Population Morphometry of Conger myriaster (Anguilliformes: Congridae) along the Coast of China: Implications for Population Structure and Fishery Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
Samples Collection and Morphometric Measurement
2.2. Data Analysis
3. Results
3.1. The One-Way Analysis of Variance
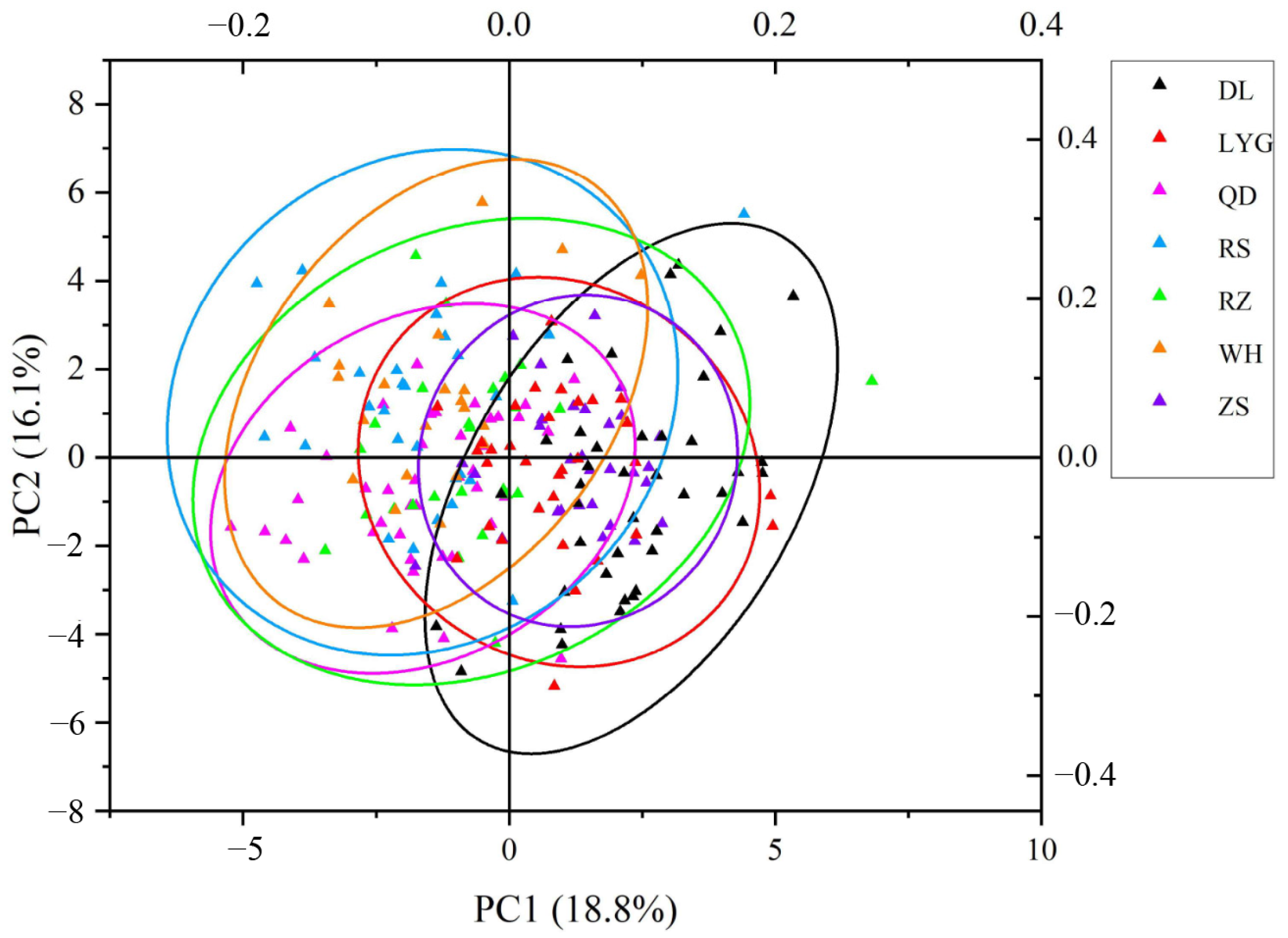
3.2. The Principal Component Analysis
3.3. The Cluster Analysis
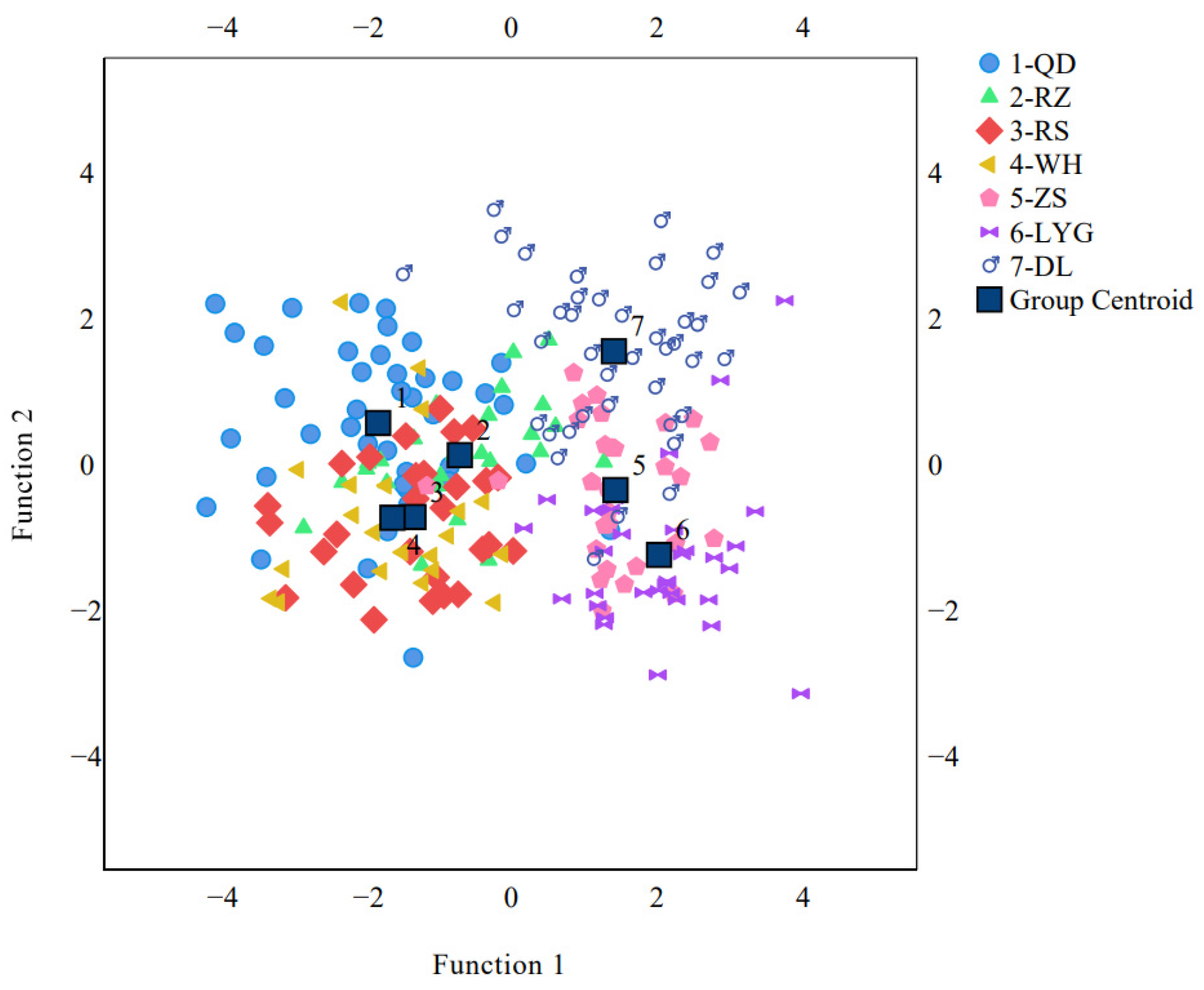
3.4. The Discriminant Function Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaneko, K. Relationship among phenotypic plasticity, phenotypic fluctuations, robustness, and evolvability; Waddington’s legacy revisited under the spirit of Einstein. J. Biosci. 2009, 34, 529–542. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 1989, 20, 249–278. [Google Scholar] [CrossRef]
- Cadrin, S.X. Advances in morphometric identification of fishery stocks. Rev. Fish Biol. Fish. 2000, 10, 91–112. [Google Scholar] [CrossRef]
- Imre, I.; McLaughlin, R.L.; Noakes, D.L.G. Phenotypic plasticity on brook charr: Changes in caudal fin induced by water flow. J. Fish Biol. 2002, 61, 1171–1181. [Google Scholar] [CrossRef]
- Armstrong, D.; Kemp, P.S.; Kennedy, G.A.; Ladle, M.; Milner, N. Habitat requirements of Atlantic salmon and brown trout in rivers and streams. Fish. Res. 2003, 62, 143–170. [Google Scholar] [CrossRef]
- Salgueiro, P.; Carvalho, G.; Collares-Pereira, M.J.; Coelho, M.M. Microsatellite analysis of genetic population structure of the endangered cyprinid Anaecypris hispanica in Portugal: Implications for conservation. Biol. Conserv. 2003, 109, 47–56. [Google Scholar] [CrossRef]
- Silva, A. Morphometric variation among sardine (Sardina pilchardus) populations from the northeastern Atlantic and the western Mediterranean. ICES J. Mar. Sci. 2003, 60, 1352–1360. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; De, K. Role of morphometrics in fish diversity assessment: Status, challenges and future prospects. Natl. Acad. Sci. Lett. 2024, 47, 123–126. [Google Scholar] [CrossRef]
- Lind, C.E.; Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L. Selective breeding in fish and conservation of genetic resources for aquaculture. Reprod. Domest. Anim. 2012, 47, 255–263. [Google Scholar] [CrossRef]
- Frederich, B.; Liu, S.V.; Dai, C.F. Morphological and genetic divergences in a coral reef damselfish, Pomacentrus coelestis. Evol. Biol. 2012, 39, 359–370. [Google Scholar] [CrossRef]
- Secor, D.H. Chapter Two—The Unit Stock Concept: Bounded Fish and Fisheries. In Stock Identification Methods, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 7–28. [Google Scholar] [CrossRef]
- Turki-Missaoui, O.; M’Hetli, M.; Kraïem, M.M.; Chriki, A. Morphological differentiation of introduced pikeperch (Sander lucioperca L., 1758) populations in Tunisian freshwaters. J. Appl. Ichthyol. 2011, 27, 1181–1189. [Google Scholar] [CrossRef]
- Myoung, S.H.; Kim, J.K. Population structure of the Korean gizzard shad, Konosirus punctatus (Clupeiformes, Clupeidae) using multivariate morphometric analysis. Ocean Sci. J. 2016, 51, 33–41. [Google Scholar] [CrossRef]
- Turan, C.; Oral, M.; Öztürk, B.; Düzgüneş, E. Morphometric and meristic variation between stocks of Bluefish (Pomatomus saltatrix) in the Black, Marmara, Aegean and northeastern Mediterranean Seas. Fish. Res. 2006, 79, 139–147. [Google Scholar] [CrossRef]
- Brevoort, J.C. Notes on Some Figures of Japanese Fish Taken from Recent Specimens by the Artists of the U. S. Japan Expedition; Creative Media Partners, LLC: Sacramento, CA, USA, 1856; pp. 253–288. [Google Scholar]
- Chen, D.G.; Zhang, M.Z. Marine Fishes of China; China Ocean University Press: Qingdao, China, 2015; pp. 187–188. (In Chinese) [Google Scholar]
- Kimura, Y.; Ishikawa, S.; Tokai, T.; Nishida, M.; Tsukamoto, K. Early life history characteristics and genetic homogeneity of Conger myriaster leptocephali along the east coast of central Japan. Fish. Res. 2004, 70, 61–69. [Google Scholar] [CrossRef]
- Tokimura, M. Conger-eel fisheries and fisheries resources in the East China Sea. Nippon Suisan Gakkaishi 2001, 67, 125–126. [Google Scholar] [CrossRef]
- Li, M.; Jiao, Y.; Bi, R.; Ren, Y. Population status and distribution of whitespotted conger (Conger myriaster) in Yellow Sea: An important migratory species along coastal China with limited data. Fish Oceanogr. 2020, 29, 32–45. [Google Scholar] [CrossRef]
- Mochioka, N.; Tabeta, O.; Kubota, T. A pre-leptocephalus larva of Conger myriaster (family Congridae) collected from Suruga Bay, Central Japan. Jpn. J. Ichthyol. 1988, 35, 184–188. [Google Scholar] [CrossRef]
- Ishikawa, S.; Kimura, Y.; Tokai, T.; Tsukamoto, K.; Nishida, M. Genetic variation in the mitochondrial and nuclear DNA of the Japanese conger Conger myriaster. Fish. Sci. 2001, 67, 1081–1087. [Google Scholar] [CrossRef]
- Miller, M.J.; Yoshinaga, T.; Aoyama, J.; Otake, T.; Mochioka, N.; Kurogi, H.; Tsukamoto, K. Offshore spawning of Conger myriaster in the western North Pacific: Evidence for convergent migration strategies of anguilliform eels in the Atlantic and Pacific. Naturwissenschaften 2011, 98, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Kurogi, H.; Mochioka, N.; Okazaki, M.; Takahashi, M.; Miller, M.J.; Tsukamoto, K.; Ambe, D.; Katayama, S.; Chow, S. Discovery of a spawning area of the common Japanese conger Conger myriaster along the Kyushu-Palau Ridge in the western North Pacific. Fish. Sci. 2012, 78, 525–532. [Google Scholar] [CrossRef]
- Kawazu, M.; Kameda, T.; Kurogi, H.; Yoda, M.; Ohshimo, S.; Sakai, T.; Tsukamoto, Y.; Mochioka, N. Biological characteristics of Conger myriaster during the initial stage of spawning migration in the East China Sea. Fish. Sci. 2015, 81, 663–671. [Google Scholar] [CrossRef]
- Yang, H.; Shi, B.; Niu, H.X.; Zhang, D.Q.; Li, J. Advances and future prospects in Conger myriaster research. Mar. Sci. 2020, 44, 152–158, (In Chinese with English abstract). [Google Scholar]
- Zhang, X.M.; Cheng, S.F. Path analysis and growth curve fitting of the morphological traits and body weight of Conger myriaster. J. Fish. Sci. China 2020, 27, 1167–1175, (In Chinese with English abstract). [Google Scholar]
- Yin, J.; Mu, X.X.; Zhang, C.L.; Xu, B.D.; Xue, Y.; Ren, Y.P. Comparison of morphological and genetic characteristics of Conger myriaster populations in China. J. Fish. China 2020, 44, 358–367, (In Chinese with English abstract). [Google Scholar]
- Ying, Y.P.; Chen, Y.; Lin, L.S.; Gao, T.X. Risks of ignoring fish population spatial structure in fisheries management. Can. J. Fish. Aquat. Sci. 2011, 68, 2101–2120. [Google Scholar] [CrossRef]
- Elliott, N.G.; Haskard, K.; Koslow, J.A. Morphometric analysis of orange roughly (Hoplostethus atianticus) off the continental slope of Southern Australia. Fish Biol. 1995, 46, 202–220. [Google Scholar] [CrossRef]
- Mayr, E.; Linsley, E.G.; Usinger, R.L. Methods and Principles of Systematic Zoology. J. Mammal. 1953, 34, 520–521. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2005; pp. 127–169. [Google Scholar]
- Fisher, R.A. The Design of Experiments; Oliver and Boyd: Edinburgh, UK, 1935; pp. 88–136. [Google Scholar]
- Tamhane, A.C. A comparison of procedures for multiple comparisons of means with unequal variances. J. Am. Stat. Assoc. 1979, 74, 471–480. [Google Scholar] [CrossRef]
- Jennrich, R.I. Statistical Methods for Digital Computers III; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1977; pp. 99–133. [Google Scholar]
- Veasey, E.A.; Schammass, E.A.; Vencovsky, R.; Martins, P.S.; Bandel, G. Germplasm characterization of Sesbania accessions based on multivariate analyses. Genet. Resour. Crop Evol. 2001, 48, 79–91. [Google Scholar] [CrossRef]
- Samaee, M.; Patzner, R.A.; Mansour, N. Morphological differentiation within the population of Siah mahi, Capoeta capoeta gracilis, (Cyprinidae, Teleostei) in a river of the south Caspian Sea basin: A pilot study. J. Appl. Ichthyol. 2006, 25, 583–590. [Google Scholar] [CrossRef]
- Mir, J.I.; Sarkar, U.K.; Dwivedi, A.K.; Gusain, O.P.; Jena, J.K. Stock structure analysis of Labeo rohita (Hamilton, 1822) across the Ganga basin (India) using a truss network system. J. Appl. Ichthyol. 2013, 29, 1097–1103. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Turan, C.; Erguden, D.; Gurlek, M.; Başusta, N.; Turan, F. Morphometric structuring of the anchovy (Engraulis encrasicolus L.) in the black, Aegean and Northeastern Mediterranean seas. Turk. J. Vet. Anim. Sci. 2004, 28, 865–871. [Google Scholar]
- Hourston, A.S. Homing by Canada’s west coast herring to management units and divisions as indicated by tag recoveries. Can. J. Fish. Aquat. Sci. 1982, 39, 1414–1422. [Google Scholar] [CrossRef]
- Iles, T.D.; Sinclair, M. Atlantic herring: Stock discreteness and abundance. Science 1982, 215, 627–633. [Google Scholar] [CrossRef]
- Wimberger, P.H. Plasticity of fish body shape. The effects of diet, development, family and age in two species of Geophagus (Pisces: Cichlidae). Biol. J. Linn. Soc. 1992, 45, 197–218. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Shan, X.J.; Yang, T.; Jin, X.S.; Wei, C. Biodiversity characteristics of bottom fishery communities in the sea Adjacent to the Miaodao Archipelago. Prog. Fish. Sci. 2022, 43, 132–147, (In Chinese with English abstract). [Google Scholar]
- Triantafyllou, M.S.; Triantafyllou, G.S. An Efficient Swimming Machine. Sci. Am. 1995, 272, 64–70. [Google Scholar] [CrossRef]
- Eisenhour, D. Systematics of Macrhybopsis tetranema (Cypriniformes: Cyprinidae). Copeia 1999, 1999, 969–980. [Google Scholar] [CrossRef]
- Zou, C.C.; Wang, L.J.; Kong, L.M.; Wang, Y.J.; Wu, Z.H.; Xu, J.H.; Song, A.H.; Liu, H.; You, F. High levels of genetic diversity and connectivity of whitespotted conger Conger myriaster in the East China Coast. Mar. Biodivers. 2020, 50, 47. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Zhang, C.; Ye, Z.J.; Xu, B.D.; Tian, Y.J.; Watanabe, Y. Stock structure analysis of the Japanese Spanish mackerel Scomberomorus niphonius (Cuvier, 1832) along the China coast based on truss network. J. Ocean Univ. China 2020, 19, 202–208. [Google Scholar] [CrossRef]
- Turan, C. Stock identification of Mediterranean horse mackerel (Trachurus medi-terraneus) using morphometric and meristic characters. ICES J. Mar. Sci. 2004, 61, 774–781. [Google Scholar] [CrossRef]
- Teng, A.K.; Zhang, M.S.; Yang, X.J.; Cui, W.Y.; Chen, C.; Zhu, L.; Shao, C.C.; Yu, Z.S. Study on assemblage structure of macrozoobenthos in the Yellow Sea. Period. Ocean Univ. China 2023, 53, 74–84, (In Chinese with English abstract). [Google Scholar]
- Katayama, S.; Ishida, T.; Shimizu, Y.; Yamanobe, A. Seasonal change in distribution of conger eel Conger myriaster off the Pacific coast south of Tohoku, north-eastern Japan. Fish. Sci. 2004, 70, 1–6. [Google Scholar] [CrossRef]
- Gorie, S.; Tanda, M.; Nagasawa, K. Habitat preference and feeding habits of juvenile white-spotted conger Conger myriaster in the eastern Seto Inland Sea, Japan. Aquac. Sci. 2010, 58, 167–179. [Google Scholar] [CrossRef]
- Takai, T. Studies on the morphology, ecology and culture of the important apodal fishes, Muraenesox cinereus (Forsskal) and Conger myriaster (Brevoort). J. Shimonoseki Coll. Fish. 1959, 8, 209–555. [Google Scholar]
- Mu, X.X.; Zhang, C.; Zhang, C.L.; Yang, J.; Ren, Y.P. Age-structured otolith chemistry profiles revealing the migration of Conger myriaster in China Seas. Fish. Res. 2021, 239, 105938. [Google Scholar] [CrossRef]
- Swain, D.P.; Foote, C.J. Stocks and chameleons: The use of phenotypic variation in stock identification. Fish. Res. 1999, 43, 113–128. [Google Scholar] [CrossRef]
- Pazhayamadom, D.G.; Chakraborty, S.K.; Jaiswar, A.K.; Sudheesan, D.; Sajina, A.M.; Jahageerdar, S. Stock structure analysis of ‘Bombay duck’ (Harpadon nehereus Hamilton, 1822) along the Indian coast using truss network morphometrics. J. Appl. Ichthyol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Myoung, S.H.; Ban, T.; Kim, J. Population structure of Liparis tanakae (Pisces, Liparidae) from Korea based on morphological and molecular traits. Korean J. Fish. Aquat. Sci. 2016, 49, 198–207, (In Korean with English abstract). [Google Scholar] [CrossRef]
- Li, H.B.; Chen, X.; Denis, M.; Zhao, Y.; Huang, L.F.; Jiang, Z.G.; Zhang, W.C.; Xiao, T. Seasonal and spatial variation of pelagic microbial food web structure in a semi-enclosed temperate bay. Front. Mar. Sci. 2020, 7, 2296–7745. [Google Scholar] [CrossRef]
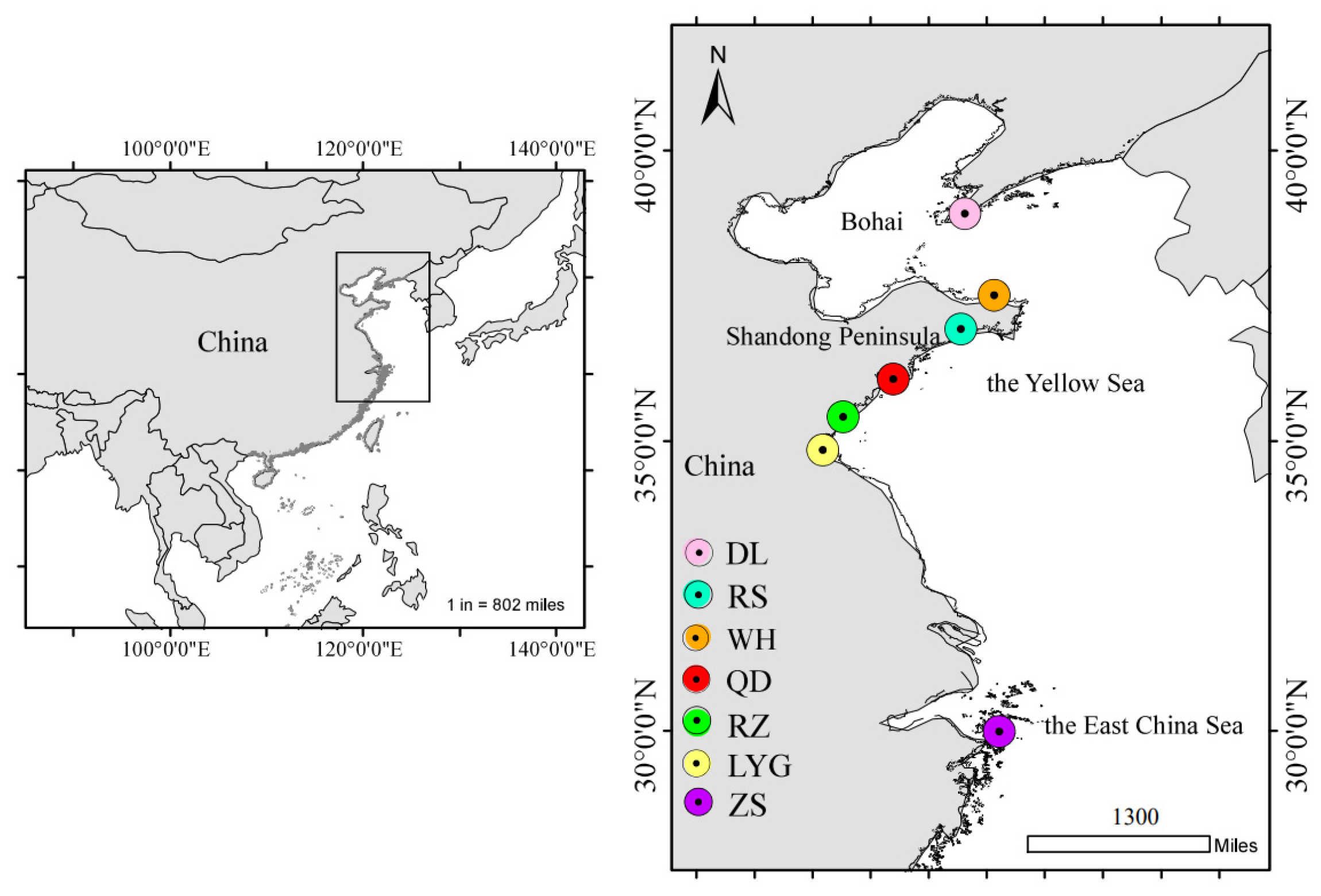


| Sampling Sites | Sea Regions | Geographical Coordinates | Sampling Time | Sample Size | Total Length (TL) | |
|---|---|---|---|---|---|---|
| Range (cm) | Mean ± SD (cm) | |||||
| Dalian (DL) | The Bohai Sea | 38°55′ N; 121°37′ E | 2023-05 | 40 | 33.7–51.7 | 45.7 ± 3.9 |
| Weihai (WH) | The Yellow Sea | 37°31′ N; 122°7′ E | 2022-09 | 21 | 28.9–36.6 | 32.7 ± 2.2 |
| Rushan (RS) | 36°56′ N; 121°32′ E | 2022-10 | 29 | 27.6–45.7 | 35.0 ± 4.3 | |
| Qingdao (QD) | 36°4′ N; 120°23′ E | 2022-09 | 41 | 30.6–39.2 | 35.0 ± 2.1 | |
| Rizhao (RZ) | 35°25′ N; 119°32′ E | 2022-08 | 25 | 33.9–48.4 | 38.2 ± 3.3 | |
| Lianyungang (LYG) | 34°36′ N; 119°13′ E | 2023-04 | 32 | 30.6–50.4 | 39.1 ± 3.7 | |
| Zhoushan (ZS) | The East China Sea | 29°59′ N; 122°12′ E | 2022-10 | 30 | 30.6–45.1 | 40.8 ± 3.9 |
| Index | Principal Component (PC) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| TV | 0.393 | −0.149 | −0.547 | −0.104 | 0.640 | −0.096 | −0.091 |
| DV | 0.596 | 0.255 | −0.568 | 0.428 | −0.007 | 0.050 | 0.047 |
| AV | 0.482 | 0.641 | −0.417 | −0.131 | 0.159 | 0.029 | −0.005 |
| AFV | 0.593 | 0.561 | −0.371 | −0.268 | 0.220 | −0.080 | −0.011 |
| DAV | 0.115 | 0.420 | 0.209 | −0.779 | 0.240 | −0.158 | 0.014 |
| DV/TV | 0.538 | 0.326 | −0.456 | 0.497 | −0.200 | 0.081 | 0.080 |
| AV/TV | 0.183 | 0.807 | 0.021 | −0.049 | −0.372 | 0.111 | 0.069 |
| AFV/TV | 0.357 | 0.786 | 0.037 | −0.221 | −0.293 | −0.010 | 0.064 |
| DAV/TV | −0.095 | 0.473 | 0.480 | −0.673 | −0.106 | −0.096 | 0.060 |
| BL | 0.153 | −0.331 | −0.095 | −0.125 | 0.075 | 0.176 | 0.716 |
| TAL | 0.293 | −0.309 | 0.131 | −0.254 | 0.274 | 0.339 | 0.006 |
| AFL | −0.406 | 0.593 | 0.417 | 0.273 | 0.322 | 0.132 | 0.060 |
| AL | −0.121 | −0.058 | 0.060 | 0.205 | −0.240 | −0.606 | 0.324 |
| DL | −0.089 | 0.456 | 0.363 | 0.572 | 0.174 | 0.202 | −0.128 |
| TRL | −0.492 | 0.594 | 0.127 | 0.181 | 0.037 | 0.299 | 0.049 |
| HL | 0.190 | 0.244 | 0.673 | 0.432 | 0.279 | −0.167 | −0.001 |
| HB | 0.436 | −0.282 | 0.081 | −0.089 | −0.352 | 0.414 | −0.141 |
| SL | 0.438 | −0.129 | 0.178 | −0.082 | −0.220 | −0.212 | −0.520 |
| ML | 0.584 | −0.025 | 0.184 | 0.242 | −0.147 | −0.384 | 0.081 |
| ED | 0.658 | −0.134 | 0.296 | −0.047 | 0.139 | −0.165 | 0.057 |
| EI | 0.606 | −0.192 | 0.217 | 0.050 | 0.043 | 0.126 | −0.088 |
| HLE | 0.525 | 0.016 | 0.550 | 0.307 | 0.224 | −0.098 | 0.068 |
| BD | 0.450 | −0.162 | 0.433 | −0.080 | 0.012 | 0.213 | 0.109 |
| BB | 0.578 | −0.205 | 0.296 | −0.095 | −0.190 | 0.303 | 0.124 |
| PL | 0.483 | −0.204 | 0.291 | 0.009 | −0.035 | −0.018 | 0.019 |
| Eigenvalues | 4.710 | 4.030 | 3.090 | 2.573 | 1.471 | 1.314 | 1.010 |
| Contribution ratio (%) | 18.839 | 16.120 | 12.361 | 10.291 | 5.884 | 5.256 | 4.038 |
| Cumulative contribution ratio (%) | 18.839 | 34.960 | 47.320 | 57.612 | 63.495 | 68.751 | 72.790 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, P.; Yang, T. Population Morphometry of Conger myriaster (Anguilliformes: Congridae) along the Coast of China: Implications for Population Structure and Fishery Management. Animals 2024, 14, 2007. https://doi.org/10.3390/ani14132007
Xiao P, Yang T. Population Morphometry of Conger myriaster (Anguilliformes: Congridae) along the Coast of China: Implications for Population Structure and Fishery Management. Animals. 2024; 14(13):2007. https://doi.org/10.3390/ani14132007
Chicago/Turabian StyleXiao, Peiyi, and Tianyan Yang. 2024. "Population Morphometry of Conger myriaster (Anguilliformes: Congridae) along the Coast of China: Implications for Population Structure and Fishery Management" Animals 14, no. 13: 2007. https://doi.org/10.3390/ani14132007





