Assessment of Ammonia Concentrations and Climatic Conditions in Calf Housing Using Stationary and Mobile Sensors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
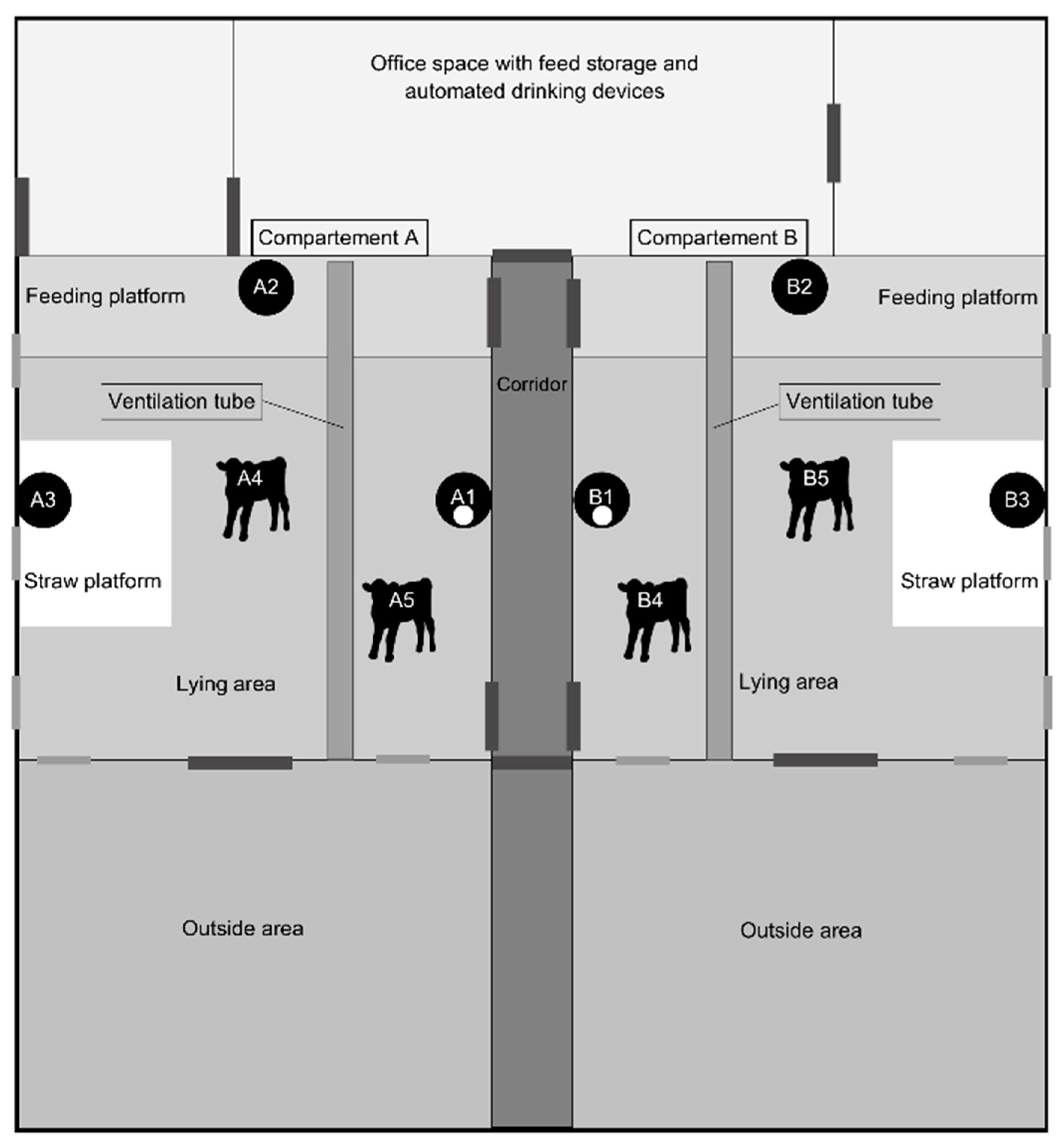
2.1. Housing and Ventilation
2.2. Animals
2.3. Sensors
2.3.1. Stationary Device for NH3 Measurement
2.3.2. Mobile Device for NH3 Measurement
2.3.3. Stationary Device for Temperature, Relative Humidity, Atmospheric Pressure, and CO2 Measurement
2.4. Set-Up Measuring Devices
2.4.1. NH3 Sensors
2.4.2. Temperature, Relative Humidity, Atmospheric Pressure, and CO2 Sensors
2.5. Statistical Analysis
3. Results
3.1. NH3 Concentrations
3.2. CO2 Concentrations, Temperature, and Relative Humidity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caja, G.; Castro-Costa, A.; Knight, C.H. Engineering to Support Wellbeing of Dairy Animals. J. Dairy Res. 2016, 83, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Alsaaod, M.; Fadul, M.; Steiner, A. Automatic Lameness Detection in Cattle. Vet. J. 2019, 246, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Vandermeulen, J.; Bahr, C.; Johnston, D.; Earley, B.; Tullo, E.; Fontana, I.; Guarino, M.; Exadaktylos, V.; Berckmans, D. Early Recognition of Bovine Respiratory Disease in Calves Using Automated Continuous Monitoring of Cough Sounds. Comput. Electron. Agric. 2016, 129, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.G.; Conceição, C.; Pereira, A.M.F.; Cerqueira, J.L.; Silva, S.R. Literature Review on Technological Applications to Monitor and Evaluate Calves’ Health and Welfare. Animals 2023, 13, 1148. [Google Scholar] [CrossRef] [PubMed]
- Costantino, A.; Fabrizio, E.; Calvet, S. The Role of Climate Control in Monogastric Animal Farming: The Effects on Animal Welfare, Air Emissions, Productivity, Health, and Energy Use. Appl. Sci. 2021, 11, 9549. [Google Scholar] [CrossRef]
- Busato, A.; Steiner, L.; Martin, S.W.; Shoukri, M.M.; Gaillard, C. Calf Health in Cow-Calf Herds in Switzerland. Prev. Vet. Med. 1997, 30, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Pardon, B.; Hostens, M.; Duchateau, L.; Dewulf, J.; Bleecker, K.; Deprez, P. Impact of Respiratory Disease, Diarrhea, Otitis and Arthritis on Mortality and Carcass Traits in White Veal Calves. BMC Vet. Res. 2013, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Van der Fels-Klerx, H.J.; Horst, H.S.; Dijkhuizen, A.A. Risk Factors for Bovine Respiratory Disease in Dairy Youngstock in The Netherlands: The Perception of Experts. Livest. Prod. Sci. 2000, 66, 35–46. [Google Scholar] [CrossRef]
- Kaske, M.; Kunz, H.-J. Gesundheits- Und Haltungsmanagement in Der Kälberaufzucht. Nutztierpraxis Aktuell. 2007, 20, 12–19. [Google Scholar]
- Bähler, C.; Steiner, A.; Luginbühl, A.; Ewy, A.; Posthaus, H.; Strabel, D.; Kaufmann, T.; Regula, G. Risk Factors for Death and Unwanted Early Slaughter in Swiss Veal Calves Kept at a Specific Animal Welfare Standard. Res. Vet. Sci. 2012, 92, 162–168. [Google Scholar] [CrossRef]
- Roland, L.; Drillich, M.; Klein-Jöbstl, D.; Iwersen, M. Invited Review: Influence of Climatic Conditions on the Development, Performance, and Health of Calves. J. Dairy Sci. 2016, 99, 2438–2452. [Google Scholar] [CrossRef] [PubMed]
- Van Leenen, K.; Jouret, J.; Demeyer, P.; Van Driessche, L.; Cremer, L.; Masmeijer, C.; Boyen, F.; Deprez, P.; Pardon, B. Associations of Barn Air Quality Parameters with Ultrasonographic Lung Lesions, Airway Inflammation and Infection in Group-Housed Calves. Prev. Vet. Med. 2020, 181, 105056. [Google Scholar] [CrossRef]
- Van Putten, G. An Ethological Definition of Animal Welfare with Special Emphasis on Pig Behaviour. In Proceedings of the Second NAHWOA Workshop; Hovi, M., Garcia Trujillo, R., Eds.; University of Reading: Cordoba, Spain, 2000. [Google Scholar]
- Hämeenoja, P. Animal Health and Welfare—Pig Production. Acta Vet. Scand. 2001, 95, 33–36. [Google Scholar] [CrossRef]
- Hillman, P.; Gebremedhin, K.; Warner, R. Ventilation System to Minimize Airborne Bacteria, Dust, Humidity, and Ammonia in Calf Nurseries. J. Dairy Sci. 1992, 75, 1305–1312. [Google Scholar] [CrossRef]
- Lago, A.; McGuirk, S.M.; Bennett, T.B.; Cook, N.B.; Nordlund, K.V. Calf Respiratory Disease and Pen Microenvironments in Naturally Ventilated Calf Barns in Winter. J. Dairy Sci. 2006, 89, 4014–4025. [Google Scholar] [CrossRef] [PubMed]
- Van Caenegem, L. Kälber Brauchen Aussenlufqualität; ART-Berichte; Forschungsantalt Agroscope Reckenholz-Tänikon ART: Ettenhausen, Switzerland, 2006. [Google Scholar]
- Caroprese, M. Sheep Housing and Welfare. Small Rumin. Res. 2008, 76, 21–25. [Google Scholar] [CrossRef]
- Cusack, P.M.V.; McMeniman, N.P.; Lean, I.J. Feedlot Entry Characteristics and Climate: Their Relationship with Cattle Growth Rate, Bovine Respiratory Disease and Mortality. Aust. Vet. J. 2007, 85, 311–316. [Google Scholar] [CrossRef]
- Bundesamt für Lebensmittelsicherheit und Veterinärwesen BLV. Fachinformation Tierschutz Stallklimawerte und Ihre Messung in der Rinderhaltung; Bundesamt für Lebensmittelsicherheit und Veterinärwesen BLV: Bern, Switzerland, 2009; Volume 2009.
- Seedorf, J. Wirkung von Atmosphärischem Ammoniak Auf Nutztiere–Eine Kurzübersicht. Impact of Atmospheric Ammonia on Livestock Animals–a Minireview. Berl. Münch. Tierärztl. Wschr. 2013, 126, 96–103. [Google Scholar] [CrossRef]
- Callan, R.J.; Garry, F.B. Biosecurity and Bovine Respiratory Disease. Vet. Clin. N. Am. Food Anim. Pract. 2002, 18, 57–77. [Google Scholar] [CrossRef]
- Zachary, J.F.; McGavin, M.D. Pathologic Basis of Veterinary Disease, 5th ed.; Mosby: St. Louis, MO, USA, 2011; ISBN 0-323-07533-9. [Google Scholar]
- Atta, A. Ammonia Emissions and Safety; Practical Information for Alberta’s Agriculture Industrie; Agriculture, Food and Rural Development: Edmonton, AB, Canada, 2006; p. 2. [Google Scholar]
- Sevi, A.; Albenzio, M.; Muscio, A.; Casamassima, D.; Centoducati, P. Effects of Litter Management on Airborne Particulates in Sheep Houses and on the Yield and Quality of Ewe Milk. Livest. Prod. Sci. 2003, 81, 1–9. [Google Scholar] [CrossRef]
- García-Ramos, F.J.; Aguirre, A.J.; Barreiro, P.; Horcas, E.; Boné, A.; Vidal, M. Applicability of Ammonia Sensors for Controlling Environmental Parameters in Accommodations for Lamb Fattening. J. Sens. 2018, 2018, 4032043. [Google Scholar] [CrossRef]
- International Commission of Agricultural Engineering. Report of Working Group on Climatization of Animal Houses; International Commission of Agricultural Engineering: Liege, Belgium, 1984. [Google Scholar]
- International Commission of Agricultural Engineering. Design Recommendations of Beef Cattle Housing; International Commission of Agricultural Engineering: Liege, Belgium, 2004. [Google Scholar]
- Wenke, C.; Pospiech, J.; Reutter, T.; Altmann, B.; Truyen, U.; Speck, S. Impact of Different Supply Air and Recirculating Air Filtration Systems on Stable Climate, Animal Health, and Performance of Fattening Pigs in a Commercial Pig Farm. PLoS ONE 2018, 13, e0194641. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, P.; Schönecker, L.; Schüpbach-Regula, G.; Meylan, M. Effects of Management Practices, Animal Transport and Barn Climate on Animal Health and Antimicrobial Use in Swiss Veal Calf Operations. Prev. Vet. Med. 2019, 167, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Xiong, Y.; Gates, R.S.; Wang, Y.; Koelkebeck, K.W. Air Temperature, Carbon Dioxide, and Ammonia Assessment inside a Commercial Cage Layer Barn with Manure-Drying Tunnels. Poult. Sci. 2020, 99, 3885–3896. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Bucher-Schnyder, P.; Schönecker, L.; Stucki, D.; Meylan, M. Evaluation of associations between barn characteristics, results of barn climate parameter measurements and health indicators in Swiss veal calf herds. Schweiz Arch Tierheilkd 2022, 164, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Leytem, A.B.; Dungan, R.S.; Bjorneberg, D.L.; Koehn, A.C. Emissions of Ammonia, Methane, Carbon Dioxide, and Nitrous Oxide from Dairy Cattle Housing and Manure Management Systems. J. Environ. Qual. 2011, 40, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Camiloti, T.V.; Fregonesi, J.A.; Keyserlingk, M.A.G.; Weary, D.M. Short Communication: Effects of Bedding Quality on the Lying Behavior of Dairy Calves. J. Dairy Sci. 2012, 95, 3380–3383. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.M.; Bateman, H.G., II; Aldrich, J.M.; Quigley, J.D.; Schlotterbeck, R.L. Short Communication: Intensive Measurements of Standing Time of Dairy Calves Housed in Individual Pens within a Naturally Ventilated, Unheated Nursery over Different Periods of the Year. J. Dairy Sci. 2013, 96, 1811–1814. [Google Scholar] [CrossRef]
- Pedersen, S.V.; Di Perta, E.S.; Hafner, S.D.; Pacholski, A.S.; Sommer, S.G. Evaluation of a Simple, Small-Plot Meteorological Technique for Measurement of Ammonia Emission: Feasibility, Costs, and Recommendations. Trans. ASABE 2018, 61, 103–115. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Heber, A.J. Sampling and Measurement of Ammonia at Animal Facilities. Adv. Agron. 2008, 98, 201–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Lisle, A.T.; Phillips, C.J.C. Development of an Effective Sampling Strategy for Ammonia, Temperature and Relative Humidity Measurement during Sheep Transport by Ship. Biosyst. Eng. 2017, 155, 12–23. [Google Scholar] [CrossRef]
- Seedorf, J.; Hartung, J.; Schröder, M.; Linkert, K.H.; Pedersen, S.; Takai, H.; Johnsen, J.O.; Metz, J.H.M.; Groot Koerkamp, P.W.G.; Uenk, G.H.; et al. Temperature and Moisture Conditions in Livestock Buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 49–57. [Google Scholar] [CrossRef]
- Seedorf, J.; Hartung, J. Survey of Ammonia Concentrations in Livestock Buildings. J. Agric. Sci. 1999, 133, 433–437. [Google Scholar] [CrossRef]
- Schüller, L.K.; Heuwieser, W. Measurement of Heat Stress Conditions at Cow Level and Comparison to Climate Conditions at Stationary Locations inside a Dairy Barn. J. Dairy Res. 2016, 83, 305–311. [Google Scholar] [CrossRef]
- Louie, A.P.; Rowe, J.D.; Love, W.J.; Lehenbauer, T.W.; Aly, S.S. Effect of the Environment on the Risk of Respiratory Disease in Preweaning Dairy Calves during Summer Months. J. Dairy Sci. 2018, 101, 10230–10247. [Google Scholar] [CrossRef]
- GoogleMaps. Ufa AG Versuchsbetrieb. Available online: https://www.google.com/maps/place/Ufa+AG/@47.3784444,8.207364,17z/data=!3m1!4b1!4m5!3m4!1s0x4790168f2ac7c10b:0x40e5c8c62547dcdf!8m2!3d47.3784408!4d8.2095527 (accessed on 30 November 2021).
- Coop Naturafarm. Richtline Coop Naturafarm Kalb; Coop Naturafarm: Basel, Switzerland, 2015. [Google Scholar]
- Von Jasmund, N.; Schmithausen, A.J.; Krommweh, M.S.; Trimborn, M.; Boeker, P.; Büscher, W. Assessment of Ammonia Sensors and Photoacoustic Measurement Systems Using a Gas Calibration Unit. Comput. Electron. Agric. 2022, 194, 106744. [Google Scholar] [CrossRef]
- Melse, R.W.; Ploegaert, J.P.M.; Ogink, N.W.M. Laboratory Test of Draeger Polytron 8000 with FL-6813260 Sensor for NH3 Measurement; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2016; p. 31. [Google Scholar] [CrossRef]
- Nauber, A.; Sick, M.; Steiner, G.; Mattern-Frühwald, M.-I.; Mett, F.; Chrzan, R.; Sommer, S. Electrochemical Gas Sensor, Liquid Electrolyte and Use of a Liquid Electrolyte. European Patent EP 3,044,576 B1, 20 July 2016. [Google Scholar]
- Teye, F.K.; Hautala, M.; Pastell, M.; Praks, J.; Veermäe, I.; Poikalainen, V.; Pajumägi, A.; Kivinen, T.; Ahokas, J. Microclimate and Ventilation in Estonian and Finnish Dairy Buildings. Energy Build. 2008, 40, 1194–1201. [Google Scholar] [CrossRef]
- Hamilton, T.D.; Roe, J.M.; Webster, A.J. Synergistic Role of Gaseous Ammonia in Etiology of Pasteurella Multocida-Induced Atrophic Rhinitis in Swine. J. Clin. Microbiol. 1996, 34, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Albright, J.L.; Stouffer, D.K.; Kenyon, N.J. Behaviour of Veal Calves in Individual Stalls and Group Pens. In New Trends in Veal Calf Production; Metz, J.H.M., Groenestein, C.M., Eds.; PUDOC: Wageningen, The Netherlands, 1991; Volume 52, pp. 44–48. [Google Scholar]
- Le Neidre, P. Effects of Breed and Early Social Environment on Calf Behaviour; Metz, J.H.M., Groenestein, C.M., Eds.; Pudoc Wageningen: Waegeningen, The Netherlands, 1991; Volume 52, pp. 32–35. [Google Scholar]
- Jungbluth, T.; Hartung, E.; Brose, G. Greenhouse Gas Emissions from Animal Houses and Manure Stores. Nutr. Cycl. Agroecosyst. 2001, 60, 133–145. [Google Scholar] [CrossRef]
- Kaufman, J.; Linington, M.; Osborne, V.R.; Wagner-Riddle, C.; Wright, T.C. Short Communication: Field Study of Air Ammonia Concentrations in Ontario Dairy Calf Housing Microenvironments. Can. J. Anim. Sci. 2015, 95, 539–542. [Google Scholar] [CrossRef]
- Bonizzi, S.; Gislon, G.; Brasca, M.; Morandi, S.; Sandrucci, A.; Zucali, M. Air Quality, Management Practices and Calf Health in Italian Dairy Cattle Farms. Animals 2022, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- Frosch, W.; Bachmann, K. Forschungsbericht Agrartechnik: Verfahrenstechnische Bewertungen Physikalisch-Chemischer Messprinzipien Zur Ammoniakquantifizierung in Stallanlagen Der Landwirtschaftlichen Nutztierhaltung Im Rahmen Des Projekts Zur „Modifizierung von Regeleingangsgrößen in Zwangsbelüfteten Anlagen Der Tierproduktion“; Bundesanstalt für Landwirtschaft und Ernhährung: Bonn, Germany, 2016. [Google Scholar]
- Urbain, B.; Gustin, P.; Charlier, G.; Coignoul, F.; Lambotte, J.L.; Grignon, G.; Foliguet, B.; Vidic, B.; Beerens, D.; Prouvost, J.F.; et al. A Morphometric and Functional Study of the Toxicity of Atmospheric Ammonia in the Extrathoracic Airways in Pigs. Vet. Res. Commun. 1996, 20, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Broucek, J.; Kisac, P.; Uhrincat, M. Effect of Hot Temperatures on the Hematological Parameters, Health and Performance of Calves. Int. J. Biometeorol. 2009, 53, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Bakony, M.; Jurkovich, V. Heat Stress in Dairy Calves from Birth to Weaning. J. Dairy Res. 2020, 87, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Wang, F.; Xiao, J.; Wang, Y.; Yang, H.; Li, S.; Cao, Z. Heat Stress on Calves and Heifers: A Review. J. Anim. Sci. Biotechnol. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Prill, R. Why Measure Carbon Dioxide Inside Buildings? Extension Energy Program; Washington State University: Washington, DC, USA, 2000. [Google Scholar]
- Sousa, P.; Pedersen, S. Ammonia Emission from Fattening Pig Houses in Relation to Animal Activity and Carbon Dioxide Production. Agric. Eng. Int. CIGR J. 2004, 6, 1–13. [Google Scholar]
- European Food Safety Authority. Scientific Report on the Effects of Farming Systems on Dairy Cow Welfare and Disease. EFSA J. 2009, 7, 1143r. [Google Scholar] [CrossRef] [PubMed]
- ASABE. Manure Storage Safety; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2011. [Google Scholar]
- Vtoryi, V.; Vtoryi, S.; Ylyin, R. Ammonia Concentration in Cow Barn under Limited Air Exchange. In Proceedings of the 18th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 22–24 May 2019; pp. 1593–1598. [Google Scholar]
- Herbut, P.; Angrecka, S. Ammonia Concentrations in a Free-Stall Dairy Barn. Ann. Anim. Sci. 2014, 14, 153–166. [Google Scholar] [CrossRef]
- Weaver, W.D.; Meijerhof, R. The Effect of Different Levels of Relative Humidity and Air Movement on Litter Conditions, Ammonia Levels, Growth, and Carcass Quality for Broiler Chickens. Poult. Sci. 1991, 70, 746–755. [Google Scholar] [CrossRef]
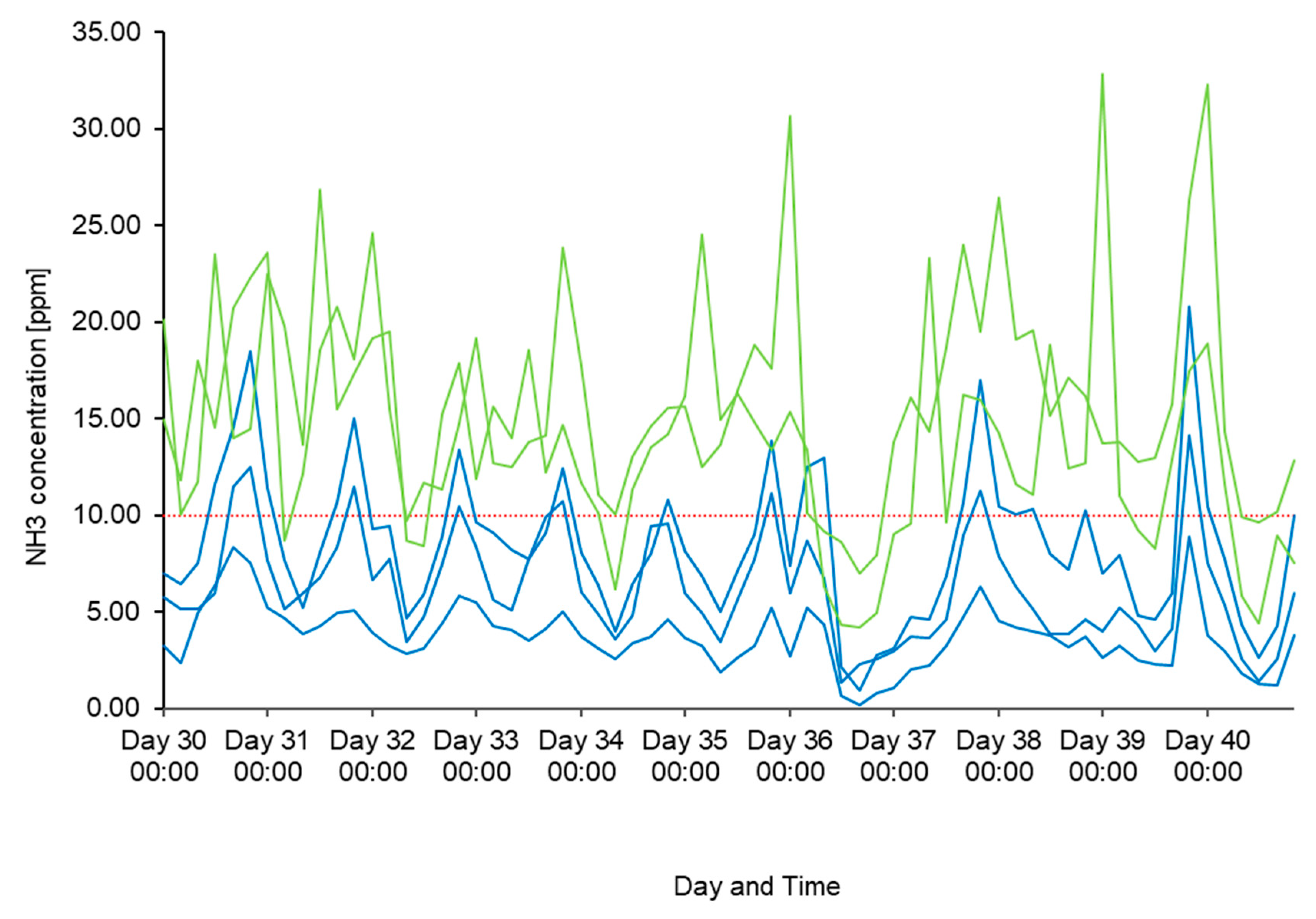


| Sensor | Overall Mean | Sd | Minimum | Maximum | n ≥ 10 ppm | % ≥ 10 ppm | n ≤ 5 ppm | % ≤ 5 ppm | n |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 8.46 | 4.76 | 0 | 43.50 | 2924 | 32.78 | 2461 | 27.59 | 8921 |
| A2 | 7.05 | 3.76 | 0 | 26.00 | 1835 | 20.58 | 3258 | 36.53 | 8918 |
| A3 | 5.94 | 4.56 | 0 | 47.00 | 1856 | 20.82 | 5219 | 59.35 | 8915 |
| A4 | 14.67 | 8.63 | 1 | 99.00 | 6376 | 72.13 | 521 | 5.89 | 8839 |
| A5 | 13.69 | 11.05 | 0.5 | 96.50 | 5178 | 58.59 | 1241 | 14.04 | 8837 |
| B1 | 7.60 | 5.41 | 0 | 65.50 | 2364 | 26.6 | 3469 | 39.03 | 8858 |
| B2 | 8.78 | 4.63 | 0.5 | 31.00 | 3005 | 33.68 | 2165 | 24.27 | 8921 |
| B3 | 9.40 | 6.49 | 0 | 45.50 | 3275 | 36.72 | 2531 | 28.37 | 8920 |
| B4 | 11.33 | 8.89 | 0.5 | 98.5 | 3484 | 47.50 | 1475 | 20.11 | 7335 |
| B5 | 13.84 | 9.92 | 1 | 97.5 | 5361 | 60.28 | 878 | 9.87 | 8894 |
| Sensor | Overall Mean | Sd | Min | Max | n ≥ 3000 ppm | % ≥ 3000 ppm | n |
|---|---|---|---|---|---|---|---|
| A1 | 1256 | 445 | 0 | 4952 | 90 | 1.02 | 8790 |
| B1 | 1340 | 502 | 0 | 4966 | 121 | 1.38 | 8759 |
| Sensor | Overall Mean | Sd | Min | Max | n ≥ 20 °C | % ≥ 20 °C | n |
|---|---|---|---|---|---|---|---|
| A1 | 24.78 | 3.36 | 16.2 | 34.0 | 8154 | 92.07 | 8857 |
| B1 | 24.7 | 3.24 | 15.1 | 33.5 | 8212 | 92.64 | 8864 |
| Sensor | Overall Mean | +/− Sd | Min | Max | n ≥ 80% rH | % ≥ 80% rH | n ≤ 50% rH | % ≤ 50% rH | n |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 73.2 | 9.11 | 39.5 | 93.9 | 2302 | 25.99 | 117 | 1.32 | 8857 |
| B1 | 74.8 | 12.10 | 34.1 | 99.4 | 1696 | 34.27 | 128 | 2.59 | 4949 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moser, J.; Kohler, S.; Hentgen, J.; Meylan, M.; Schüpbach-Regula, G. Assessment of Ammonia Concentrations and Climatic Conditions in Calf Housing Using Stationary and Mobile Sensors. Animals 2024, 14, 2001. https://doi.org/10.3390/ani14132001
Moser J, Kohler S, Hentgen J, Meylan M, Schüpbach-Regula G. Assessment of Ammonia Concentrations and Climatic Conditions in Calf Housing Using Stationary and Mobile Sensors. Animals. 2024; 14(13):2001. https://doi.org/10.3390/ani14132001
Chicago/Turabian StyleMoser, Julia, Samuel Kohler, Jérémy Hentgen, Mireille Meylan, and Gertraud Schüpbach-Regula. 2024. "Assessment of Ammonia Concentrations and Climatic Conditions in Calf Housing Using Stationary and Mobile Sensors" Animals 14, no. 13: 2001. https://doi.org/10.3390/ani14132001
APA StyleMoser, J., Kohler, S., Hentgen, J., Meylan, M., & Schüpbach-Regula, G. (2024). Assessment of Ammonia Concentrations and Climatic Conditions in Calf Housing Using Stationary and Mobile Sensors. Animals, 14(13), 2001. https://doi.org/10.3390/ani14132001






