Dietary Supplementation with Rumen-Protected Arginine or N-Carbamylglutamate Enhances Fetal Liver Development in Nutrient-Restricted Pregnant Hu Ewes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection, Handling, and Chemical Components
2.3. Liver Cellularity Estimates
2.4. Detection of Fetal Liver Functional Indicators and Antioxidation Ability
2.5. Sample Preparation for Histology Staining
2.6. Quantification of Hepatocellular-Apoptosis-Regulating Factors
2.7. Quantification of Apoptosis-Related Regulating Proteins
2.8. Statistical Analysis
3. Results
3.1. Basic Chemical Components of Fetal Liver
3.2. Fetal Liver Cellular Indicators
3.3. Antioxidation Activities of the Fetal Liver and Liver Function Indicators
3.4. TUNEL Staining to Identify Apoptotic Cells
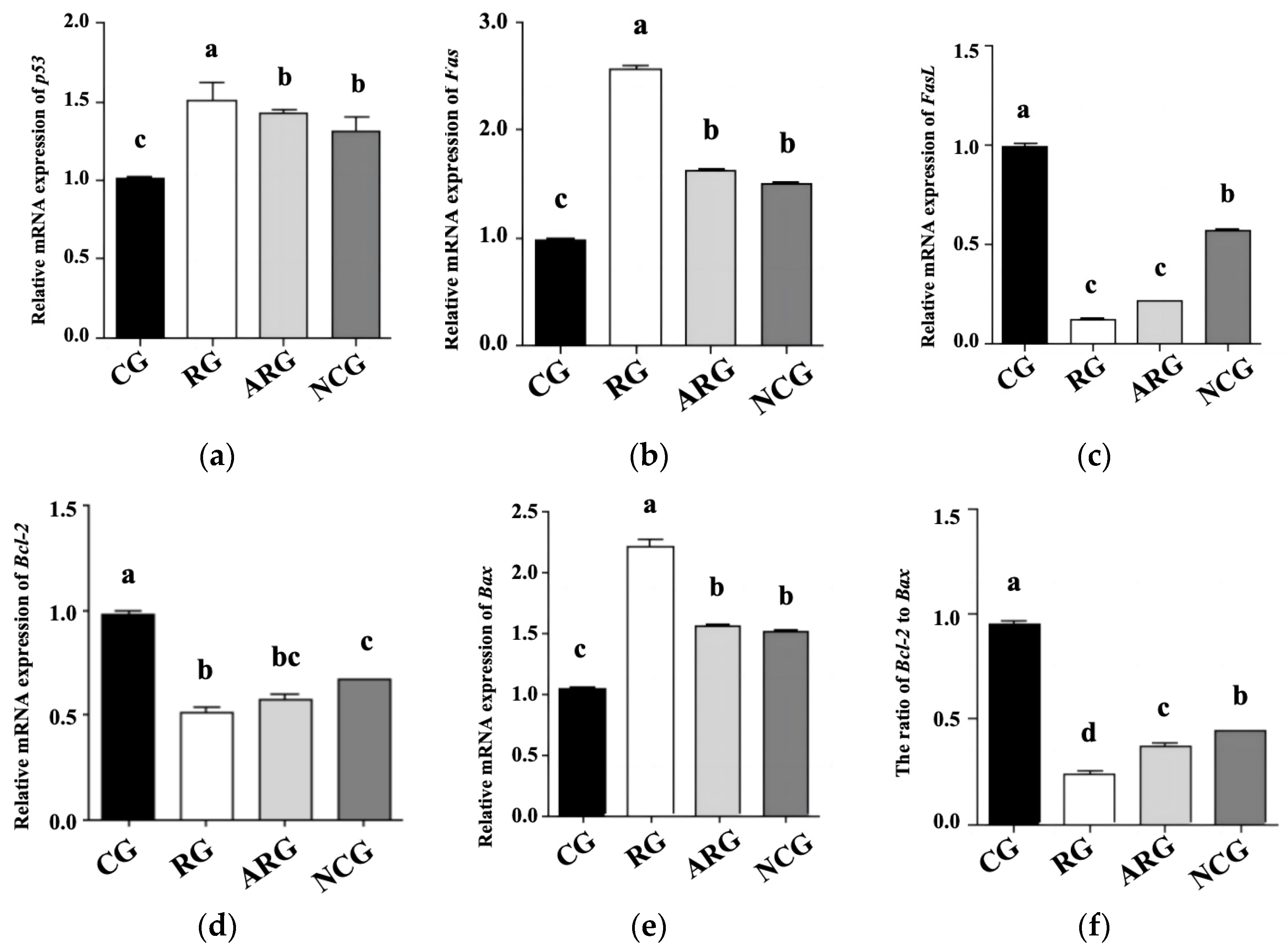
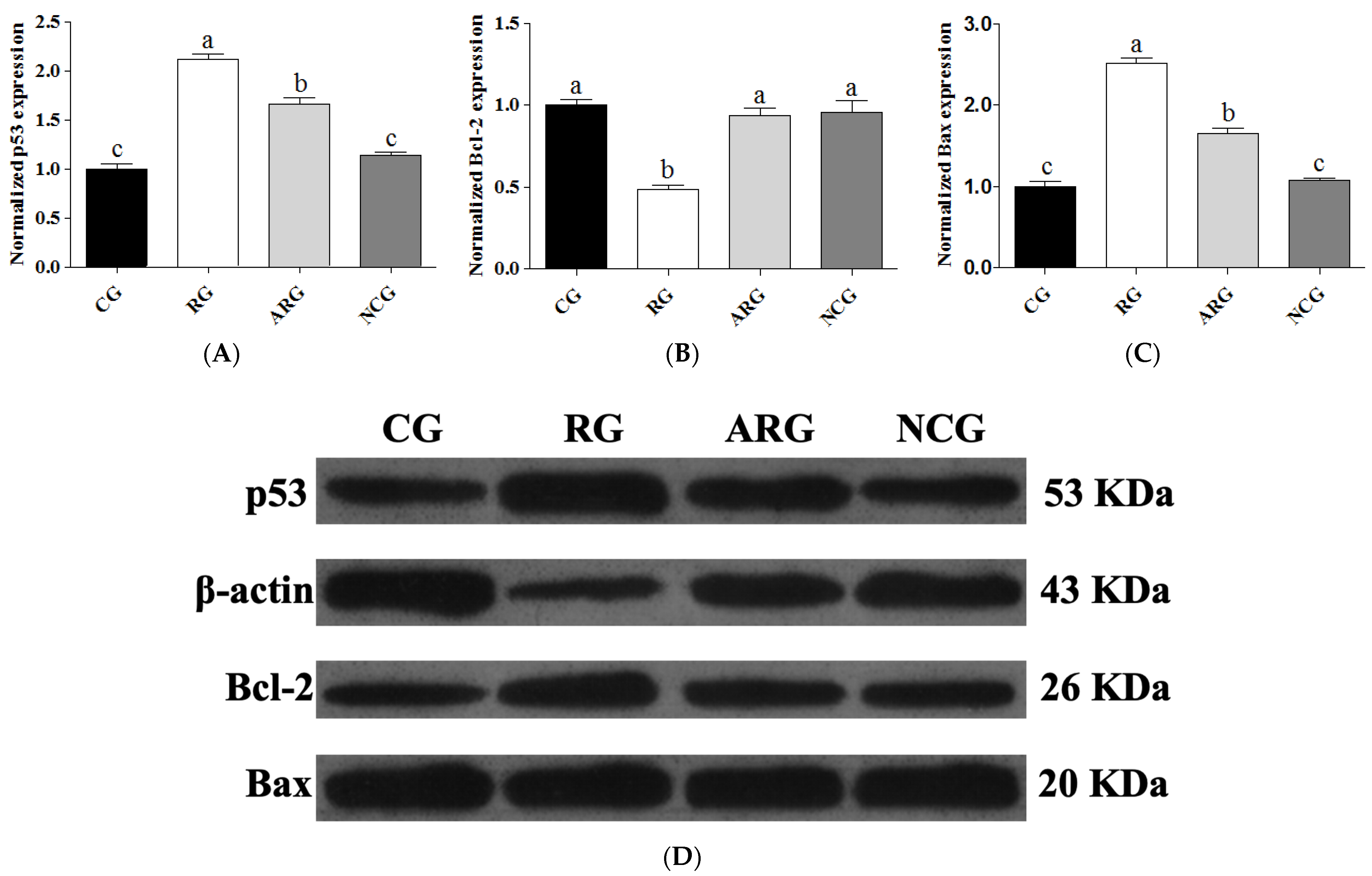
3.5. Apoptosis Genes and Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, S.; Maheshwari, A.; Jain, S.K. Maternal Nutrition and Fetal/Infant Development. Clin. Perinatol. 2022, 49, 313–330. [Google Scholar] [CrossRef]
- Prinz, N.; Putri, R.R.; Reinehr, T.; Danielsson, P.; Weghuber, D.; Norman, M.; Rochow, N.; Marcus, C.; Holl, R.W.; Hagman, E. The association between perinatal factors and cardiometabolic risk factors in children and adolescents with overweight or obesity: A retrospective two-cohort study. PLoS Med. 2023, 20, e1004165. [Google Scholar] [CrossRef]
- Hyatt, M.A.; Budge, H.; Symonds, M.E. Early developmental influences on hepatic organogenesis. Organogenesis 2008, 4, 170–175. [Google Scholar] [CrossRef]
- Sutherland, A.E.; White, T.A.; Rock, C.R.; Piscopo, B.R.; Dudink, I.; Inocencio, I.M.; Azman, Z.; Pham, Y.; Nitsos, I.; Malhotra, A.; et al. Phenotype of early-onset fetal growth restriction in sheep. Front. Endocrinol. 2024, 15, 1374897. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Satterfield, M.C.; Li, X.; Wang, X.; Johnson, G.A.; Burghardt, R.C.; Dai, Z.; Wang, J.; Wu, Z. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 2013, 45, 241–256. [Google Scholar] [CrossRef]
- Chavez, J.A.; Guzman, A.; Zamora-Gutierrez, D.; Mendoza, G.D.; Melgoza, L.M.; Montes, S.; Rosales-Torres, A.M. Supplementation with rumen-protected L-arginine-HCl increased fertility in sheep with synchronized estrus. Trop. Anim. Health Prod. 2015, 47, 1067–1073. [Google Scholar] [CrossRef]
- Wu, X.; Yin, Y.L.; Liu, Y.Q.; Liu, X.D.; Liu, Z.Q.; Li, T.J.; Huang, R.L.; Ruan, Z.; Deng, Z.Y. Effect of dietary arginine and N-carbamoylglutamate supplementation on reproduction and gene expression of eNOS, VEGFA and PlGF1 in placenta in late pregnancy of sows. Anim. Reprod. Sci. 2012, 132, 187–192. [Google Scholar] [CrossRef]
- Chacher, B.; Liu, H.; Wang, D.; Liu, J. Potential role of N-carbamoyl glutamate in biosynthesis of arginine and its significance in production of ruminant animals. J. Anim. Sci. Biotechnol. 2013, 10, 16. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.; Wang, Z.; Deng, M.; Nie, H.; Zhang, G.; Ma, T.; Wang, F. N-carbamylglutamate and L-arginine improved maternal and placental development in underfed ewes. Reprod. Suppl. 2016, 151, 623–635. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.; Wang, Z.Y.; Deng, M.; Zhang, G.; Guo, R.; Ma, T.; Wang, F. Dietary-carbamylglutamate and rumen-protected-arginine supplementation ameliorate fetal growth restriction in undernourished ewes. J. Anim. Sci. 2016, 94, 2072–2085. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Fan, Y.; Guo, Y.; Zhang, G.; Nie, H.; Wang, F. Metabolomic profiling in umbilical venous plasma reveals effects of dietary rumen-protected arginine or N-carbamylglutamate supplementation in nutrient-restricted Hu sheep during pregnancy. Reprod. Domest. Anim. 2017, 52, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Wang, Z.; Fan, Y.; Guo, Y.; Wang, F. Dietary rumen-protected arginine and N-carbamylglutamate supplementation enhances fetal growth in underfed ewes. Reprod. Fertil. Dev. 2018, 30, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- NRC-National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; National Academy of Science: Washington, DC, USA, 2007; 347p. [Google Scholar]
- Chacher, B.; Wang, D.M.; Liu, H.Y.; Liu, J.X. Degradation of L-arginine and N-carbamoyl glutamate and their effect on rumen fermentation in vitro. Ital. J. Anim. Sci. 2012, 11, 4693–4696. [Google Scholar] [CrossRef]
- Satterfield, M.C.; Dunlap, K.A.; Keisler, D.H.; Bazer, F.W.; Wu, G. Arginine nutrition and fetal brown adipose tissue development in nutrient-restricted sheep. Amino Acids 2013, 45, 489. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhong, X.; Zhang, L.; Wang, Y.; Wang, T. Heat Shock Protein 70 Expression is Increased in the Liver of Neonatal Intrauterine Growth Retardation Piglets. Asian-Australas. J. Anim. Sci. 2012, 25, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Rockville, MD, USA, 2003; Volume 2. [Google Scholar]
- Johnson, M.L.; Redmer, D.A.; Reynolds, L.P. Uterine growth, cell proliferation, and c-fos proto-oncogene expression throughout the estrous cycle in ewes. Biol. Reprod. 1997, 56, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Millaway, D.S.; Kirsch, J.D.; Infeld, J.E.; Redmer, D.A. Growth and in-vitro metabolism of placental tissues of cows from day 100 to day 250 of gestation. J. Reprod. Fertil. 1990, 89, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Neville, T.L.; Ward, M.A.; Reed, J.J.; Soto-Navarro, S.A.; Julius, S.L.; Borowicz, P.P.; Taylor, J.B.; Redmer, D.A.; Reynolds, L.P.; Caton, J. Effects of level and source of dietary selenium on maternal and fetal body weight, visceral organ mass, cellularity estimates, and jejunal vascularity in pregnant ewe lambs. J. Anim. Sci. 2008, 86, 890–901. [Google Scholar] [CrossRef]
- Lin, Y.S.; Ginsberg, G.; Caffrey, J.L.; Xue, J.; Vulimiri, S.V.; Nath, R.G.; Sonawane, B. Association of body burden of mercury with liver function test status in the U.S. population. Environ. Int. 2014, 70, 88–94. [Google Scholar] [CrossRef]
- Zhang, J.; You, H.; Wang, T.; Wang, B.; Jia, J.; Katayama, H.; Maeda, S.; Wang, R.; Asano, G.; Ishiwata, T.; et al. Triple-staining to identify apoptosis of hepatic cells in situ. J. Nippon. Med. Sch. 2000, 67, 280–283. [Google Scholar] [CrossRef]
- Heras-Molina, A.; Escudero, R.; Pesántez-Pacheco, J.L.; García-Contreras, C.; Vázquez-Gómez, M.; Astiz, S.; Óvilo, C.; González-Bulnes, A.; Isabel, B. Maternal Supplementation with Polyphenols and Omega-3 Fatty Acids during Pregnancy: Prenatal Effects on Fetal Fatty Acid Composition in the Iberian Pig. Animals 2022, 12, 2140. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, Y.; Li, L.; Li, M.; Zhang, C.; Ao, C.; Hou, X. Effects of maternal undernutrition during late pregnancy on the development and function of ovine fetal liver. Anim. Reprod. Sci. 2014, 147, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Limdi, J.K.; Hyde, G.M. Evaluation of abnormal liver function tests. Postgrad. Med. J. 2003, 79, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Kasztelan-Szczerbinska, B.; Rycyk-Bojarzynska, A.; Szczerbinska, A.; Cichoz-Lach, H. Selected Aspects of the Intricate Background of Immune-Related Cholangiopathies—A Critical Overview. Nutrients 2023, 15, 760. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.K.; Hayashi, T.; Maeda, I.; Koh, H.; Harita, N.; Uehara, S.; Onishi, Y.; Oue, K.; Nakamura, Y.; Endo, G.; et al. Serum butyrylcholinesterase and the risk of future type 2 diabetes: The Kansai Healthcare Study. Clin. Endocrinol. 2014, 80, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Poli, G. Pathogenesis of liver fibrosis: Role of oxidative stress. Mol. Aspects. Med. 2000, 21, 49–98. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nikaido, T.; Toki, T.; Kita, N.; Kanai, M.; Ashida, T.; Ohira, S.; Konishi, I. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows. Arch. 2004, 444, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Haugen, G.; Kiserud, T.; Godfrey, K.; Crozier, S.; Hanson, M. Portal and umbilical venous blood supply to the liver in the human fetus near term. Ultrasound Obstet. Gynecol. 2004, 24, 599–605. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, X.; Lin, L.; Li, S.; Li, Q.; Zhong, S.; Peng, J.; Cui, Z. Genetic ablation of solute carrier family 7a3a leads to hepatic steatosis in zebrafish during fasting. Hepatology 2014, 60, 1929–1941. [Google Scholar] [CrossRef]
- Tipton, K.F.; Boyce, S.; O’Sullivan, J.; Davey, G.P.; Healy, J. Monoamine oxidases: Certainties and uncertainties. Curr. Med. Chem. 2004, 11, 1965–11982. [Google Scholar] [CrossRef]
- Erboga, M.; Kanter, M. Effect of Cadmium on Trophoblast Cell Proliferation and Apoptosis in Different Gestation Periods of Rat Placenta. Biol. Trace. Elem. Res. 2016, 169, 285–293. [Google Scholar] [CrossRef]
- Yamauchi, H.; Katayama, K.; Ueno, M.; Uetsuka, K.; Nakayama, H.; Doi, K. Involvement of p53 in 1-beta-D-arabinofuranosylcytosine-induced trophoblastic cell apoptosis and impaired proliferation in rat placenta. Biol. Reprod. 2004, 70, 1762–1767. [Google Scholar] [CrossRef]
- Ge, J.; Yang, N.; Yang, Y.; Yu, H.; Yang, X.; Wang, Y.; Wang, T.; Cheng, S.; Wang, Y.; Han, Z.; et al. The combination of eddy thermal effect of biodegradable magnesium with immune checkpoint blockade shows enhanced efficacy against osteosarcoma. Bioact. Mater. 2023, 25, 73–85. [Google Scholar] [CrossRef]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef]
- Khodapasand, E.; Jafarzadeh, N.; Farrokhi, F.; Kamalidehghan, B.; Houshmand, M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran. Biomed. J. 2015, 19, 69–75. [Google Scholar]
- Rauf, A.; Khatri, M.; Murgia, M.V.; Saif, Y.M. Fas/FasL and perforin-granzyme pathways mediated T cell cytotoxic responses in infectious bursal disease virus infected chickens. Results Immunol. 2012, 2, 112–119. [Google Scholar] [CrossRef]
- Nkongolo, S.; Mahamed, D.; Kuipery, A.; Sanchez Vasquez, J.D.; Kim, S.C.; Mehrotra, A.; Patel, A.; Hu, C.; McGilvray, I.; Feld, J.J.; et al. Longitudinal liver sampling in patients with chronic hepatitis B starting antiviral therapy reveals hepatotoxic CD8+ T cells. J. Clin. Investig. 2023, 133, e158903. [Google Scholar] [CrossRef] [PubMed]

| Items | Treatment Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CG | RG | ARG | NCG | |||
| n. | 16 | 16 | 16 | 16 | ||
| Fetal body weight, g | 1877.88 a | 1400.75 c | 1659.25 b | 1681.75 b | 23.53 | 0.03 |
| Liver, g | 60.12 a | 51.18 c | 51.73 c | 55.34 b | 3.73 | 0.03 |
| Body/liver weight (%) | 3.19 b | 3.72 a | 3.14 b | 3.28 b | 0.41 | 0.02 |
| Chemical components (g) | ||||||
| Dry matter | 12.56 a | 11.40 b | 11.44 b | 12.10 a | 0.31 | 0.03 |
| Water | 47.73 a | 40.69 c | 40.71 c | 43.13 b | 0.52 | <0.01 |
| Fat | 1.17 b | 1.12 c | 1.19 bc | 1.27 a | 0.10 | 0.04 |
| Protein | 8.88 a | 8.29 b | 8.63 ab | 9.02 a | 0.38 | 0.03 |
| Ash | 0.61 a | 0.55 b | 0.60 a | 0.62 a | 0.07 | 0.50 |
| Chemical components expressed as percentage of fetal liver weights (%) | ||||||
| Dry matter | 20.89 b | 22.27 a | 22.11 a | 21.86 a | 0.22 | 0.04 |
| Water | 79.39 | 79.50 | 78.70 | 77.94 | 0.32 | 0.49 |
| Fat | 1.95 b | 2.19 a | 2.30 a | 2.29 a | 0.05 | <0.01 |
| Protein | 14.77 c | 16.20 b | 16.68 a | 16.30 b | 0.69 | <0.01 |
| Ash | 1.01 | 1.07 | 1.16 | 1.12 | 0.05 | 0.73 |
| Items | Treatment Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CG | RG | ARG | NCG | |||
| DNA, mg/g | 4.57 a | 4.32 c | 4.28 c | 4.50 b | 0.05 | 0.03 |
| DNA, mg | 0.26 | 0.21 | 0.22 | 0.24 | 0.01 | 0.57 |
| RNA, mg/g | 3.92 b | 3.63 c | 3.80 b | 4.10 a | 0.05 | <0.01 |
| RNA, mg | 0.26 a | 0.20 b | 0.22 ab | 0.23 ab | 0.01 | 0.03 |
| RNA/DNA | 1.06 | 1.04 | 1.10 | 1.13 | 0.02 | 0.30 |
| Protein, mg/g | 54.63 a | 48.53 c | 51.53 b | 55.83 a | 0.88 | <0.01 |
| Protein, mg | 3.28 a | 2.53 b | 2.72 b | 3.15 a | 0.10 | <0.01 |
| Protein/DNA | 11.17 a | 10.34 b | 10.58 b | 10.92 ab | 0.11 | 0.02 |
| Items | Treatment Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CG | RG | ARG | NCG | |||
| Fetal serum | ||||||
| ALT (IU/L) | 5.36 a | 3.28 c | 4.11 b | 4.78 a | 0.25 | <0.01 |
| AST (IU/L) | 25.56 c | 37.11 a | 31.83 b | 26.86 c | 1.38 | <0.01 |
| TP (g/L) | 41.48 a | 31.03 c | 33.91 bc | 36.04 b | 1.17 | <0.01 |
| Fetal liver extract | ||||||
| CHE (U/mg) | 11.81 a | 8.57 b | 11.96 a | 11.41 a | 0.43 | <0.01 |
| GSH-Px (U/mg) | 26.88 b | 34.01 a | 27.23 b | 25.56 b | 1.03 | <0.01 |
| MAO (U/mg) | 2.13 | 2.10 | 2.11 | 2.10 | 0.02 | 0.94 |
| MDA (nmol/mg) | 4.07 b | 5.18 a | 4.43 b | 4.06 b | 0.14 | 0.04 |
| NO (umol/g) | 2.29 a | 1.69 b | 2.38 a | 2.68 a | 0.11 | <0.01 |
| NOS (U/mg) | 0.84 a | 0.65 c | 0.70 bc | 0.78 ab | 0.03 | 0.03 |
| SOD (U/mg) | 26.39 a | 21.61 b | 22.87 b | 24.92 ab | 0.59 | 0.01 |
| TAC (U/mg) | 2.65 a | 1.67 c | 2.28 b | 2.59 a | 0.12 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Sun, L.; He, M.; Xu, J.; Wu, C.; Gao, J.; Dai, J. Dietary Supplementation with Rumen-Protected Arginine or N-Carbamylglutamate Enhances Fetal Liver Development in Nutrient-Restricted Pregnant Hu Ewes. Animals 2024, 14, 1988. https://doi.org/10.3390/ani14131988
Lin Y, Sun L, He M, Xu J, Wu C, Gao J, Dai J. Dietary Supplementation with Rumen-Protected Arginine or N-Carbamylglutamate Enhances Fetal Liver Development in Nutrient-Restricted Pregnant Hu Ewes. Animals. 2024; 14(13):1988. https://doi.org/10.3390/ani14131988
Chicago/Turabian StyleLin, Yuexia, Lingwei Sun, Mengqian He, Jiehuan Xu, Caifeng Wu, Jun Gao, and Jianjun Dai. 2024. "Dietary Supplementation with Rumen-Protected Arginine or N-Carbamylglutamate Enhances Fetal Liver Development in Nutrient-Restricted Pregnant Hu Ewes" Animals 14, no. 13: 1988. https://doi.org/10.3390/ani14131988
APA StyleLin, Y., Sun, L., He, M., Xu, J., Wu, C., Gao, J., & Dai, J. (2024). Dietary Supplementation with Rumen-Protected Arginine or N-Carbamylglutamate Enhances Fetal Liver Development in Nutrient-Restricted Pregnant Hu Ewes. Animals, 14(13), 1988. https://doi.org/10.3390/ani14131988






