Pig-Derived Probiotic Bacillus tequilensis YB-2 Alleviates Intestinal Inflammation and Intestinal Barrier Damage in Colitis Mice by Suppressing the TLR4/NF-κB Signaling Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
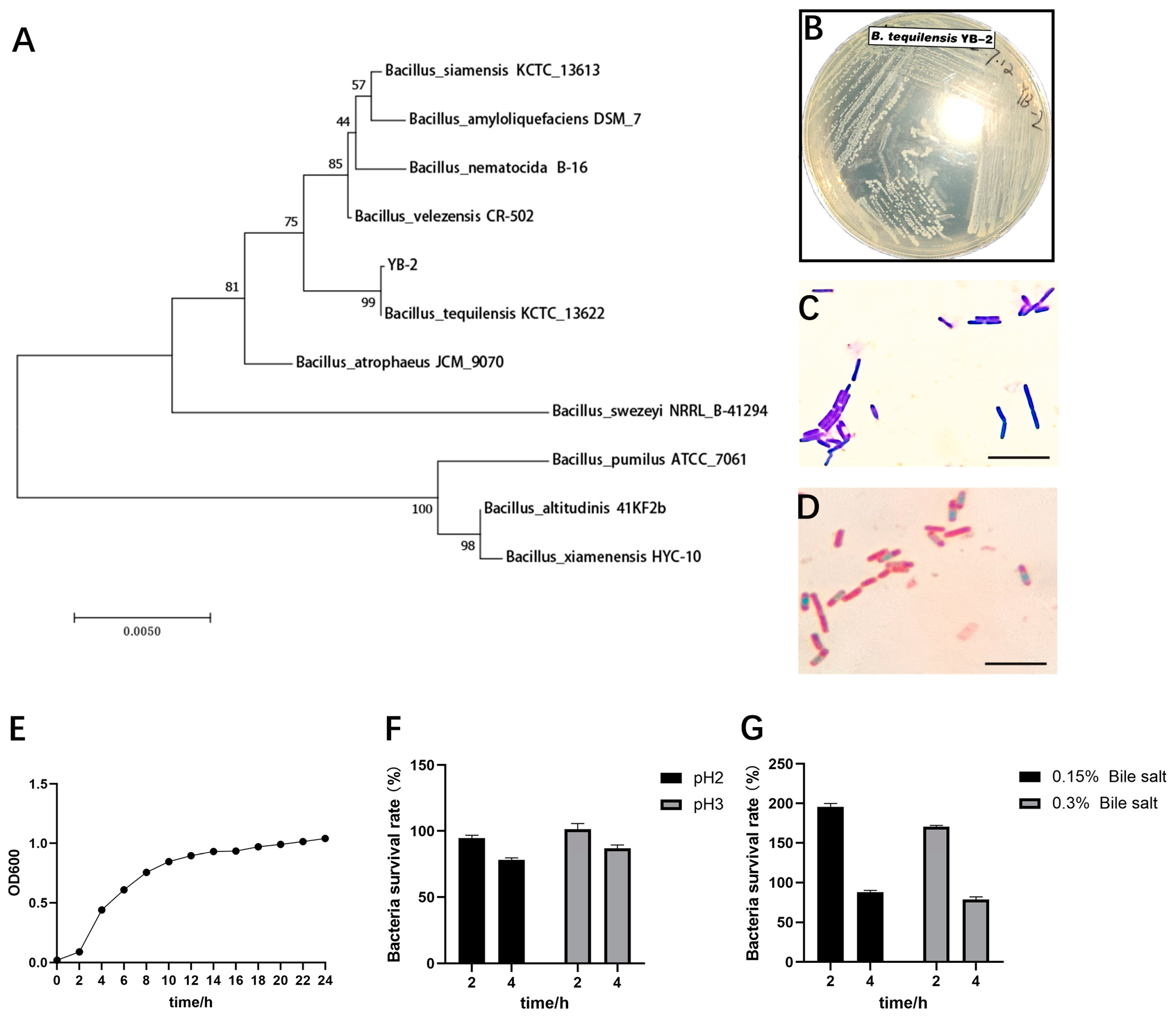
2.1. Isolation and Identification of Pig-Derived Bacillus
2.2. Growth Characteristics and Acid and Bile Salts Tolerance of B. tequilensis YB-2
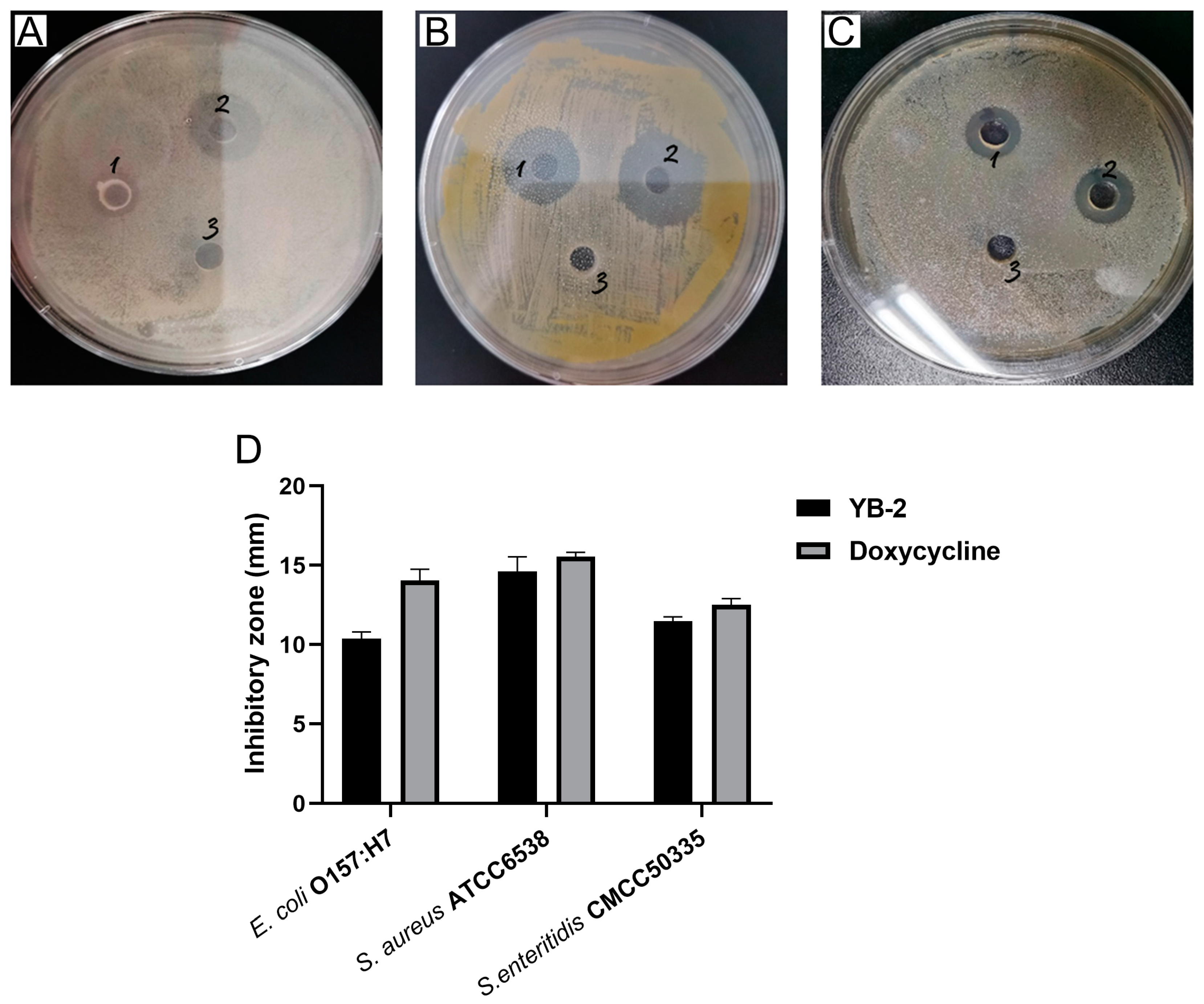
2.3. Antibacterial Activities of B. tequilensis YB-2
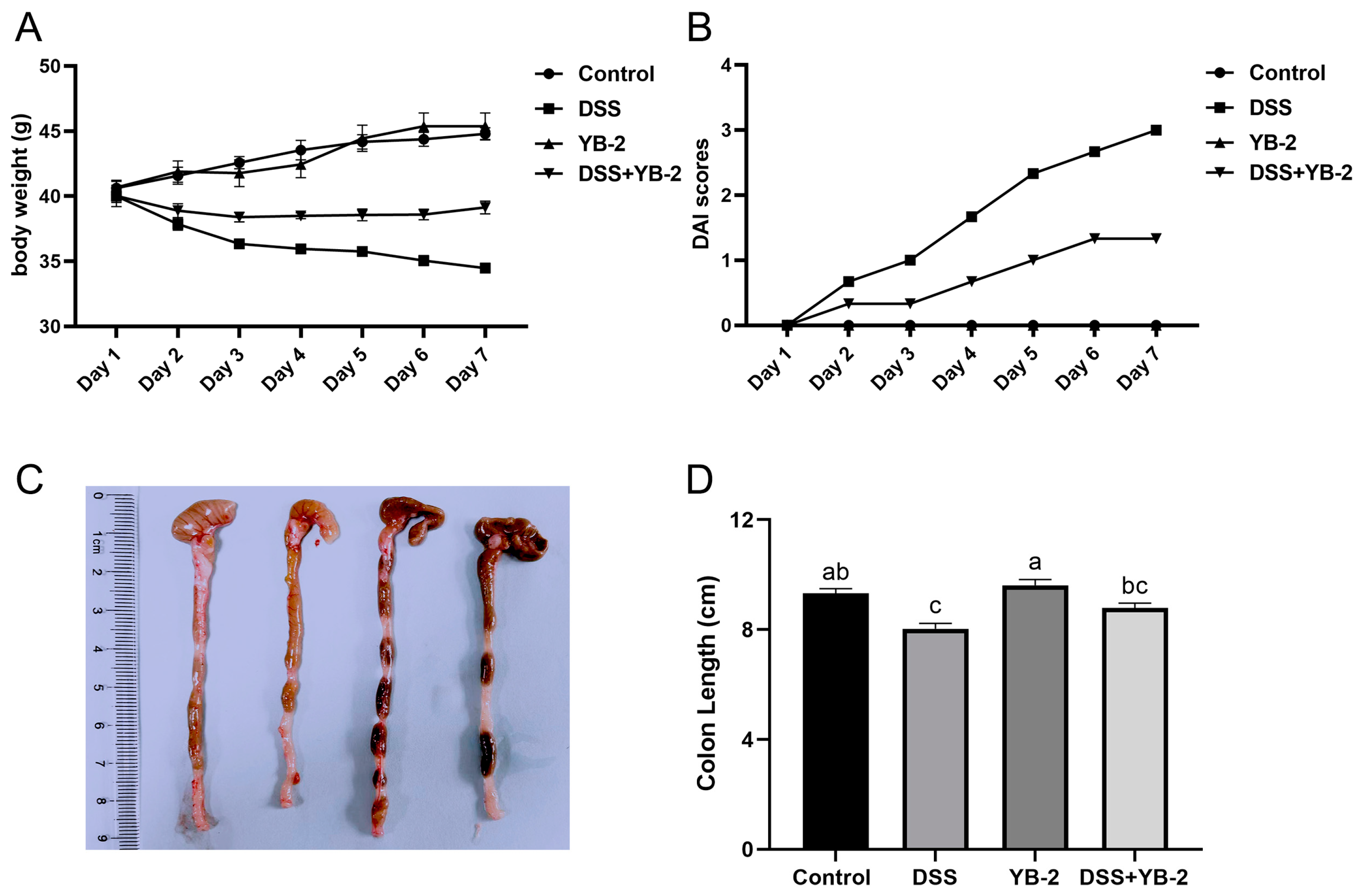
2.4. Animals and Study Design
2.5. Assessment of Disease Activity Index
2.6. Histological Evaluation of Colon Tissue
2.7. Transmission Electron Microscope Observation
2.8. Alcian Blue/Periodic Acid-Schiff (PAS) Staining
2.9. Detection of Nitric Oxide in the Serum and Colon
2.10. Serum Cytokine Concentration
2.11. Quantitative Real-Time PCR (qRT-PCR)
2.12. Western Blotting
2.13. Statistical Analysis
3. Results
3.1. Identification of B. tequilensis YB-2
3.2. Probiotic Properties of B. tequilensis YB-2 In Vitro
3.3. Antibacterial Activity of B. tequilensis YB-2
3.4. B. tequilensis YB-2 Alleviates Weight Loss and Lowers DAI Scores
3.5. B. tequilensis YB-2 Alleviates Colon Damage
3.6. B. tequilensis YB-2 Decreases Expression of Inflammatory Cytokines
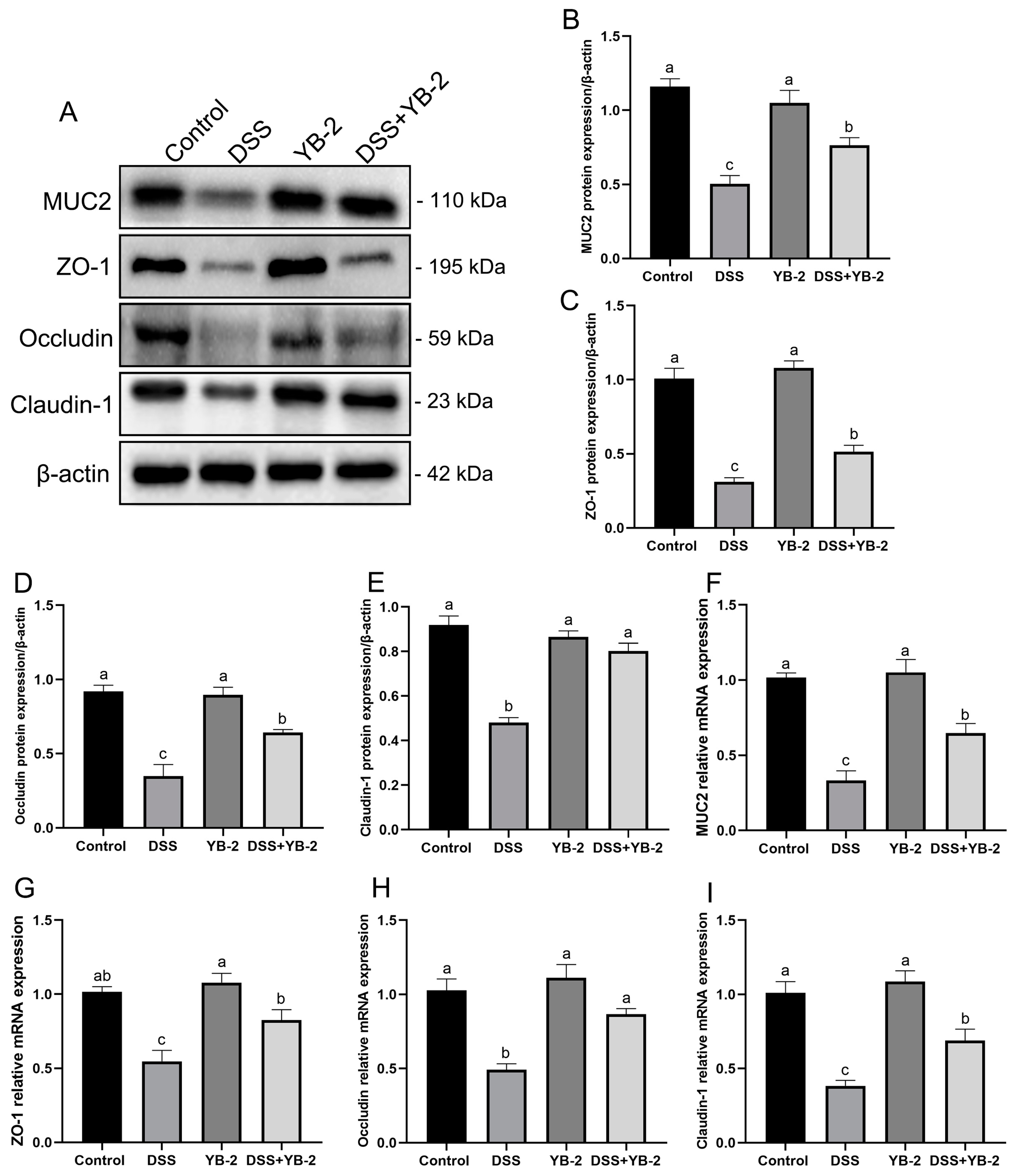
3.7. B. tequilensis YB-2 Repairs the Intestinal Mucosal Barrier
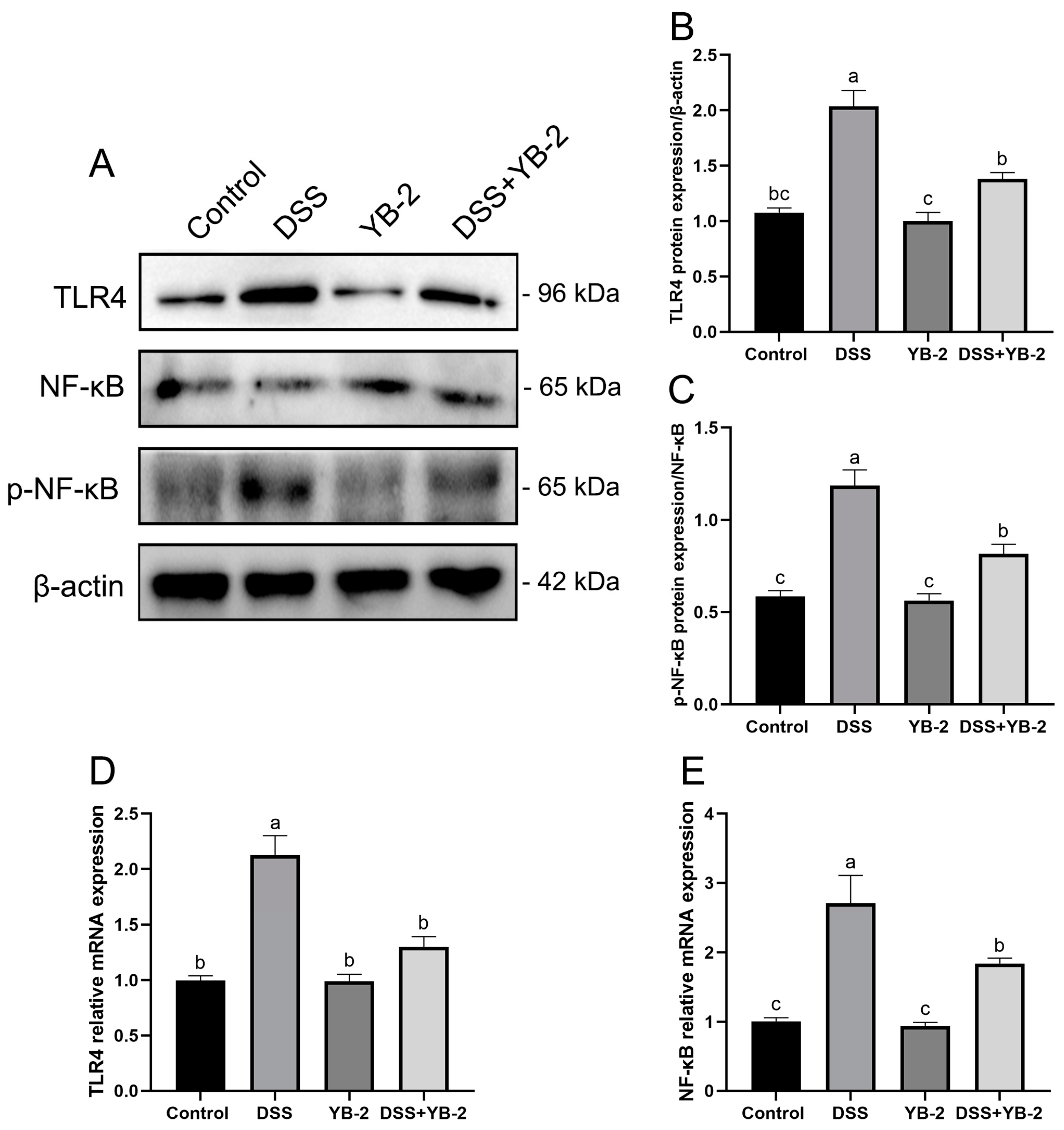
3.8. Effect of B. tequilensis YB-2 on the TLR4/NF-κB Signaling Pathway of Colitis Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khmaissa, M.; Zouari-Mechichi, H.; Sciara, G.; Record, E.; Mechichi, T. Pollution from livestock farming antibiotics an emerging environmental and human health concern: A Review. J. Hazard. Mater. Adv. 2024, 13, 100410. [Google Scholar] [CrossRef]
- Ngangom, B.L.; Tamunjoh, S.S.A.; Boyom, F.F. Antibiotic residues in food animals: Public health concern. Acta Ecol. Sin. 2019, 39, 411–415. [Google Scholar]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Rabetafika, H.N.; Razafindralambo, A.; Ebenso, B.; Razafindralambo, H.L. Probiotics as antibiotic alternatives for human and animal applications. Encyclopedia 2023, 3, 561–581. [Google Scholar] [CrossRef]
- Kapse, N.G.; Engineer, A.S.; Gowdaman, V.; Wagh, S.; Dhakephalkar, P.K. Functional annotation of the genome unravels probiotic potential of Bacillus coagulans HS243. Genomics 2019, 111, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Hotel, A.C.P.; Cordoba, A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 2001, 5, 1–10. [Google Scholar]
- Ruiz, S.S.; Bueno, T.; de Oliveira, A.; Karp, S.G.; Soccol, C.R. Bacillus subtilis natto as a potential probiotic in animal nutrition. Crit. Rev. Biotechnol. 2021, 41, 355–369. [Google Scholar] [CrossRef]
- Khalid, F.; Khalid, A.; Fu, Y.; Hu, Q.; Zheng, Y.; Khan, S.; Wang, Z. Potential of Bacillus velezensis as a probiotic in animal feed: A review. J. Microbiol. 2021, 59, 627–633. [Google Scholar] [CrossRef]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- Bank, S.; Andersen, P.S.; Burisch, J.; Pedersen, N.; Roug, S.; Galsgaard, J.; Turino, S.Y.; Brodersen, J.B.; Rashid, S.; Rasmussen, B.K.; et al. Genetically determined high activity of IL-12 and IL-18 in ulcerative colitis and TLR5 in Crohns disease were associated with non-response to anti-TNF therapy. Pharmacogenom. J. 2018, 18, 87–97. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, T.; Ding, H.; Chen, J.; Li, B.; Zhang, Q.; Yang, S.; Zhang, S.; Guan, W. Exploring the Benefits of Probiotics in Gut Inflammation and Diarrhea-From an Antioxidant Perspective. Antioxidants 2023, 12, 1342. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Xue, Y.; Wei, S.; Wu, B.; Wang, H.; Zeng, D.; Zhao, Y.; Khalique, A.; Pan, K.; Zeng, Y.; et al. Compound Probiotics Improve Body Growth Performance by Enhancing Intestinal Development of Broilers with Subclinical Necrotic Enteritis. Probiotics Antimicrob. Proteins 2023, 15, 558–572. [Google Scholar] [CrossRef]
- Hossen, I.; Hua, W.; Mehmood, A.; Raka, R.N.; Jingyi, S.; Jian-Ming, J.; Min, X.; Shakoor, A.; Yanping, C.; Wang, C.; et al. Glochidion ellipticum Wight extracts ameliorate dextran sulfate sodium-induced colitis in mice by modulating nuclear factor kappa-light-chain-enhancer of activated B cells signalling pathway. J. Pharm. Pharmacol. 2021, 73, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jian, W.; Wang, L.; Yang, S.; Niu, Y.; Xie, S.; Hayer, K.; Chen, K.; Zhang, Y.; Guo, Y. Alleviation of DSS-induced colitis in mice by a new-isolated Lactobacillus acidophilus C4. Front. Microbiol. 2023, 14, 1137701. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, Y.; Tan, Z.; Hong, Z.; Yao, L.; Huang, S.; Li, Z.; Zhang, L.; Li, H. Resveratrol ameliorates DSS-induced ulcerative colitis by acting on mouse gut microbiota. Inflammopharmacology 2024, 32, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yan, Y.; Zhong, J.; Zhao, Y.; Xu, Y.; Zhang, T.; Xiong, H.; Chen, Y.; Wang, X.; Zhang, K. Enterococcus durans 98D alters gut microbial composition and function to improve DSS-induced colitis in mice. Heliyon 2024, 10, e28486. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Wong, W.T.; Hsu, H.T.; Cheng, Y.H.; Yu, Y.H.; Chen, W.J.; Ho, C.L.; Hsu, H.C.; Hua, K.F. Surfactin Containing Bacillus licheniformis-Fermented Products Alleviate Dextran Sulfate Sodium-Induced Colitis by Inhibiting Colonic Inflammation and the NLRP3 Inflammasome in Mice. Animals 2022, 12, 3456. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zuo, Z.; Chen, K.; Gao, C.; Yang, Z.; Zhao, S.; Li, J.; Song, H.; Peng, X.; Fang, J.; et al. Histopathological Injuries, Ultrastructural Changes, and Depressed TLR Expression in the Small Intestine of Broiler Chickens with Aflatoxin B(1). Toxins 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.J.; Schetter, A.J.; Yfantis, H.G.; Ridnour, L.A.; Horikawa, I.; Khan, M.A.; Robles, A.I.; Hussain, S.P.; Goto, A.; Bowman, E.D.; et al. Macrophages, nitric oxide and microRNAs are associated with DNA damage response pathway and senescence in inflammatory bowel disease. PLoS ONE 2012, 7, e44156. [Google Scholar] [CrossRef]
- Xie, J.; Yu, R.; Qi, J.; Zhang, G.; Peng, X.; Luo, J. Pectin and inulin stimulated the mucus formation at a similar level: An omics-based comparative analysis. J. Food Sci. 2020, 85, 1939–1947. [Google Scholar] [CrossRef]
- Soni, A.; Oey, I.; Silcock, P.; Bremer, P. Bacillus Spores in the Food Industry: A Review on Resistance and Response to Novel Inactivation Technologies. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, Z.; Xu, Y.; Ying, J.; Wang, B.; Majeed, M.; Majeed, S.; Pande, A.; Li, W. Application of Bacillus coagulans in Animal Husbandry and Its Underlying Mechanisms. Animals 2020, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Oh, H.H.; Kim, Y. Probiotic characteristics of Bacillus tequilensis JBC17126 isolated from Korean traditional soybean paste. J. Korean Soc. Food Sci. Nutr. 2019, 7, 710–717. [Google Scholar] [CrossRef]
- Khan, Z.; Shafique, M.; Nawaz, H.R.; Jabeen, N.; Naz, S.A. Bacillus tequilensis ZMS-2: A novel source of alkaline protease with antimicrobial, anti-coagulant, fibrinolytic and dehairing potentials. Pak. J. Pharm. Sci. 2019, 32, 1913–1918. [Google Scholar]
- Wirtz, S.; Neurath, M.F. Animal models of intestinal inflammation: New insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int. J. Color. Dis. 2000, 15, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Li, Y.; Wang, Y.; Guo, Y.; Liu, J.; Zhao, S.; Wang, J.; Guan, G.; Luo, J.; Yin, H.; et al. Probiotic Bacillus licheniformis ZW3 Alleviates DSS-Induced Colitis and Enhances Gut Homeostasis. Int. J. Mol. Sci. 2024, 25, 561. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.; Zhou, J.; Hou, B.; Su, X.; Liu, Z.; Yuan, J.; Li, M. Bacillus licheniformis Zhengchangsheng(R) attenuates DSS-induced colitis and modulates the gut microbiota in mice. Benef. Microbes 2019, 10, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; LaRusso, N.F.; Gores, G.J. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: Linking inflammation to oncogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G626–G634. [Google Scholar] [CrossRef]
- Liang, T.Y.; Deng, R.M.; Li, X.; Xu, X.; Chen, G. The role of nitric oxide in peptic ulcer: A narrative review. Med. Gas. Res. 2021, 11, 42–45. [Google Scholar]
- Bamias, G.; Cominelli, F. Cytokines and intestinal inflammation. Curr. Opin. Gastroenterol. 2016, 32, 437–442. [Google Scholar] [CrossRef]
- Nakanishi, T.; Yamanaka, K.; Kakeda, M.; Tsuda, K.; Mizutani, H. Mutant interleukin-4/13 signaling blockade successfully suppresses acute phase inflammation. Arch. Dermatol. Res. 2013, 305, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qin, S.; Yang, C.; Niu, Y.; Feng, J. The protective effects of Bacillus licheniformis against inflammatory responses and intestinal barrier damage in broilers with necrotic enteritis induced by Clostridium perfringens. J. Sci. Food Agric. 2023, 103, 6958–6965. [Google Scholar] [CrossRef]
- Jung, H.; Lee, H.; Kim, K.; Lee, N.; Paik, H. Anti-inflammatory, antioxidant effects, and antimicrobial effect of Bacillus subtilis P223. Food Sci. Biotechnol. 2023, 1–9. [Google Scholar] [CrossRef]
- Deng, L.; Guo, H.; Wang, S.; Liu, X.; Lin, Y.; Zhang, R.; Tan, W. The Attenuation of Chronic Ulcerative Colitis by (R)-salbutamol in Repeated DSS-Induced Mice. Oxid. Med. Cell. Longev. 2022, 2022, 9318721. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Papageorgiou, I.; Charonis, A. Enterocytes’ tight junctions: From molecules to diseases. World J. Gastrointest. Pathophysiol. 2011, 2, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Goswami, P.; Das, T.K.; Nag, T.; Sreenivas, V.; Ahuja, V.; Panda, S.K.; Gupta, S.D.; Makharia, G.K. Comparative tight junction protein expressions in colonic Crohn’s disease, ulcerative colitis, and tuberculosis: A new perspective. Virchows Arch. 2012, 460, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef]
- Alipour, M.; Zaidi, D.; Valcheva, R.; Jovel, J.; Martínez, I.; Sergi, C.; Walter, J.; Mason, A.L.; Wong, G.; Dieleman, L.A. Mucosal barrier depletion and loss of bacterial diversity are primary abnormalities in paediatric ulcerative colitis. J Crohns Colitis 10: 462–471. Nature 2016, 567, 49–55. [Google Scholar]
- Prakash, R.; Bharathi, R.S.; Devaraj, H.; Devaraj, S.N. Up-regulation of MUC2 and IL-1beta expression in human colonic epithelial cells by Shigella and its interaction with mucins. PLoS ONE 2011, 6, e27046. [Google Scholar] [CrossRef] [PubMed]
- Bankole, E.; Read, E.; Curtis, M.A.; Neves, J.F.; Garnett, J.A. The Relationship between Mucins and Ulcerative Colitis: A Systematic Review. J. Clin. Med. 2021, 10, 1935. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, N.; Boerner, K.; Waschke, J. Targeting desmosomal adhesion and signalling for intestinal barrier stabilization in inflammatory bowel diseases-Lessons from experimental models and patients. Acta Physiol. 2021, 231, e13492. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, N.G. Effects of Indigenous Spore-Forming Probiotic as Feed Supplement on Performance and Safety in Broilers. J. Hell. Vet. Med. Soc. 2019, 70, 70. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, F.; Huang, L.; Teng, H.; Shen, T.; Qin, H. Long-term and continuous administration of Bacillus subtilis during remission effectively maintains the remission of inflammatory bowel disease by protecting intestinal integrity, regulating epithelial proliferation, and reshaping microbial structure and function. Food Funct. 2021, 12, 2201–2210. [Google Scholar] [PubMed]
- Tang, Y.; Clayburgh, D.R.; Mittal, N.; Goretsky, T.; Dirisina, R.; Zhang, Z.; Kron, M.; Ivancic, D.; Katzman, R.B.; Grimm, G. Epithelial NF-κB enhances transmucosal fluid movement by altering tight junction protein composition after T cell activation. Am. J. Pathol. 2010, 176, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Zhao, P.; Li, F.; Bao, H.; Ding, S.; Ji, L.; Yan, J. MicroRNA-16 inhibits the TLR4/NF-kappaB pathway and maintains tight junction integrity in irritable bowel syndrome with diarrhea. J. Biol. Chem. 2022, 298, 102461. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; You, M.; Shi, H.; Hou, Y. Ubiquitin-mediated NFkappaB degradation pathway. Cell. Mol. Immunol. 2015, 12, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.L.; O’Neill, L.A. Toll-like receptors: From the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 2006, 72, 1102–1113. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Notas, G.; Panagiotopoulos, A.; Vamvoukaki, R.; Kalyvianaki, K.; Kiagiadaki, F.; Deli, A.; Kampa, M.; Castanas, E. ERalpha36-GPER1 Collaboration Inhibits TLR4/NFkappaB-Induced Pro-Inflammatory Activity in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 7603. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Y.; Lai, X.; Wang, W.; Zhang, J.; Chen, S. Genetic Deletion of Fbw7 in the mouse intestinal epithelium aggravated dextran sodium sulfate-induced colitis by modulating the inflammatory response of NF-kappaB pathway. Biochem. Biophys. Res. Commun. 2018, 498, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tang, R.; Zhao, C.; Mu, R.; Wang, Y.; Cao, Y.; Zhang, N.; Fu, Y. The Prevention Effect of Bacillus subtilis on Escherichia coli-Induced Mastitis in Mice by Suppressing the NF-kappaB and MAPK Signaling Pathways. Probiotics Antimicrob. Proteins 2023, 15, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Niu, H.; Sun, M.; Miao, X.; Jin, X.; Xu, X.; Yanping, C.; Mei, H.; Wang, J.; Da, L.; et al. Effects of Bacillus subtilis natto JLCC513 on gut microbiota and intestinal barrier function in obese rats. J. Appl. Microbiol. 2022, 133, 3634–3644. [Google Scholar] [CrossRef] [PubMed]
 ); LY: lysosomes (□); JC: junctional complexes (
); LY: lysosomes (□); JC: junctional complexes ( )); (D) Alcian blue/PAS staining of colonic tissue, ×400, bar = 50 μm; (E) the number of goblet cells in colon. Values are presented with the means ± SEM (n = 6). Significant difference was expressed with different letters among different groups at p < 0.05.
)); (D) Alcian blue/PAS staining of colonic tissue, ×400, bar = 50 μm; (E) the number of goblet cells in colon. Values are presented with the means ± SEM (n = 6). Significant difference was expressed with different letters among different groups at p < 0.05.
 ); LY: lysosomes (□); JC: junctional complexes (
); LY: lysosomes (□); JC: junctional complexes ( )); (D) Alcian blue/PAS staining of colonic tissue, ×400, bar = 50 μm; (E) the number of goblet cells in colon. Values are presented with the means ± SEM (n = 6). Significant difference was expressed with different letters among different groups at p < 0.05.
)); (D) Alcian blue/PAS staining of colonic tissue, ×400, bar = 50 μm; (E) the number of goblet cells in colon. Values are presented with the means ± SEM (n = 6). Significant difference was expressed with different letters among different groups at p < 0.05.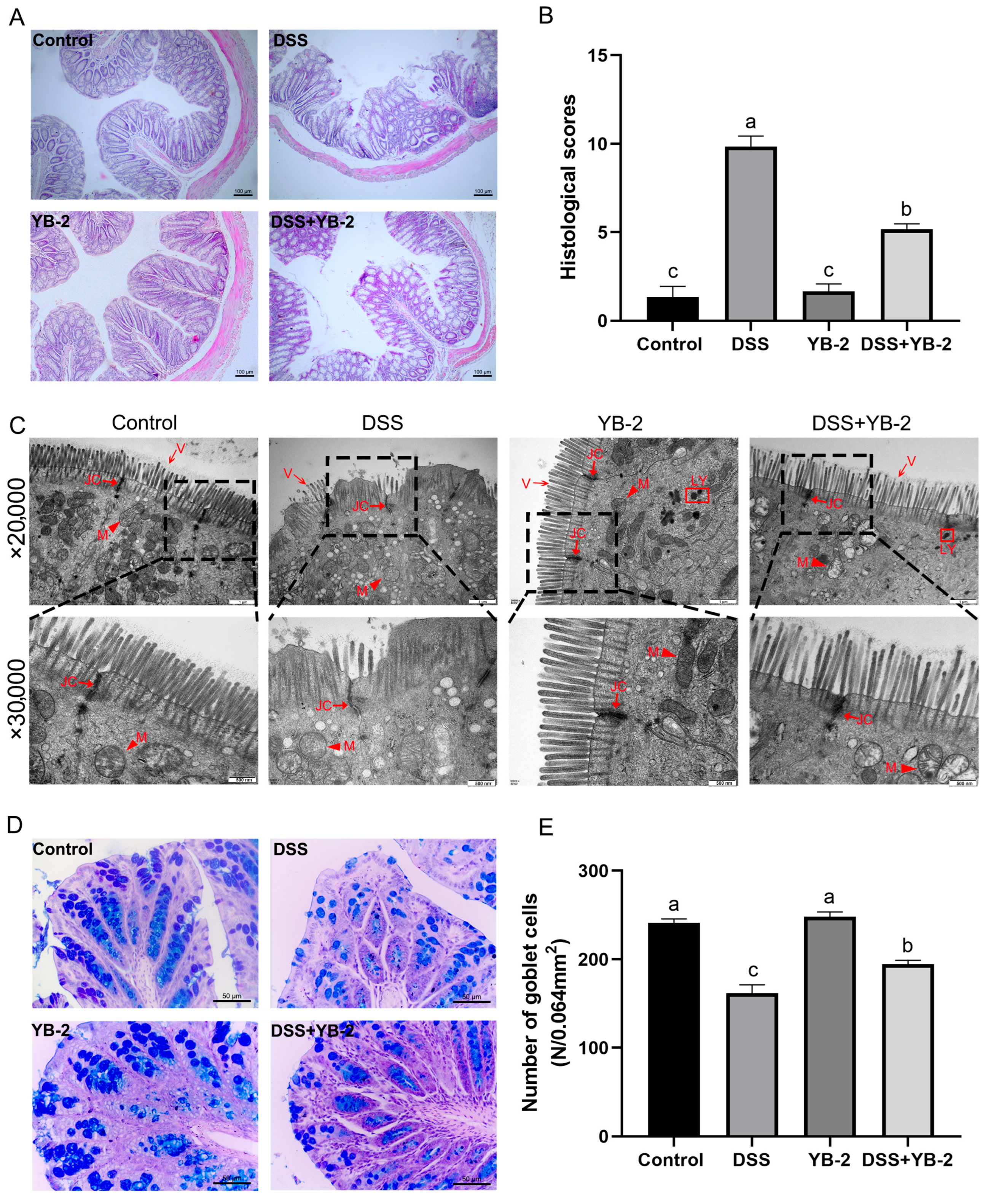


| Control Group | DSS Group | YB-2 Group | DSS + YB-2 Group | |
|---|---|---|---|---|
| 18–22 g Male ICR Mice, n = 18/Group | ||||
| 1th to 4th week | Sterile saline (i.g) | Sterile saline (i.g) | B. tequilensis YB-2 suspension (i.g) | B. tequilensis YB-2 suspension (i.g) |
| 5th week | The normal drinking water | 3% DSS in the drinking water | The normal drinking water | 3% DSS in the drinking water |
| Target Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| IL-1β | TCGGCAAAGAAATCAAGATGGC | GTGCAAGTCTCATGAAGTGAGC |
| IL-6 | ACAGAAGGAGTGGCTAAGGA | AGGCATAACGCACTAGGTTT |
| TNF-α | CGTCGTAGCAAACCACCAAG | TTGAAGAGAACCTGGGAGTAGACA |
| IL-4 | CTTCCAAGGTGCTTCGCATA | GATGAATCCAGGCATCGAAA |
| IL-10 | AATTCCCTGGGTGAGAAGCTGAAG | CTGCTCCACTGCCTTGCTCTTAT |
| MUC2 | AGTCTGCTCGTGAAGTGCC | GGCAAACACAGTCCTTGCAG |
| ZO-1 | CTTCTCTTGCTGGCCCTAAAC | TGGCTTCACTTGAGGTTTCTG |
| Occludin | CACACTTGCTTGGGACAGAG | TAGCCATAGCCTCCATAGCC |
| Claudin-1 | GGTTATCGGAACTGTGGTAGAA | GTGCTCAGGGAAGATGGTAAG |
| TLR4 | GCCGGAAAGTTATTGTGGTG | ATGGGTTTTAGGCGCAGAGTT |
| NF-κB | GGGCATGCGTTTCCGTTACA | ATGTGGATGAGGCCGGTGAG |
| β-actin | GGAGATTACTGCCCTGGCTCCTA | GACTCATCGTACTCCTGCTTGCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Wang, C.; Shuai, Y.; Xie, Z.; Liu, J. Pig-Derived Probiotic Bacillus tequilensis YB-2 Alleviates Intestinal Inflammation and Intestinal Barrier Damage in Colitis Mice by Suppressing the TLR4/NF-κB Signaling Pathway. Animals 2024, 14, 1989. https://doi.org/10.3390/ani14131989
Yin H, Wang C, Shuai Y, Xie Z, Liu J. Pig-Derived Probiotic Bacillus tequilensis YB-2 Alleviates Intestinal Inflammation and Intestinal Barrier Damage in Colitis Mice by Suppressing the TLR4/NF-κB Signaling Pathway. Animals. 2024; 14(13):1989. https://doi.org/10.3390/ani14131989
Chicago/Turabian StyleYin, Heng, Chengbi Wang, Yi Shuai, Zhuoya Xie, and Jingbo Liu. 2024. "Pig-Derived Probiotic Bacillus tequilensis YB-2 Alleviates Intestinal Inflammation and Intestinal Barrier Damage in Colitis Mice by Suppressing the TLR4/NF-κB Signaling Pathway" Animals 14, no. 13: 1989. https://doi.org/10.3390/ani14131989
APA StyleYin, H., Wang, C., Shuai, Y., Xie, Z., & Liu, J. (2024). Pig-Derived Probiotic Bacillus tequilensis YB-2 Alleviates Intestinal Inflammation and Intestinal Barrier Damage in Colitis Mice by Suppressing the TLR4/NF-κB Signaling Pathway. Animals, 14(13), 1989. https://doi.org/10.3390/ani14131989






