in felines: A preliminary investigation of the domestic cat (Felis silvestris catus). J. Ethol. 2009, 27, 289–293. [Google Scholar] [CrossRef]
- Rosenkilde, C.E.; Divac, I. Discrimination of time intervals in cats. Acta Neurobiol. Exp. 1976, 36, 311–317. [Google Scholar]
- Whitt, E.; Douglas, M.; Osthaus, B.; Hocking, I. Domestic cats (Felis catus) do not show causal understanding in a string-pulling task. Anim. Cogn. 2009, 12, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Collard, R.R. Fear of strangers and play behavior in kittens with varied social experience. Child. Dev. 1967, 38, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Karsh, E.B.; Turner, D.C. The human-cat relationship. In The Domestic Cat: The Biology of Its Behaviour; Turner, D.C., Bateson, P.P.G., Eds.; Cambridge University Press Cambridge: Cambridge, UK, 1988. [Google Scholar]
- Lowe, S.; Bradshaw, J. Responses of pet cats to being held by an unfamiliar person, from weaning to three years of age. Anthrozoos A Multidiscip. J. Interact. People Anim. 2002, 15, 69–79. [Google Scholar] [CrossRef]
- McCune, S. The impact of paternity and early socialisation on the development of cats’ behaviour to people and novel objects. Appl. Anim. Behav. Sci. 1995, 45, 109–124. [Google Scholar] [CrossRef]
- Howell, T.J.; King, T.; Bennett, P.C. Puppy parties and beyond: The role of early age socialization practices on adult dog behavior. Vet. Med. Res. Rep. 2015, 6, 143–153. [Google Scholar] [CrossRef]
- Meier, G.W. Infantile handling and development in Siamese kittens. J. Comp. Physiol. Psychol. 1961, 54, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, M.; Carpenter, M. Shared intentionality. Dev. Sci. 2007, 10, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Miklósi, A.; Pongrácz, P.; Lakatos, G.; Topál, J.; Csányi, V. A comparative study of the use of visual communicative signals in interactions between dogs (Canis familiaris) and humans and cats (Felis catus) and humans. J. Comp. Psychol. 2005, 119, 179–186. [Google Scholar] [CrossRef]
- Bray, E.E.; Gnanadesikan, G.E.; Horschler, D.J.; Levy, K.M.; Kennedy, B.S.; Famula, T.R.; MacLean, E.L. Early-emerging and highly heritable sensitivity to human communication in dogs. Curr. Biol. 2021, 31, 3132–3136.e5. [Google Scholar] [CrossRef]
- Bray, E.E.; Gruen, M.E.; Gnanadesikan, G.E.; Horschler, D.J.; Levy, K.M.; Kennedy, B.S.; Hare, B.A.; MacLean, E.L. Dog cognitive development: A longitudinal study across the first 2 years of life. Anim. Cogn. 2021, 24, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Salomons, H.; Smith, K.C.M.; Callahan-Beckel, M.; Callahan, M.; Levy, K.; Kennedy, B.S.; Bray, E.E.; Gnanadesikan, G.E.; Horschler, D.J.; Gruen, M.; et al. Cooperative Communication with Humans Evolved to Emerge Early in Domestic Dogs. Curr. Biol. 2021, 31, 3137–3144.e11. [Google Scholar] [CrossRef] [PubMed]
- Virányi, Z.; Gácsi, M.; Kubinyi, E.; Topál, J.; Belényi, B.; Ujfalussy, D.; Miklósi, Á. Comprehension of human pointing gestures in young human-reared wolves (Canis lupus) and dogs (Canis familiaris). Anim. Cogn. 2008, 11, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Hare, B.; Brown, M.; Williamson, C.; Tomasello, M. The domestication of social cognition in dogs. Science 2002, 298, 1634–1636. [Google Scholar] [CrossRef] [PubMed]
- MacLean, E.L.; Herrmann, E.; Suchindran, S.; Hare, B. Individual differences in cooperative communicative skills are more similar between dogs and humans than chimpanzees. Anim. Behav. 2017, 126, 41–51. [Google Scholar] [CrossRef]
- Pongrácz, P.; Szapu, J.S.; Faragó, T. Cats (Felis silvestris catus) read human gaze for referential information. Intelligence 2019, 74, 43–52. [Google Scholar] [CrossRef]
- Itakura, S. Gaze-following and joint visual attention in nonhuman animals. Jpn. Psychol. Res. 2004, 46, 216–226. [Google Scholar] [CrossRef]
- Téglás, E.; Gergely, A.; Kupán, K.; Miklósi, Á.; Topál, J. Dogs’ gaze following is tuned to human communicative signals. Curr. Biol. 2012, 22, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.; Ellis, S. The Trainable Cat: A Practical Guide to Making Life Happier for You and Your Cat; Basic Books: New York, NY, USA, 2016. [Google Scholar]
- Kogan, L.; Kolus, C.; Schoenfeld-Tacher, R. Assessment of Clicker Training for Shelter Cats. Animals 2017, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Feuerbacher, E.N.; Wynne, C.D. Most domestic dogs (Canis lupus familiaris) prefer food to petting: Population, context, and schedule effects in concurrent choice. J. Exp. Anal. Behav. 2014, 101, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Willson, E.K.; Stratton, R.B.; Bolwell, C.F.; Stafford, K.J. Comparison of positive reinforcement training in cats: A pilot study. J. Vet. Behav. 2017, 21, 64–70. [Google Scholar] [CrossRef]
- Chiandetti, C.; Avella, S.; Fongaro, E.; Cerri, F. Can clicker training facilitate conditioning in dogs? Appl. Anim. Behav. Sci. 2016, 184, 109–116. [Google Scholar] [CrossRef]
- Feng, L.C.; Hodgens, N.H.; Woodhead, J.K.; Howell, T.J.; Bennett, P.C. Is clicker training (clicker + food) better than food-only training for novice companion dogs and their owners? Appl. Anim. Behav. Sci. 2018, 204, 81–93. [Google Scholar] [CrossRef]
- Smith, S.M.; Davis, E.S. Clicker increases resistance to extinction but does not decrease training time of a simple operant task in domestic dogs (Canis familiaris). Appl. Anim. Behav. Sci. 2008, 110, 318–329. [Google Scholar] [CrossRef]
- Saito, A.; Shinozuka, K. Vocal recognition of owners by domestic cats (Felis catus). Anim. Cogn. 2013, 16, 685–690. [Google Scholar] [CrossRef]
- Saito, A.; Shinozuka, K.; Ito, Y.; Hasegawa, T. Domestic cats (Felis catus) discriminate their names from other words. Sci. Rep. 2019, 9, 5394. [Google Scholar] [CrossRef]
- de Mouzon, C.; Gonthier, M.; Leboucher, G. Discrimination of cat-directed speech from human-directed speech in a population of indoor companion cats (Felis catus). Anim. Cogn. 2023, 26, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aderet, T.; Gallego-Abenza, M.; Reby, D.; Mathevon, N. Dog-directed speech: Why do we use it and do dogs pay attention to it? Proc. Biol. Sci. 2017, 284, 20162429. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.P.; Aslin, R.N. Preference for infant-directed speech in the first month after birth. Child. Dev. 1990, 61, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Watanabe, A.; Takagi, S.; Arahori, M.; Saito, A. Cats beg for food from the human who looks at and calls to them: Ability to understand humans’ attentional states. Psychologia 2016, 59, 112–120. [Google Scholar] [CrossRef]
- Nicastro, N. Perceptual and acoustic evidence for species-level differences in meow vocalizations by domestic cats (Felis catus) and African wild cats (Felis silvestris lybica). J. Comp. Psychol. 2004, 118, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.C.; Kim, Y.K.; Park, S.J.; Lee, S.S.; Lee, S.Y.; Suh, E.H.; Houpt, K.A.; Chang, H.H.; Lee, H.C.; Yang, B.G.; et al. Differences between vocalization evoked by social stimuli in feral cats and house cats. Behav. Process. 2011, 87, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Finkler, H.; Hatna, E.; Terkel, J. The Impact of Anthropogenic Factors on the Behavior, Reproduction, Management and Welfare of Urban, Free-Roaming Cat Populations. Anthrozoös 2011, 24, 31–49. [Google Scholar] [CrossRef]
- Mumme, D.L.; Fernald, A.; Herrera, C. Infants’ responses to facial and vocal emotional signals in a social referencing paradigm. Child. Dev. 1996, 67, 3219–3237. [Google Scholar] [CrossRef] [PubMed]
- Merola, I.; Prato-Previde, E.; Marshall-Pescini, S. Dogs’ social referencing towards owners and strangers. PLoS ONE 2012, 7, e47653. [Google Scholar] [CrossRef]
- Merola, I.; Prato-Previde, E.; Marshall-Pescini, S. Social referencing in dog-owner dyads? Anim. Cogn. 2012, 15, 175–185. [Google Scholar] [CrossRef]
- Goodwin, D.; Bradshaw, J.W.S. Regulation of interactions between cats and humans by gaze and mutual gaze. In The International Society for Anthrozoology (ISAZ ’98) Abstracts; 1998; p. 5. [Google Scholar]
- Quaranta, A.; d’Ingeo, S.; Amoruso, R.; Siniscalchi, M. Emotion Recognition in Cats. Animals 2020, 10, 1107. [Google Scholar] [CrossRef] [PubMed]
- Rieger, G.; Turner, D.C. How Depressive Moods Affect the Behavior of Singly Living Persons Toward their Cats. Anthrozoös 1999, 12, 224–233. [Google Scholar] [CrossRef]
- Turner, D.C.; Rieger, G. Singly Living People and Their Cats: A Study of Human Mood and Subsequent Behavior. Anthrozoös 2001, 14, 38–46. [Google Scholar] [CrossRef]
- Ainsworth, M.D.; Bell, S.M. Attachment, exploration, and separation: Illustrated by the behavior of one-year-olds in a strange situation. Child. Dev. 1970, 41, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.R.; Behnke, A.C.; Udell, M.A.R. Attachment bonds between domestic cats and humans. Curr. Biol. 2019, 29, R864–R865. [Google Scholar] [CrossRef]
- Edwards, C.; Heiblum, M.; Tejeda, A.; Galindo, F. Experimental evaluation of attachment behaviors in owned cats. J. Vet. Behav.-Clin. Appl. Res. 2007, 2, 119–125. [Google Scholar] [CrossRef]
- Topál, J.; Gácsi, M.; Miklósi, Á.; Virányi, Z.; Kubinyi, E.; Csányi, V. Attachment to humans: A comparative study on hand-reared wolves and differently socialized dog puppies. Anim. Behav. 2005, 70, 1367–1375. [Google Scholar] [CrossRef]
- Schöberl, I.; Beetz, A.; Solomon, J.; Wedl, M.; Gee, N.; Kotrschal, K. Social factors influencing cortisol modulation in dogs during a strange situation procedure. J. Vet. Behav. 2016, 11, 77–85. [Google Scholar] [CrossRef]
- Wanser, S.H.; Udell, M.A.R. Does attachment security to a human handler influence the behavior of dogs who engage in animal assisted activities? Appl. Anim. Behav. Sci. 2019, 210, 88–94. [Google Scholar] [CrossRef]
- Schwartz, S. Separation anxiety syndrome in cats: 136 cases (1991–2000). J. Am. Vet. Med. Assoc. 2002, 220, 1028–1033. [Google Scholar] [CrossRef]
- de Souza Machado, D.; Oliveira, P.M.B.; Machado, J.C.; Ceballos, M.C.; Sant’Anna, A.C. Identification of separation-related problems in domestic cats: A questionnaire survey. PLoS ONE 2020, 15, e0230999. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.R.; Udell, M.A.R. The quality of being sociable: The influence of human attentional state, population, and human familiarity on domestic cat sociability. Behav. Process. 2019, 158, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Koski, S.E. Broader horizons for animal personality research. Front. Ecol. Evol. 2014, 2, 70. [Google Scholar] [CrossRef]
- Urrutia, A.; Bánszegi, O.; Szenczi, P.; Hudson, R. Development of “personality” in the domestic cat: A longitudinal study. Dev. Psychobiol. 2023, 65, e22427. [Google Scholar] [CrossRef] [PubMed]
- Beaver, B.V. Fractious cats and feline aggression. J. Feline Med. Surg. 2004, 6, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.C.; Rutter, N.J.; Woodhead, J.K.; Howell, T.J. Assessment of domestic cat personality, as perceived by 416 owners, suggests six dimensions. Behav. Process. 2017, 141 Pt 3, 273–283. [Google Scholar] [CrossRef]
- Casey, R.A.; Bradshaw, J.W.S. The effects of additional socialisation for kittens in a rescue centre on their behaviour and suitability as a pet. Appl. Anim. Behav. Sci. 2008, 114, 196–205. [Google Scholar] [CrossRef]
- Casey, R.A.; Vandenbussche, S.; Bradshaw, J.W.; Roberts, M.A. Reasons for Relinquishment and Return of Domestic Cats (Felis silvestris catus) to Rescue Shelters in the UK. Anthrozoös 2009, 22, 347–358. [Google Scholar] [CrossRef]
- Serpell, J.A. Evidence for an association between pet behavior and owner attachment levels. Appl. Anim. Behav. Sci. 1996, 47, 49–60. [Google Scholar] [CrossRef]
- Turner, D.C. Treating canine and feline behaviour problems and advising clients. Appl. Anim. Behav. Sci. 1997, 52, 199–204. [Google Scholar] [CrossRef]
- Feaver, J.; Mendl, M.; Bateson, P. A method for rating the individual distinctiveness of domestic cats. Anim. Behav. 1986, 34, 1016–1025. [Google Scholar] [CrossRef]
- Lowe, S.E.; Bradshaw, J.W. Ontogeny of individuality in the domestic cat in the home environment. Anim. Behav. 2001, 61, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.C.; Mertens, C.; Size, H.R. Overlap and Exploitation in Domestic Farm Cats (Felis catus). Behaviour 1986, 99, 22–45. [Google Scholar]
- Weiss, E.; Gramann, S.; Drain, N.; Dolan, E.; Slater, M. Modification of the Feline-Ality™ Assessment and the Ability to Predict Adopted Cats’ Behaviors in Their New Homes. Animals 2015, 5, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Finka, L.R. Conspecific and Human Sociality in the Domestic Cat: Consideration of Proximate Mechanisms, Human Selection and Implications for Cat Welfare. Animals 2022, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Leech, L.E.; Preziosi, R.; Stoycheva, R.; Pastorino, G.Q. The Effects of Owner and Domestic Cat (Felis catus) Demographics on Cat Personality Traits. Appl. Anim. Behav. Sci. 2022, 248, 105570. [Google Scholar] [CrossRef]
- Litchfield, C.A.; Quinton, G.; Tindle, H.; Chiera, B.; Kikillus, K.H.; Roetman, P. The ‘Feline Five’: An exploration of personality in pet cats (Felis catus). PLoS ONE 2017, 12, e0183455. [Google Scholar] [CrossRef] [PubMed]
- Raihani, G.; Rodríguez, A.; Saldaña, A.; Guarneros, M.; Hudson, R. A proposal for assessing individual differences in behaviour during early development in the domestic cat. Appl. Anim. Behav. Sci. 2014, 154, 48–56. [Google Scholar] [CrossRef]
- Travnik, I.D.C.; Machado, D.D.S.; Gonçalves, L.D.S.; Ceballos, M.C.; Sant’Anna, A.C. Temperament in Domestic Cats: A Review of Proximate Mechanisms, Methods of Assessment, Its Effects on Human-Cat Relationships, and One Welfare. Animals 2020, 10, 1516. [Google Scholar] [CrossRef]
- Ilska, J.; Haskell, M.J.; Blott, S.C.; Sánchez-Molano, E.; Polgar, Z.; Lofgren, S.E.; Clements, D.N.; Wiener, P. Genetic Characterization of Dog Personality Traits. Genetics 2017, 206, 1101–1111. [Google Scholar] [CrossRef]
- Reisner, I.R.; Houpt, K.A.; Erb, H.N.; Quimby, F.W. Friendliness to humans and defensive aggression in cats: The influence of handling and paternity. Physiol. Behav. 1994, 55, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Ha, J. A subjective domestic cat (Felis silvestris catus) temperament assessment results in six independent dimensions. Behav. Process. 2017, 141 Pt 3, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Finkler, D.; Terkel, J. Cortisol levels and aggression in neutered and intact free-roaming female cats living in urban social groups. Physiol. Behav. 2010, 99, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Calipari, S.; Guelfi, G.; Catanzaro, A.; Diverio, S. My Dog Is Not My Cat: Owner Perception of the Personalities of Dogs and Cats Living in the Same Household. Animals 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Templer, V.L.; Hampton, R.R. Episodic memory in nonhuman animals. Curr. Biol. 2013, 23, R801–R806. [Google Scholar] [CrossRef] [PubMed]
- Babb, S.J.; Crystal, J.D. Episodic-like memory in the rat. Curr. Biol. 2006, 16, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Clayton, N.S.; Griffiths, D.P.; Emery, N.J.; Dickinson, A. Elements of episodic-like memory in animals. Philos. Trans. R. Soc. Lond B Biol. Sci. 2001, 356, 1483–1491. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; McEwen, B.S. Psychobiological allostasis: Resistance, resilience and vulnerability. Trends. Cogn. Sci. 2011, 15, 576–584. [Google Scholar] [CrossRef]
- Rigterink, A. Fear, Anxiety, Stress Behaviors in Cats. In Clinical Handbook of Feline Behavior Medicine; Stelow, E., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2022; pp. 129–141. [Google Scholar]
- Calvo, M.G.; Gutiérrez-García, A. Chapter 16—Cognition and Stress. In Stress: Concepts, Cognition, Emotion, and Behavior; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 139–144. [Google Scholar]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef]
- Amat, M.; Camps, T.; Manteca, X. Stress in owned cats: Behavioural changes and welfare implications. J. Feline Med. Surg. 2016, 18, 577–586. [Google Scholar] [CrossRef]
- Beata, C.; Beaumont-Graff, E.; Coll, V.; Cordel, J.; Marion, M.; Massal, N.; Marlois, N.; Tauzin, J. Effect of alpha-casozepine (Zylkene) on anxiety in cats. J. Vet. Behav. 2007, 2, 40–46. [Google Scholar] [CrossRef]
- Kessler, M.R.; Turner, D.C. Stress and Adaptation of Cats (Felis silvestris catus) Housed Singly. In Pairs and in Groups in Boarding Catteries; Animal Welfare: Snohomish, WA, USA, 1997; Volume 6, pp. 243–254. [Google Scholar]
- Duffy, D.L.; de Moura, R.T.D.; Serpell, J.A. Development and evaluation of the Fe-BARQ: A new survey instrument for measuring behavior in domestic cats (Feliss catus). Behav. Process. 2017, 141 Pt 3, 329–341. [Google Scholar] [CrossRef]
- Behnke, A.; Vitale, K.; Udell, M. The Effect of Owner Presence and Scent on Stress Resilience in Cats. Appl. Anim. Behav. Sci. 2021, 243, 105444. [Google Scholar] [CrossRef]
- Bowen, J.R. Chapter 3—An overview of canine social behaviour and communication. In The Domestic Dog: Its Evolution, Behavior and Interactions with People; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Azadian, A.; Gunn-Moore, D.A. Age-related cognitive impairments in domestic cats naturally infected with feline immunodeficiency virus. Vet. Rec. 2022, 191, e1683. [Google Scholar] [CrossRef]
- Dow, S.W.; Poss, M.L.; Hoover, E.A. Feline immunodeficiency virus: A neurotropic lentivirus. J. Acquir. Immune. Defic. Syndr. 1990, 3, 658–668. [Google Scholar]
- Gunn-Moore, D.A. Cognitive dysfunction in cats: Clinical assessment and management. Top Companion. Anim. Med. 2011, 26, 17–24. [Google Scholar] [CrossRef]
- Maingat, F.; Vivithanaporn, P.; Zhu, Y.; Taylor, A.; Baker, G.; Pearson, K.; Power, C. Neurobehavioral performance in feline immunodeficiency virus infection: Integrated analysis of viral burden, neuroinflammation, and neuronal injury in cortex. J. Neurosci. 2009, 29, 8429–8437. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Nichol, J.; Araujo, J.A. Cognitive dysfunction syndrome: A disease of canine and feline brain aging. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 749–768. [Google Scholar] [CrossRef]
- Moffat, K.; Landsberg, G.M. An investigation of the prevalence of clinical signs of cognitive dysfunction syndrome (CDS) in cats. J. Am. Animal. Hospital. Assoc. 2003, 39, 512. [Google Scholar]
- Chapagain, D.; Range, F.; Huber, L.; Virányi, Z. Cognitive Aging in Dogs. Gerontology 2018, 64, 165–171. [Google Scholar] [CrossRef]
- Sordo, L.; Gunn-Moore, D.A. Cognitive Dysfunction in Cats: Update on Neuropathological and Behavioural Changes Plus Clinical Management. Vet. Rec. 2021, 188, e3. [Google Scholar] [CrossRef]
- Černá, P.; Gardiner, H.; Sordo, L.; Tørnqvist-Johnsen, C.; Gunn-Moore, D.A. Potential Causes of Increased Vocalisation in Elderly Cats with Cognitive Dysfunction Syndrome as Assessed by Their Owners. Animals 2020, 10, 1092. [Google Scholar] [CrossRef]
- Gunn-Moore, D.; Moffat, K.; Christie, L.A.; Head, E. Cognitive dysfunction and the neurobiology of ageing in cats. J. Small Anim. Pract. 2007, 48, 546–553. [Google Scholar] [CrossRef]
- Volicer, L.; Crino, P.B. Involvement of free radicals in dementia of the Alzheimer type: A hypothesis. Neurobiol. Aging 1990, 11, 567–571. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Denenberg, S.; Araujo, J.A. Cognitive dysfunction in cats: A syndrome we used to dismiss as ‘old age’. J. Feline Med. Surg. 2010, 12, 837–848. [Google Scholar] [CrossRef]
- Bellows, J.; Center, S.; Daristotle, L.; Estrada, A.H.; Flickinger, E.A.; Horwitz, D.F.; Lascelles, B.D.X.; Lepine, A.; Perea, S.; Scherk, M.; et al. Aging in cats: Common physical and functional changes. J. Feline Med. Surg. 2016, 18, 533–550. [Google Scholar] [CrossRef]
- Milgram, N.M. Neuropsychological function and aging in cats. In Proceedings of the 15th Annual Conference on Canine Cognition and Aging, Laguna Beach, CA, USA, 10–12 November 2010. [Google Scholar]
- Provoost, L. Cognitive Changes Associated with Aging and Physical Disease in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2024, 54, 101–119. [Google Scholar] [CrossRef]
- Watson, P.E.; Thomas, D.G.; Bermingham, E.N.; Schreurs, N.M.; Parker, M.E. Drivers of Palatability for Cats and Dogs-What It Means for Pet Food Development. Animals 2023, 13, 1134. [Google Scholar] [CrossRef]
- Aldrich, G.C.; Koppel, K. Pet Food Palatability Evaluation: A Review of Standard Assay Techniques and Interpretation of Results with a Primary Focus on Limitations. Animals 2015, 5, 43–55. [Google Scholar] [CrossRef]
- Houpt, K.A.; Zicker, S. Dietary effects on canine and feline behavior. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 405–416. [Google Scholar] [CrossRef]
- Zaghini, G.; Biagi, G. Nutritional peculiarities and diet palatability in the cat. Vet. Res. Commun. 2005, 29 (Suppl. 2), 39–44. [Google Scholar] [CrossRef]
- Pickering, G.J. Optimizing the sensory characteristics and acceptance of canned cat food: Use of a human taste panel. J. Anim. Physiol. Anim. Nutr. 2009, 93, 52–60. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Maller, O.; Rogers, J.G. Flavor preferences in cats (Felis catus and Panthera sp.). J. Comp. Physiol. Psychol. 1977, 91, 1118–1127. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Wang, H.; Bayley, D.L.; Cao, J.; Reed, D.R.; Bachmanov, A.A.; Huang, L.; Legrand-Defretin, V.; Beauchamp, G.K.; et al. Cats lack a sweet taste receptor. J. Nutr. 2006, 136 (Suppl. 7), 1932s–1934s. [Google Scholar] [CrossRef]
- Alegría-Morán, R.A.; Guzmán-Pino, S.A.; Egaña, J.I.; Sotomayor, V.; Figueroa, J. Food Preferences in Cats: Effect of Dietary Composition and Intrinsic Variables on Diet Selection. Animals 2019, 9, 372. [Google Scholar] [CrossRef]
- Salaun, F.; Blanchard, G.; Le Paih, L.; Roberti, F.; Niceron, C. Impact of macronutrient composition and palatability in wet diets on food selection in cats. J. Anim. Physiol. Anim. Nutr. 2017, 101, 320–328. [Google Scholar] [CrossRef]
- Bourgeois, H.; Elliott, D.; Marniquet, P.; Soulard, Y. Dietary behavior of dogs and cats. Bull. L’académie Vétérinaire Fr. 2006, 159, 301–308. [Google Scholar] [CrossRef]
- Bradshaw, J.W. Mere exposure reduces cats’ neophobia to unfamiliar food. Anim. Behav. 1986, 34, 613–614. [Google Scholar] [CrossRef]
- Cheney, C.D.; Miller, E.R. Effects of forced flavor exposure on food neophobia. Appl. Anim. Behav. Sci. 1997, 53, 213–217. [Google Scholar] [CrossRef]
- WSAVA nutritional assessment guidelines. J. Feline Med. Surg. 2011, 13, 516–525. [CrossRef]
- Hall, J.A.; Jackson, M.I.; Vondran, J.C.; Vanchina, M.A.; Jewell, D.E. Comparison of circulating metabolite concentrations in dogs and cats when allowed to freely choose macronutrient intake. Biol. Open 2018, 7, bio036228. [Google Scholar] [CrossRef]
- Hewson-Hughes, A.K.; Hewson-Hughes, V.L.; Miller, A.T.; Hall, S.R.; Simpson, S.J.; Raubenheimer, D. Geometric analysis of macronutrient selection in the adult domestic cat, Felis catus. J. Exp. Biol. 2011, 214 Pt 6, 1039–1051. [Google Scholar] [CrossRef]
- Tynes, V.V.; Landsberg, G.M. Nutritional Management of Behavior and Brain Disorders in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 711–727. [Google Scholar] [CrossRef]
- Choy, O. Nutritional factors associated with aggression. Front. Psychiatry 2023, 14, 1176061. [Google Scholar] [CrossRef]
- Kaplan, B.J.; Crawford, S.G.; Field, C.J.; Simpson, S.J. Vitamins, minerals, and mood. Psychol. Bull. 2007, 133, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Totten, M.S.; Davenport, T.S.; Edwards, L.F.; Howell, J.M. Trace Minerals and Anxiety: A Review of Zinc, Copper, Iron, and Selenium. Dietetics 2023, 2, 83–103. [Google Scholar] [CrossRef]
- Bosch, G.; Beerda, B.; Hendriks, W.H.; Van der Poel, A.F.B.; Verstegen, M.W.A. Impact of nutrition on canine behaviour: Current status and possible mechanisms. Nutr. Res. Rev. 2007, 20, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Markus, C.R.; Olivier, B.; Panhuysen, G.E.; Van der Gugten, J.; Alles, M.S.; Tuiten, A.; Westenberg, H.G.M.; Fekkes, D.; Koppeschaar, H.F.; de Haan, E.E. The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am. J. Clin. Nutr. 2000, 71, 1536–1544. [Google Scholar] [CrossRef]
- DeNapoli, J.S.; Dodman, N.H.; Shuster, L.; Rand, W.M.; Gross, K.L. Effect of dietary protein content and tryptophan supplementation on dominance aggression, territorial aggression, and hyperactivity in dogs. J. Am. Vet. Med. Assoc. 2000, 217, 504–508. [Google Scholar] [CrossRef]
- Beata, C.; Beaumont-Graff, E.; Diaz, C.; Marion, M.; Massal, N.; Marlois, N.; Muller, G.; Lefranc, C. Effects of alpha-casozepine (Zylkene) versus selegiline hydrochloride (Selgian, Anipryl) on anxiety disorders in dogs. J. Vet. Behav. Clin. Appl. Res. 2007, 2, 175. [Google Scholar] [CrossRef]
- Wakabayashi, C.; Numakawa, T.; Ninomiya, M.; Chiba, S.; Kunugi, H. Behavioral and molecular evidence for psychotropic effects in L-theanine. Psychopharmacology 2012, 219, 1099–1109. [Google Scholar] [CrossRef]
- DePorter, T.L.; Bledsoe, D.L.; Conley, J.R.; Warner, C.W.; Linn, E.; Griffin, D. Case Report Series of Clinical Effectiveness and Safety of Solliquin® for Behavioral Support in Dogs and Cats. In Proceedings of the Veterinary Behavior Symposium Proceedings, San Antonio, TX, USA, 5 August 2016. [Google Scholar]
- Landsberg, G.; Milgram, B.; Mougeot, I.; Kelly, S.; de Rivera, C. Therapeutic effects of an alpha-casozepine and L-tryptophan supplemented diet on fear and anxiety in the cat. J. Feline Med. Surg. 2017, 19, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, K.; Kato, M.; Ohtani, N.; Ohta, M. Experimental Verification of the Effects on Normal Domestic Cats by Feeding Prescription Diet for Decreasing Stress. J. Appl. Anim. Welf Sci. 2015, 18, 355–362. [Google Scholar] [CrossRef]
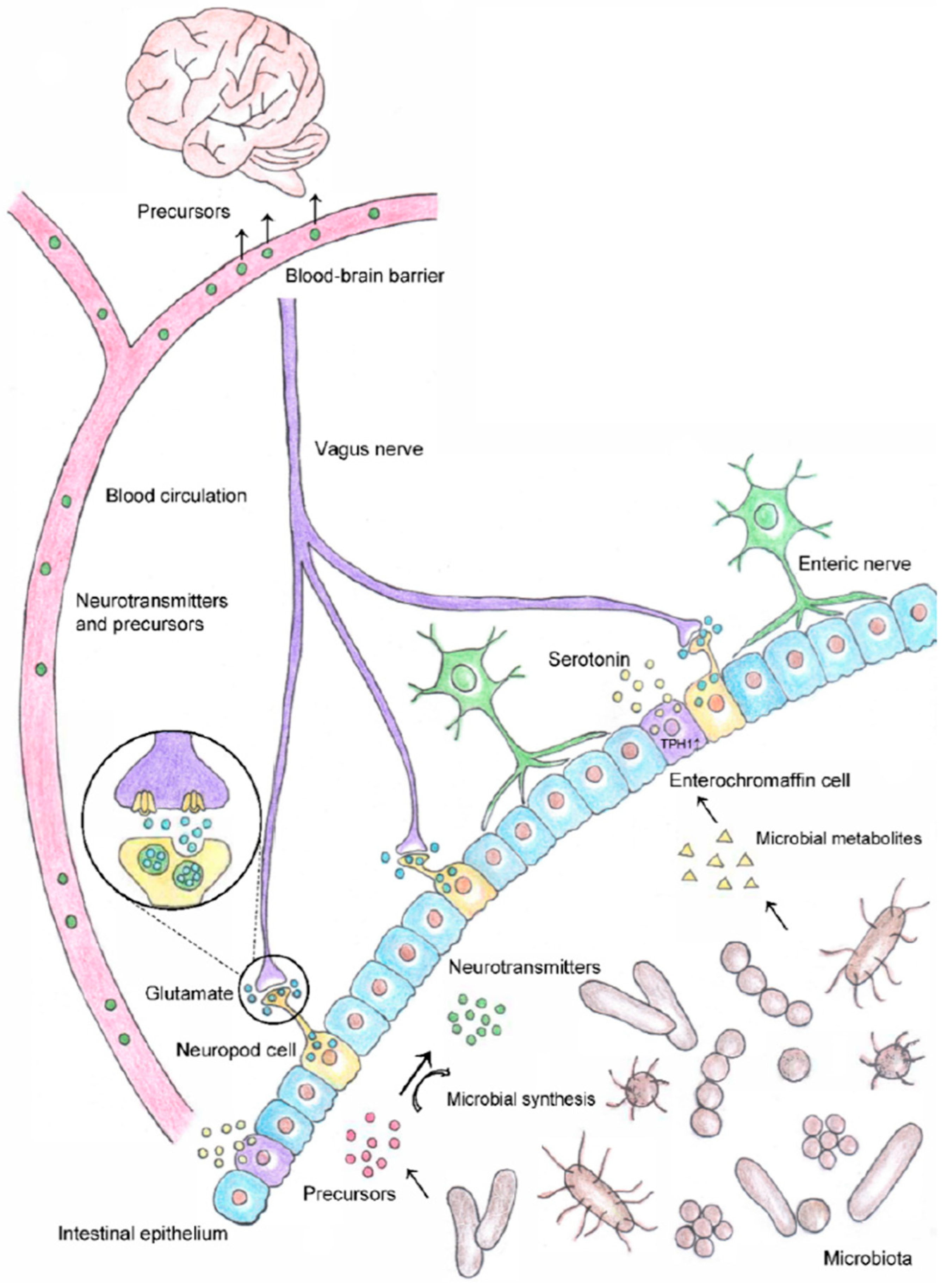
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Mondo, E.; Barone, M.; Soverini, M.; D’amico, F.; Cocchi, M.; Petrulli, C.; Mattioli, M.; Marliani, G.; Candela, M.; Accorsi, P.A. Gut microbiome structure and adrenocortical activity in dogs with aggressive and phobic behavioral disorders. Heliyon 2020, 6, e03311. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012, 37, 1369–1378. [Google Scholar] [CrossRef]
- Foster, J.A.; Neufeld, K.A.M. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Lee, J.; del Carmen Rosas, M.; Chen, J.; Henderson, W.A.; Starkweather, A.R.; Cong, X.S. Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndrome. Sci. Rep. 2022, 12, 1648. [Google Scholar] [CrossRef]
- Ray, M.; Carney, H.C.; Boynton, B.; Quimby, J.; Robertson, S.; St. Denis, K.; Tuzio, H.; Wright, B. 2021 AAFP Feline Senior Care Guidelines. J. Feline Med. Surg. 2021, 23, 613–638. [Google Scholar] [CrossRef]
- Miele, A.; Sordo, L.; Gunn-Moore, D.A. Feline Aging: Promoting Physiologic and Emotional Well-Being. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 719–748. [Google Scholar] [CrossRef]
- Pan, Y.; Araujo, J.A.; Burrows, J.; de Rivera, C.; Gore, A.; Bhatnagar, S.; Milgram, N.W. Cognitive enhancement in middle-aged and old cats with dietary supplementation with a nutrient blend containing fish oil, B vitamins, antioxidants and arginine. Br. J. Nutr. 2013, 110, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Head, E.; Zicker, S.C. Nutraceuticals, aging, and cognitive dysfunction. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Heath, S.E.; Barabas, S.; Craze, P.G. Nutritional supplementation in cases of canine cognitive dysfunction—A clinical trial. Appl. Anim. Behav. Sci. 2007, 105, 284–296. [Google Scholar] [CrossRef]
- Ikeda-Douglas, C.J.; Zicker, S.C.; Estrada, J.; Jewell, D.E.; Milgram, N.W. Prior experience, antioxidants, and mitochondrial cofactors improve cognitive function in aged beagles. Vet. Ther. 2004, 5, 5–16. [Google Scholar] [PubMed]
- Landsberg, G. Therapeutic options for cognitive decline in senior pets. J. Am. Anim. Hosp. Assoc. 2006, 42, 407–413. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Deporter, T.; Araujo, J.A. Clinical signs and management of anxiety, sleeplessness, and cognitive dysfunction in the senior pet. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 565–590. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Kennedy, A.D.; Jönsson, T.J.; Milgram, N.W. Cognitive enhancement in old dogs from dietary supplementation with a nutrient blend containing arginine, antioxidants, B vitamins and fish oil. Br. J. Nutr. 2018, 119, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Landsberg, G.; Mougeot, I.; Kelly, S.; Xu, H.; Bhatnagar, S.; Gardner, C.L.; Milgram, N.W. Efficacy of a Therapeutic Diet on Dogs With Signs of Cognitive Dysfunction Syndrome (CDS): A Prospective Double Blinded Placebo Controlled Clinical Study. Front Nutr. 2018, 5, 127. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Larson, B.; Araujo, J.A.; Lau, W.; De Rivera, C.; Santana, R.; Gore, A.; Milgram, N.W. Dietary supplementation with medium-chain TAG has long-lasting cognition-enhancing effects in aged dogs. Br. J. Nutr. 2010, 103, 1746–1754. [Google Scholar] [CrossRef]
- Pop, V.; Head, E.; Hill, M.A.; Gillen, D.; Berchtold, N.C.; Muggenburg, B.A.; Milgram, N.W.; Murphy, P.M.; Cotman, C.W. Synergistic effects of long-term antioxidant diet and behavioral enrichment on beta-amyloid load and non-amyloidogenic processing in aged canines. J. Neurosci. 2010, 30, 9831–9839. [Google Scholar] [CrossRef]
- Roudebush, R.; Zicker, S.C.; Cotman, C.W.; Milgram, N.W.; Muggenburg, B.A.; Head, E. Nutritional management of brain aging in dogs. J. Am. Vet. Med. Assoc. 2005, 227, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Head, E.; Rofina, J.; Zicker, S. Oxidative stress, aging, and central nervous system disease in the canine model of human brain aging. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 167–178. [Google Scholar] [CrossRef] [PubMed]
- May, K.A.; Laflamme, D.P. Nutrition and the aging brain of dogs and cats. J. Am. Vet. Med. Assoc. 2019, 255, 1245–1254. [Google Scholar] [CrossRef]
- Buffenstein, R.; Edrey, Y.H.; Yang, T.; Mele, J. The oxidative stress theory of aging: Embattled or invincible? Insights from non-traditional model organisms. Age 2008, 30, 99–109. [Google Scholar] [PubMed]
- Vite, C.H.; Head, E. Aging in the canine and feline brain. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 1113–1129. [Google Scholar] [CrossRef] [PubMed]
- Head, E.; Liu, J.; Hagen, T.M.; Muggenburg, B.A.; Milgram, N.W.; Ames, B.N.; Cotman, C.W. Oxidative damage increases with age in a canine model of human brain aging. J. Neurochem. 2002, 82, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Head, E.; Nukala, V.N.; Fengolio, K.A.; Muggenburg, B.A.; Cotman, C.W.; Sullivan, P.G. Effects of age, dietary, and behavioral enrichment on brain mitochondria in a canine model of human aging. Exp. Neurol. 2009, 220, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Yoon, Y.S.; Yoo, K.Y.; Li, H.; Choi, J.H.; Kim, D.W.; Yi, S.S.; Seong, J.K.; Lee, I.S.; Won, M.H. Differences in lipid peroxidation and Cu,Zn-superoxide dismutase in the hippocampal CA1 region between adult and aged dogs. J. Vet. Med. Sci. 2008, 70, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Opii, W.O.; Joshi, G.; Head, E.; Milgram, N.W.; Muggenburg, B.A.; Klein, J.B.; Pierce, W.M.; Cotman, C.W.; Butterfield, D.A. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: Relevance to Alzheimer’s disease. Neurobiol. Aging 2008, 29, 51–70. [Google Scholar] [CrossRef]
- Skoumalova, A.; Rofina, J.; Schwippelova, Z.; Gruys, E.; Wilhelm, J. The role of free radicals in canine counterpart of senile dementia of the Alzheimer type. Exp. Gerontol. 2003, 38, 711–719. [Google Scholar] [CrossRef]
- Cotman, C.W.; Head, E. The canine (dog) model of human aging and disease: Dietary, environmental and immunotherapy approaches. J. Alzheimers Dis. 2008, 15, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Rofina, J.E.; Van Ederen, A.M.; Toussaint, M.J.M.; Secreve, M.; Van Der Spek, A.; Van Der Meer, I.; Van Eerdenburg, F.J.C.M.; Gruys, E. Cognitive disturbances in old dogs suffering from the canine counterpart of Alzheimer’s disease. Brain Res. 2006, 1069, 216–226. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Weninger, S.C.; Yankner, B.A. Inflammation and Alzheimer disease: The good, the bad, and the ugly. Nat. Med. 2001, 7, 527–528. [Google Scholar] [CrossRef]
- Cotman, C.W.; Head, E.; Muggenburg, B.A.; Zicker, S.; Milgram, N.W. Brain aging in the canine: A diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol. Aging 2002, 23, 809–818. [Google Scholar] [CrossRef]
- Milgram, N.W.; Zicker, S.C.; Head, E.; Muggenburg, B.A.; Murphey, H.; Ikeda-Douglas, C.J.; Cotman, C.W. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiol. Aging 2002, 23, 737–745. [Google Scholar] [CrossRef]
- Piletz, J.E.; Aricioglu, F.; Cheng, J.T.; Fairbanks, C.A.; Gilad, V.H.; Haenisch, B.; Halaris, A.; Hong, S.; Lee, J.E.; Li, J.; et al. Agmatine: Clinical applications after 100 years in translation. Drug. Discov. Today 2013, 18, 880–893. [Google Scholar] [CrossRef]
- Zicker, S.C. Cognitive and behavioral assessment in dogs and pet food market applications. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Agosti, P.; Lozupone, M.; Custodero, C.; Schilardi, A.; Valiani, V.; Santamato, A.; Sardone, R.; Dibello, V.; Di Lena, L.; et al. Nutritional interventions and cognitive-related outcomes in patients with late-life cognitive disorders: A systematic review. Neurosci. Biobehav. Rev. 2018, 95, 480–498. [Google Scholar] [CrossRef]
- Chapagain, D.; Virányi, Z.; Huber, L.; Serra, J.; Schoesswender, J.; Range, F. Effect of Age and Dietary Intervention on Discrimination Learning in Pet Dogs. Front Psychol. 2018, 9, 2217. [Google Scholar] [CrossRef]
- Milgram, N.W.; Head, E.; Zicker, S.C.; Ikeda-Douglas, C.J.; Murphey, H.; Muggenburg, B.; Siwak, C.; Tapp, D.; Cotman, C.W. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: A two-year longitudinal study. Neurobiol. Aging 2005, 26, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Milgram, N.W.; Siwak, C.T.; Gruet, P.; Atkinson, P.; Woehrlé, F.; Callahan, H. Oral administration of adrafinil improves discrimination learning in aged beagle dogs. Pharmacol. Biochem. Behav. 2000, 66, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Singla, A.; Singh, S.; Shilwant, S.; Kaur, R. Role of Omega-3 Fatty Acids in Canine Health: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2283–2293. [Google Scholar] [CrossRef]
- Young, G.; Conquer, J. Omega-3s and Their Impact on Brain Health. In Marine Nutraceuticals and Functional Foods; Barrow, C.J., Shahidi, F., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 63–88. [Google Scholar]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N., Jr.; Stedman, M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement 2010, 6, 456–464. [Google Scholar] [PubMed]
- Lenox, C.E.; Bauer, J.E. Potential adverse effects of omega-3 Fatty acids in dogs and cats. J. Vet. Int. Med. 2013, 27, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Cupp, C.J.; Jean-Philippe, C.; Kerr, W.W.; Patil, A.R.; Perez-Camargo, G. Effect of nutritional interventions on longevity of senior cats. Int. J. Appl. Res. Vet. Med. 2006, 4, 34–50. [Google Scholar]
- Cupp, C.J.; Kerr, W.W.; Patil, A. The Role of Nutritional Interventions in the Longevity and Maintenance of Long-Term Health in Aging Cats; CABI: Wallingford, UK, 2008. [Google Scholar]
- Shiefelbein, H.M.J. Behavior and health in aged cats fed a food with antioxidants, phytonutrients, and fatty acids. J. Vet. Int. Med. 2017, 31, 1327. [Google Scholar]
- Jewell, D.B.J.; Brockman, J. Anti-Aging Foods for Companion Animals. U.S. Patent WO2014092716, 19 June 2014. [Google Scholar]
- Houpt, K.; Levine, E.; Landsberg, G.; Moffat, K.S.; Zicker, S.C. Antioxidant fortified food improves owner perceived behavior in the aging cat. In Proceedings of the ESFM Conference, Prague, Czech Republic, 29–31 October 2007. [Google Scholar]
- Ephraim, E.; Jewell, D.E. Effect of Nutrition on Age-Related Metabolic Markers and the Gut Microbiota in Cats. Microorganisms 2021, 9, 2430. [Google Scholar] [CrossRef] [PubMed]
- Surai, P. Antioxidant Action of Carnitine: Molecular Mechanisms and Practical Applications. EC Vet. Sci. 2015, 2, 66–84. [Google Scholar]
- MacDonald, M.L.; Rogers, Q.R.; Morris, J.G. Aversion of the cat to dietary medium-chain triglycerides and caprylic acid. Physiol. Behav. 1985, 35, 371–375. [Google Scholar] [CrossRef]
- Orlando, J.M. Behavioral Nutraceuticals and Diets. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 473–495. [Google Scholar] [CrossRef]
- Finno, C.J. Veterinary Pet Supplements and Nutraceuticals. Nutr. Today 2020, 55, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Dzanis, D.A. Understanding regulations affecting pet foods. Top Comp. Anim. Med. 2008, 23, 117–120. [Google Scholar] [CrossRef]
- Tedesco, D.E.A.; Cagnardi, P. Regulatory Guidelines for Nutraceuticals in the European Union. In Nutraceuticals in Veterinary Medicine; Gupta, R., Srivastava, A., Lall, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 793–805. [Google Scholar]
- Bottiglieri, T. S-Adenosyl-L-methionine (SAMe): From the bench to the bedside--molecular basis of a pleiotrophic molecule. Am. J. Clin. Nutr. 2002, 76, 1151s–1157s. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.; Faubert, M.L.; Brooks, M.L.; Landsberg, G.M.; Lobprise, H. NOVIFIT® (NoviSAMe®) Tablets Improve Executive Function in Aged Dogs and Cats: Implications for Treatment of Cognitive Dysfunction Syndrome. Int. J. Appl. Vet. Med. 2012, 10, 90. [Google Scholar]
- Rème, C.A.; Dramard, V.; Kern, L.; Hofmans, J.; Halsberghe, C.; Mombiela, D.V. Effect of S-adenosylmethionine tablets on the reduction of age-related mental decline in dogs: A double-blinded, placebo-controlled trial. Vet. Ther. 2008, 9, 69–82. [Google Scholar]
- Berk, B.A.; Packer, R.M.A.; Law, T.H.; Wessmann, A.; Bathen-Noethen, A.; Jokinen, T.S.; Knebel, A.; Tipold, A.; Pelligand, L.; Volk, H.A. Medium-chain triglycerides dietary supplement improves cognitive abilities in canine epilepsy. Epilepsy Behav. 2021, 114 Pt A, 107608. [Google Scholar] [CrossRef] [PubMed]
- Trevizan, L.; de Mello Kessler, A.; Bigley, K.E.; Anderson, W.H.; Waldron, M.K.; Bauer, J.E. Effects of dietary medium-chain triglycerides on plasma lipids and lipoprotein distribution and food aversion in cats. Am. J. Vet. Res. 2010, 71, 435–440. [Google Scholar] [CrossRef]
- Meyer, H.P.; Bečvářová, I. Effects of a Urinary Food Supplemented with Milk Protein Hydrolysate and L-tryptophan on Feline Idiopathic Cystitis—Results of a Case Series in 10 Cats. Int. J. Appl. Res. Vet. Med. 2016, 14, 59–65. [Google Scholar]
- Laflamme, D.; Gunn-Moore, D. Nutrition of aging cats. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 761–774. [Google Scholar] [CrossRef]
- Landsberg, G.; Araujo, J.A. Behavior problems in geriatric pets. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 675–698. [Google Scholar] [CrossRef] [PubMed]
- Studzinski, C.M.; Araujo, J.A.; Milgram, N.W. The canine model of human cognitive aging and dementia: Pharmacological validity of the model for assessment of human cognitive-enhancing drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Kume, K.; Hanyu, H.; Sakurai, H.; Takada, Y.; Onuma, T.; Iwamoto, T. Effects of telmisartan on cognition and regional cerebral blood flow in hypertensive patients with Alzheimer’s disease. Geriatr. Gerontol. Int. 2012, 12, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wincewicz, D.; Braszko, J.J. Telmisartan attenuates cognitive impairment caused by chronic stress in rats. Pharmacol. Rep. 2014, 66, 436–441. [Google Scholar] [CrossRef]
- Araujo, J.A.; Greig, N.H.; Ingram, D.K.; Sandin, J.; de Rivera, C.; Milgram, N.W. Cholinesterase inhibitors improve both memory and complex learning in aged beagle dogs. J. Alzheimers Dis. 2011, 26, 143–155. [Google Scholar] [CrossRef]
- Casey, R.; Bradshaw, J. The Assessment of Welfare; Springer: Dordrecht, The Netherlands, 2007; pp. 23–46. [Google Scholar]
| Letter | Sign |
|---|---|
| V | Increased Vocalization, especially at night |
| I | Altered social Interaction with the family and/or pets |
| S | Changes in Sleep/wake patterns |
| H | House soiling |
| D | Spatial and temporal Disorientation |
| A | Changes in Activity (e.g., aimless wandering) |
| A | Anxiety |
| L | Learning and memory deficits |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGrath, A.P.; Horschler, D.J.; Hancock, L. Feline Cognition and the Role of Nutrition: An Evolutionary Perspective and Historical Review. Animals 2024, 14, 1967. https://doi.org/10.3390/ani14131967
McGrath AP, Horschler DJ, Hancock L. Feline Cognition and the Role of Nutrition: An Evolutionary Perspective and Historical Review. Animals. 2024; 14(13):1967. https://doi.org/10.3390/ani14131967
Chicago/Turabian StyleMcGrath, Allison P., Daniel J. Horschler, and Leslie Hancock. 2024. "Feline Cognition and the Role of Nutrition: An Evolutionary Perspective and Historical Review" Animals 14, no. 13: 1967. https://doi.org/10.3390/ani14131967





