Molecular and Serological Surveillance for Mycobacterium leprae and Mycobacterium lepromatosis in Wild Red Squirrels (Sciurus vulgaris) from Scotland and Northern England
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Squirrel Samples
2.2. DNA Extraction
2.3. Real-Time PCR
2.4. Anti-PGL-I UCP-LFA
2.5. Anti-PGL-I Antibody ELISA
2.6. M. leprae Genotyping
2.7. Statistical Analysis
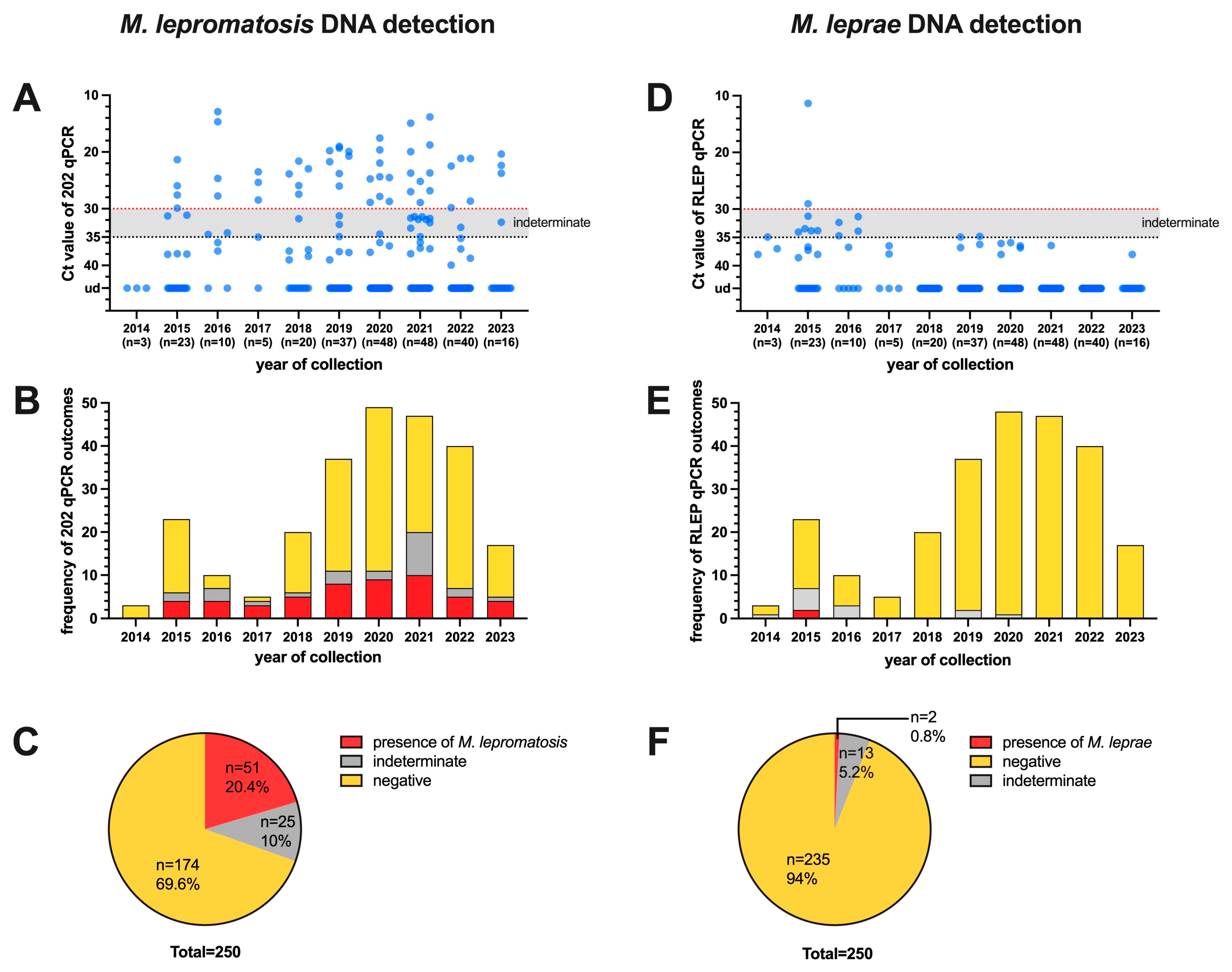
3. Results
3.1. Detection of M. leprae and M. lepromatosis DNA in Red Squirrels
3.2. Genotyping of M. leprae
3.3. Detection of Anti-M. leprae PGL-I Antibodies in the Blood/Body Cavity Fluid
3.4. Summary of Molecular and Serological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global leprosy (Hansen disease) update, 2021: Moving towards interruption of transmission–Situation de la lèpre (maladie de Hansen) dans le monde, 2021: Vers l’interruption de la transmission. Wkly. Epidemiol. Rec. Relev. Épidémiol. Hebd. 2022, 97, 429–450. [Google Scholar]
- Han, X.Y.; Seo, Y.H.; Sizer, K.C.; Schoberle, T.; May, G.S.; Spencer, J.S.; Li, W.; Nair, R.G. A new Mycobacterium species causing diffuse lepromatous leprosy. Am. J. Clin. Pathol. 2008, 130, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Romero-Navarrete, M.; Arenas, R.; Han, X.Y.; Vega-Memije, M.E.; Castillo-Solana, A.D. Leprosy Caused by Mycobacterium lepromatosis. Am. J. Clin. Pathol. 2022, 158, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Aung, F.M.; Choon, S.E.; Werner, B. Analysis of the leprosy agents Mycobacterium leprae and Mycobacterium lepromatosis in four countries. Am. J. Clin. Pathol. 2014, 142, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Dhople, A.M.; Green, K.J.; Osborne, L.J. Limited in vitro multiplication of Mycobacterium leprae. Ann. Inst. Pasteur Microbiol. 1988, 139, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.; Hendrickson, D.; Tolli, N.; Mehaffy, C.; Peña, M.; Nick, J.A.; Knabenbaur, P.; Watkins, J.; Simpson, A.; Amin, A.G.; et al. Culturing Mycobacteria. In Mycobacteria Protocols; Parish, T., Kumar, A., Eds.; Springer: New York, NY, USA, 2021; pp. 1–58. [Google Scholar]
- Woods, S.A.; Cole, S.T. A family of dispersed repeats in Mycobacterium leprae. Mol. Microbiol. 1990, 4, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, P.; McCoy, R.C.; Lenz, S.M.; Donovan, K.; Ochoa, M.T.; Estrada-Garcia, I.; Silva-Miranda, M.; Jurado-Santa Cruz, F.; Balagon, M.F.; et al. Isolation of Mycobacterium lepromatosis and Development of Molecular Diagnostic Assays to Distinguish Mycobacterium leprae and M. lepromatosis. Clin. Infect. Dis. 2020, 71, e262–e269. [Google Scholar] [CrossRef] [PubMed]
- Tio-Coma, M.; Sprong, H.; Kik, M.; van Dissel, J.T.; Han, X.Y.; Pieters, T.; Geluk, A. Lack of evidence for the presence of leprosy bacilli in red squirrels from North-West Europe. Transbound. Emerg. Dis. 2020, 67, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Ridley, D.S.; Jopling, W.H. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 1966, 34, 255–273. [Google Scholar]
- Salgame, P.; Abrams, J.S.; Clayberger, C.; Goldstein, H.; Convit, J.; Modlin, R.L.; Bloom, B.R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 1991, 254, 279–282. [Google Scholar] [CrossRef]
- Quaresma, J.A.; Aarão, T.L.; Sousa, J.R.; Botelho, B.S.; Barros, L.F.; Araujo, R.S.; Rodrigues, J.L.; Prudente, D.L.; Pinto, D.S.; Carneiro, F.R.; et al. T-helper 17 cytokines expression in leprosy skin lesions. Br. J. Dermatol. 2015, 173, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Aarão, T.L.; de Sousa, J.R.; Botelho, B.S.; Fuzii, H.T.; Quaresma, J.A. Correlation between nerve growth factor and tissue expression of IL-17 in leprosy. Microb. Pathog. 2016, 90, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Gormus, B.J.; Xu, K.; Cho, S.N.; Baskin, G.B.; Bohm, R.P.; Martin, L.N.; Blanchard, J.L.; Mack, P.A.; Ratterree, M.S.; Meyers, W.M.; et al. Experimental leprosy in monkeys. II. Longitudinal serological observations in sooty mangabey monkeys. Lepr. Rev. 1995, 66, 105–125. [Google Scholar] [CrossRef]
- Han, X.Y.; Jessurun, J. Severe leprosy reactions due to Mycobacterium lepromatosis. Am. J. Med. Sci. 2013, 345, 65–69. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chemotherapy of leprosy for control programmes. World Health Organ. Tech. Rep. Ser. 1982, 675, 1–33. [Google Scholar]
- Hungria, E.M.; Bührer-Sékula, S.; Oliveira, R.M.; Aderaldo, L.C.; Pontes, M.A.A.; Cruz, R.; de Gonçalves, H.S.; Penna, M.L.F.; Penna, G.O.; Stefani, M.M.A. Mycobacterium leprae-specific antibodies in multibacillary leprosy patients decrease during and after treatment with either the regular 12 doses multidrug therapy (MDT) or the uniform 6 doses MDT. Front. Immunol. 2018, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Adriyani, R.; Wahyuni, C.U.; Yudhastuti, R.; Mahmudah, M.; Notobroto, H.B.; Iswahyudi, I.; Adriaty, A.D. Serological IgM antibody profile of M. Leprae PGL-1 and characteristics of leprosy contacts from an endemic area in East Java, Indonesia. J. Public Health Afr. 2023, 14 (Suppl. 2), 2581. [Google Scholar] [CrossRef]
- Deps, P.; Collin, S.M. Mycobacterium lepromatosis as a Second Agent of Hansen’s Disease. Front. Microbiol. 2021, 12, 698588. [Google Scholar] [CrossRef] [PubMed]
- Tió-Coma, M.; Avanzi, C.; Verhard, E.M.; Pierneef, L.; van Hooij, A.; Benjak, A.; Roy, J.C.; Khatun, M.; Alam, K.; Corstjens, P.; et al. Genomic Characterization of Mycobacterium leprae to Explore Transmission Patterns Identifies New Subtype in Bangladesh. Front. Microbiol. 2020, 11, 1220. [Google Scholar] [CrossRef]
- van Hooij, A.; Tió-Coma, M.; Verhard, E.M.; Khatun, M.; Alam, K.; Tjon Kon Fat, E.; de Jong, D.; Sufian Chowdhury, A.; Corstjens, P.; Richardus, J.H.; et al. Household contacts of leprosy patients in endemic areas display a specific innate immunity profile. Front. Immunol. 2020, 11, 1811. [Google Scholar] [CrossRef]
- Levis, W.R.; Meeker, H.C.; Schuller-Levis, G.B.; Gillis, T.P.; Marino, L.J., Jr.; Zabriskie, J. Serodiagnosis of leprosy: Relationships between antibodies to Mycobacterium leprae phenolic glycolipid I and protein antigens. J. Clin. Microbiol. 1986, 24, 917–921. [Google Scholar] [CrossRef] [PubMed]
- van Hooij, A.; van den Eeden, S.; Richardus, R.; Fat, E.T.K.; Wilson, L.; Franken, K.; Faber, R.; Khatun, M.; Alam, K.; Chowdhury, A.S.; et al. Application of new host biomarker profiles in quantitative point-of-care tests facilitates leprosy diagnosis in the field. Ebiomedicine 2019, 47, 301–308. [Google Scholar] [CrossRef]
- van Hooij, A.; Tjon Kon Fat, E.M.; Batista da Silva, M.; Carvalho Bouth, R.; Cunha Messias, A.C.; Gobbo, A.R.; Lema, T.; Bobosha, K.; Li, J.; Weng, X.; et al. Evaluation of immunodiagnostic tests for leprosy in Brazil, China and Ethiopia. Sci. Rep. 2018, 8, 17920. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Pena, M.; van Hooij, A.; Pierneef, L.; de Jong, D.; Stevenson, R.; Walley, R.; Corstjens, P.L.A.M.; Truman, R.; Adams, L.; et al. Detection and Monitoring of Mycobacterium leprae Infection in Nine Banded Armadillos (Dasypus novemcinctus) Using a Quantitative Rapid Test. Front. Microbiol. 2021, 12, 763289. [Google Scholar] [CrossRef] [PubMed]
- Schilling, A.-K.; van Hooij, A.; Lurz, P.W.; Shaw, D.J.; Geluk, A.; Corstjens, P.L.; Stevenson, K.; Meredith, A.L. Clinical progression of leprosy in Eurasian red squirrels (Sciurus vulgaris) in a naturally infected wild population. J. Zoo Wildl. Med. 2021, 52, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Schilling, A.-K.; van Hooij, A.; Corstjens, P.; Lurz, P.W.W.; DelPozo, J.; Stevenson, K.; Meredith, A.; Geluk, A. Detection of humoral immunity to mycobacteria causing leprosy in Eurasian red squirrels (Sciurus vulgaris) using a quantitative rapid test. Eur. J. Wildl. Res. 2019, 65, 49. [Google Scholar] [CrossRef]
- Wauters, L.A.; Gurnell, J. The mechanism of replacement of red squirrels by grey squirrels: A test of the interference competition hypothesis. Ethology 1999, 105, 1053–1071. [Google Scholar] [CrossRef]
- Tompkins, D.M.; Sainsbury, A.W.; Nettleton, P.; Buxton, D.; Gurnell, J. Parapoxvirus causes a deleterious disease in red squirrels associated with UK population declines. Proc. Biol. Sci. 2002, 269, 529–533. [Google Scholar] [CrossRef]
- Carroll, B.; Russell, P.; Gurnell, J.; Nettleton, P.; Sainsbury, A.W. Epidemics of squirrelpox virus disease in red squirrels (Sciurus vulgaris): Temporal and serological findings. Epidemiol. Infect. 2009, 137, 257–265. [Google Scholar] [CrossRef]
- Stokstad, E. Red squirrels rising. Science 2016, 352, 1268–1271. [Google Scholar] [CrossRef]
- Rushton, S.; Lurz, P.; Gurnell, J.; Fuller, R. Modelling the spatial dynamics of parapoxvirus disease in red and grey squirrels: A possible cause of the decline in the red squirrel in the UK? J. Appl. Ecol. 2000, 37, 997–1012. [Google Scholar] [CrossRef]
- Council of Europe. Convention on the Conservation of European Wildlife and Natural Habitats (ETS No. 104), Appendix III. Available online: https://rm.coe.int/168097eb57 (accessed on 3 July 2024).
- Gurnell, J.; Lurz, P.; Bertoldi, W. The changing patterns in the distribution of red and grey squirrels in the North of England and Scotland between 1991 and 2010 based on volunteer surveys. Hystrix Ital. J. Mammal. 2014, 25, 83–89. [Google Scholar] [CrossRef]
- Lloyd, H. Past and present distribution of red and grey squirrels. Mammal Rev. 1983, 13, 69–80. [Google Scholar] [CrossRef]
- Simpson, V.; Hargreaves, J.; Butler, H.; Blackett, T.; Stevenson, K.; McLuckie, J. Leprosy in red squirrels on the Isle of Wight and Brownsea Island. Vet. Rec. 2015, 177, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, C.M.; Everest, D.; Holmes, P.; Bell, S.; Cripps, R. An Opportunistic Assessment of the Impact of Squirrelpox Disease Outbreaks upon a Red Squirrel Population Sympatric with Grey Squirrels in Wales. Animals 2022, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Magris, L.; Gurnell, J. Population ecology of the red squirrel (Sciurus vulgaris) in a fragmented woodland ecosystem on the Island of Jersey, Channel Islands. J. Zool. 2002, 256, 99–112. [Google Scholar] [CrossRef]
- Meredith, A.; Del Pozo, J.; Smith, S.; Milne, E.; Stevenson, K.; McLuckie, J. Leprosy in red squirrels in Scotland. Vet. Rec. 2014, 175, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Schilling, A.K.; Del-Pozo, J.; Lurz, P.W.W.; Stevenson, K.; Avanzi, C.; Shuttleworth, C.M.; Cole, S.T.; Meredith, A.L. Leprosy in red squirrels in the UK. Vet. Rec. 2019, 184, 416. [Google Scholar] [CrossRef] [PubMed]
- Avanzi, C.; Del-Pozo, J.; Benjak, A.; Stevenson, K.; Simpson, V.R.; Busso, P.; McLuckie, J.; Loiseau, C.; Lawton, C.; Schoening, J.; et al. Red squirrels in the British Isles are infected with leprosy bacilli. Science 2016, 354, 744–747. [Google Scholar] [CrossRef]
- Schilling, A.K.; McCurdy, K.; Fish, A.; Lurz, P.W.W.; Geluk, A.; Van Hooij, A.; Farish, M.; Mitchell, M.; Stevenson, K.; Meredith, A.L. Diagnosing and categorizing leprosy in live eurasian red squirrels (Sciurus vulgaris) for management, surveillance, and translocation purposes. J. Zoo Wildl. Med. 2021, 52, 648–659. [Google Scholar] [CrossRef]
- Fulton, N.; Anderson, L.F.; Watson, J.M.; Abubakar, I. Leprosy in England and Wales 1953-2012: Surveillance and challenges in low incidence countries. BMJ Open 2016, 6, e010608. [Google Scholar] [CrossRef] [PubMed]
- Ploemacher, T.; Faber, W.R.; Menke, H.; Rutten, V.; Pieters, T. Reservoirs and transmission routes of leprosy; A systematic review. PLoS Negl. Trop. Dis. 2020, 14, e0008276. [Google Scholar] [CrossRef]
- LaRose, J.P.; Meredith, A.L.; Everest, D.J.; Fiegna, C.; McInnes, C.J.; Shaw, D.J.; Milne, E.M. Epidemiological and postmortem findings in 262 red squirrels (Sciurus vulgaris) in Scotland, 2005 to 2009. Vet. Rec. 2010, 167, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Corstjens, P.; van Hooij, A.; Tjon Kon Fat, E.M.; Alam, K.; Vrolijk, L.B.; Dlamini, S.; da Silva, M.B.; Spencer, J.S.; Salgado, C.G.; Richardus, J.H.; et al. Fingerstick test quantifying humoral and cellular biomarkers indicative for M. leprae infection. Clin. Biochem. 2019, 66, 76–82. [Google Scholar] [CrossRef]
- van Hooij, A.; Tjon Kon Fat, E.M.; Richardus, R.; van den Eeden, S.J.; Wilson, L.; de Dood, C.J.; Faber, R.; Alam, K.; Richardus, J.H.; Corstjens, P.L.; et al. Quantitative lateral flow strip assays as user-friendly tools to detect biomarker profiles for leprosy. Sci. Rep. 2016, 6, 34260. [Google Scholar] [CrossRef] [PubMed]
- van Hooij, A.; Tjon Kon Fat, E.M.; van den Eeden, S.J.F.; Wilson, L.; Batista da Silva, M.; Salgado, C.G.; Spencer, J.S.; Corstjens, P.; Geluk, A. Field-friendly serological tests for determination of M. leprae-specific antibodies. Sci. Rep. 2017, 7, 8868. [Google Scholar] [CrossRef] [PubMed]
- Pierneef, L.; Malaviya, P.; van Hooij, A.; Sundar, S.; Singh, A.K.; Kumar, R.; de Jong, D.; Meuldijk, M.; Kumar, A.; Zhou, Z.; et al. Field-friendly anti-PGL-I serosurvey in children to monitor Mycobacterium leprae transmission in Bihar, India. Front. Med. 2023, 10, 1260375. [Google Scholar] [CrossRef]
- Monot, M.; Honoré, N.; Garnier, T.; Araoz, R.; Coppée, J.Y.; Lacroix, C.; Sow, S.; Spencer, J.S.; Truman, R.W.; Williams, D.L.; et al. On the origin of leprosy. Science 2005, 308, 1040–1042. [Google Scholar] [CrossRef]
- Tio-Coma, M.; Wijnands, T.; Pierneef, L.; Schilling, A.K.; Alam, K.; Roy, J.C.; Faber, W.R.; Menke, H.; Pieters, T.; Stevenson, K.; et al. Detection of Mycobacterium leprae DNA in soil: Multiple needles in the haystack. Sci. Rep. 2019, 9, 3165. [Google Scholar] [CrossRef]
- Gurnell, J. Squirrel numbers and the abundance of tree seeds. Mammal Rev. 1983, 13, 133–148. [Google Scholar] [CrossRef]
- Hockings, K.J.; Mubemba, B.; Avanzi, C.; Pleh, K.; Düx, A.; Bersacola, E.; Bessa, J.; Ramon, M.; Metzger, S.; Patrono, L.V. Leprosy in wild chimpanzees. Nature 2021, 598, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Valverde, C.R.; Canfield, D.; Tarara, R.; Esteves, M.I.; Gormus, B.J. Spontaneous leprosy in a wild-caught cynomolgus macaque. Int. J. Lepr. Other Mycobact. Dis. 1998, 66, 140–148. [Google Scholar] [PubMed]
- Truman, R.W.; Singh, P.; Sharma, R.; Busso, P.; Rougemont, J.; Paniz-Mondolfi, A.; Kapopoulou, A.; Brisse, S.; Scollard, D.M.; Gillis, T.P.; et al. Probable zoonotic leprosy in the southern United States. N. Engl. J. Med. 2011, 364, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, P.; Loughry, W.J.; Lockhart, J.M.; Inman, W.B.; Duthie, M.S.; Pena, M.T.; Marcos, L.A.; Scollard, D.M.; Cole, S.T.; et al. Zoonotic Leprosy in the Southeastern United States. Emerg. Infect. Dis. 2015, 21, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.B.; Portela, J.M.; Li, W.; Jackson, M.; Gonzalez-Juarrero, M.; Hidalgo, A.S.; Belisle, J.T.; Bouth, R.C.; Gobbo, A.R.; Barreto, J.G.; et al. Evidence of zoonotic leprosy in Pará, Brazilian Amazon, and risks associated with human contact or consumption of armadillos. PLoS Negl. Trop. Dis. 2018, 12, e0006532. [Google Scholar] [CrossRef]
- Urban, C.; Blom, A.A.; Avanzi, C.; Walker-Meikle, K.; Warren, A.K.; White-Iribhogbe, K.; Turle, R.; Marter, P.; Dawson-Hobbis, H.; Roffey, S.; et al. Ancient Mycobacterium leprae genome reveals medieval English red squirrels as animal leprosy host. Curr. Biol. 2024, 34, 2221–2230. [Google Scholar] [CrossRef] [PubMed]
- Avanzi, C.; Singh, P.; Truman, R.W.; Suffys, P.N. Molecular epidemiology of leprosy: An update. Infect. Genet. Evol. 2020, 86, 104581. [Google Scholar] [CrossRef] [PubMed]
- Schilling, A.K.; Avanzi, C.; Ulrich, R.G.; Busso, P.; Pisanu, B.; Ferrari, N.; Romeo, C.; Mazzamuto, M.V.; McLuckie, J.; Shuttleworth, C.M.; et al. British Red Squirrels Remain the Only Known Wild Rodent Host for Leprosy Bacilli. Front. Vet. Sci. 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Silva, F.J. On the age of leprosy. PLoS Negl. Trop. Dis. 2014, 8, e2544. [Google Scholar] [CrossRef]
- Schuenemann, V.J.; Singh, P.; Mendum, T.A.; Krause-Kyora, B.; Jäger, G.; Bos, K.I.; Herbig, A.; Economou, C.; Benjak, A.; Busso, P.; et al. Genome-wide comparison of medieval and modern Mycobacterium leprae. Science 2013, 341, 179–183. [Google Scholar] [CrossRef]
- Truman, R.W.; Andrews, P.K.; Robbins, N.Y.; Adams, L.B.; Krahenbuhl, J.L.; Gillis, T.P. Enumeration of Mycobacterium leprae using real-time PCR. PLoS Negl. Trop. Dis. 2008, 2, e328. [Google Scholar] [CrossRef] [PubMed]
- da Silva Ferreira, J.; de Carvalho, F.M.; Vidal Pessolani, M.C.; de Paula Antunes, J.M.A.; de Medeiros Oliveira, I.V.P.; Ferreira Moura, G.H.; Truman, R.W.; Peña, M.T.; Sharma, R.; Duthie, M.S.; et al. Serological and molecular detection of infection with Mycobacterium leprae in Brazilian six banded armadillos (Euphractus sexcinctus). Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101397. [Google Scholar] [CrossRef] [PubMed]





| Code | Locus 1 | Locus 2 | Locus 3 | Genotype |
|---|---|---|---|---|
| R25/15 | C | T | C | 3 |
| R26/15 | ud | T | C | 3 or 4 |
| RLEP qPCR (M. leprae) | 202 qPCR (M. lepromatosis) | UCP-LFA (Anti-PGL-I ANTIBODIES) | Squirrels (n) |
|---|---|---|---|
| + | − | − | 2 |
| − | + | + | 18 |
| − | + | − | 16 |
| − | − | + | 11 |
| − | − | − | 127 |
| − | + | n.a. | 17 |
| − | − | n.a. | 59 |
| n.a. | n.a. | + | 5 |
| n.a. | n.a. | − | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; van Hooij, A.; Wassenaar, G.N.; Seed, E.; Verhard-Seymonsbergen, E.M.; Corstjens, P.L.A.M.; Meredith, A.L.; Wilson, L.A.; Milne, E.M.; Beckmann, K.M.; et al. Molecular and Serological Surveillance for Mycobacterium leprae and Mycobacterium lepromatosis in Wild Red Squirrels (Sciurus vulgaris) from Scotland and Northern England. Animals 2024, 14, 2005. https://doi.org/10.3390/ani14132005
Zhou Z, van Hooij A, Wassenaar GN, Seed E, Verhard-Seymonsbergen EM, Corstjens PLAM, Meredith AL, Wilson LA, Milne EM, Beckmann KM, et al. Molecular and Serological Surveillance for Mycobacterium leprae and Mycobacterium lepromatosis in Wild Red Squirrels (Sciurus vulgaris) from Scotland and Northern England. Animals. 2024; 14(13):2005. https://doi.org/10.3390/ani14132005
Chicago/Turabian StyleZhou, Zijie, Anouk van Hooij, Gaby N. Wassenaar, Emma Seed, Els M. Verhard-Seymonsbergen, Paul L. A. M. Corstjens, Anna L. Meredith, Liam A. Wilson, Elspeth M. Milne, Katie M. Beckmann, and et al. 2024. "Molecular and Serological Surveillance for Mycobacterium leprae and Mycobacterium lepromatosis in Wild Red Squirrels (Sciurus vulgaris) from Scotland and Northern England" Animals 14, no. 13: 2005. https://doi.org/10.3390/ani14132005
APA StyleZhou, Z., van Hooij, A., Wassenaar, G. N., Seed, E., Verhard-Seymonsbergen, E. M., Corstjens, P. L. A. M., Meredith, A. L., Wilson, L. A., Milne, E. M., Beckmann, K. M., & Geluk, A. (2024). Molecular and Serological Surveillance for Mycobacterium leprae and Mycobacterium lepromatosis in Wild Red Squirrels (Sciurus vulgaris) from Scotland and Northern England. Animals, 14(13), 2005. https://doi.org/10.3390/ani14132005






