Effects of Hypoxia on the Antibacterial Activity of Epidermal Mucus from Chilean Meagre (Cilus gilberti)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
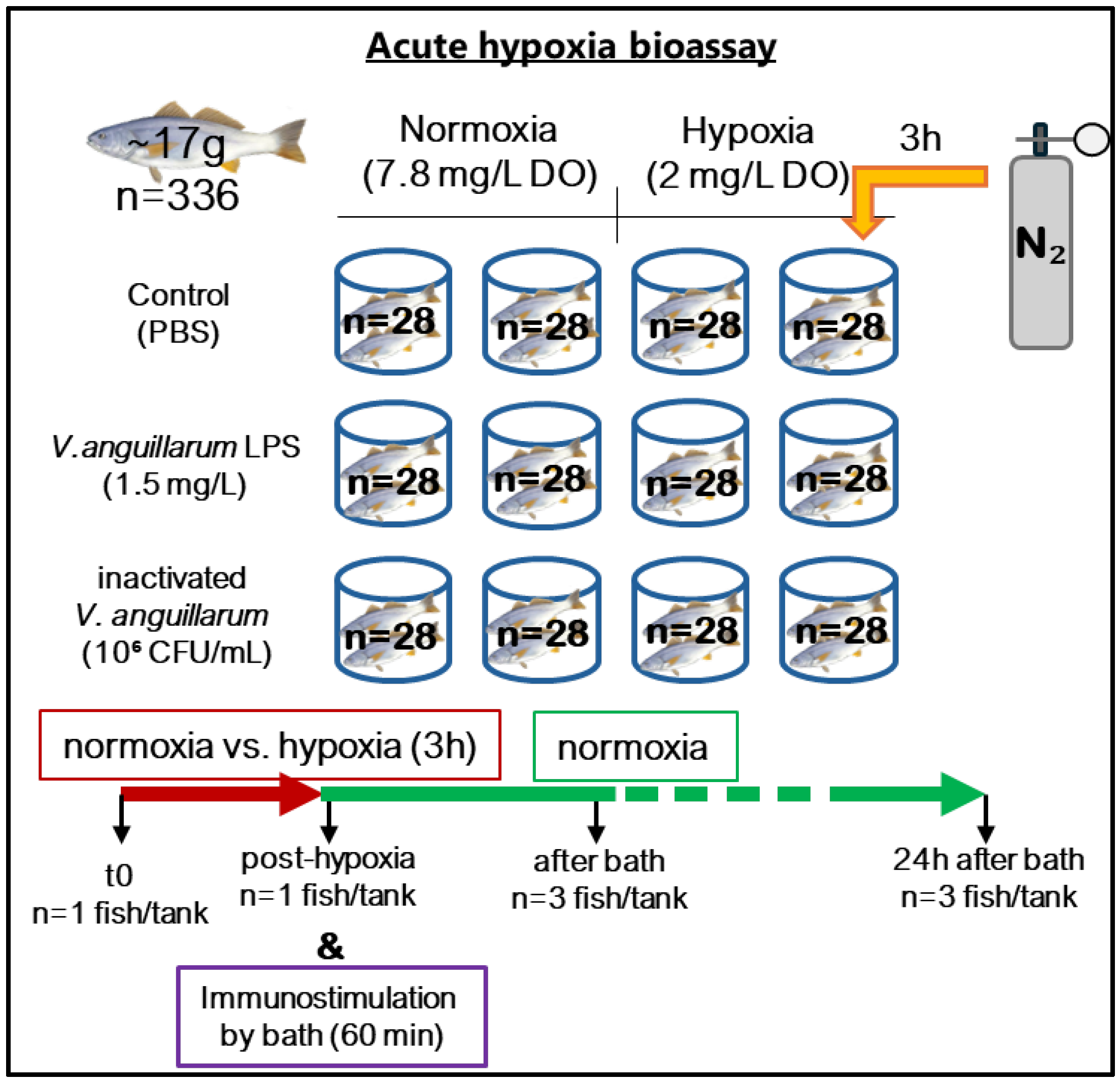
2.2. Acute Hypoxia and Bath Challenge
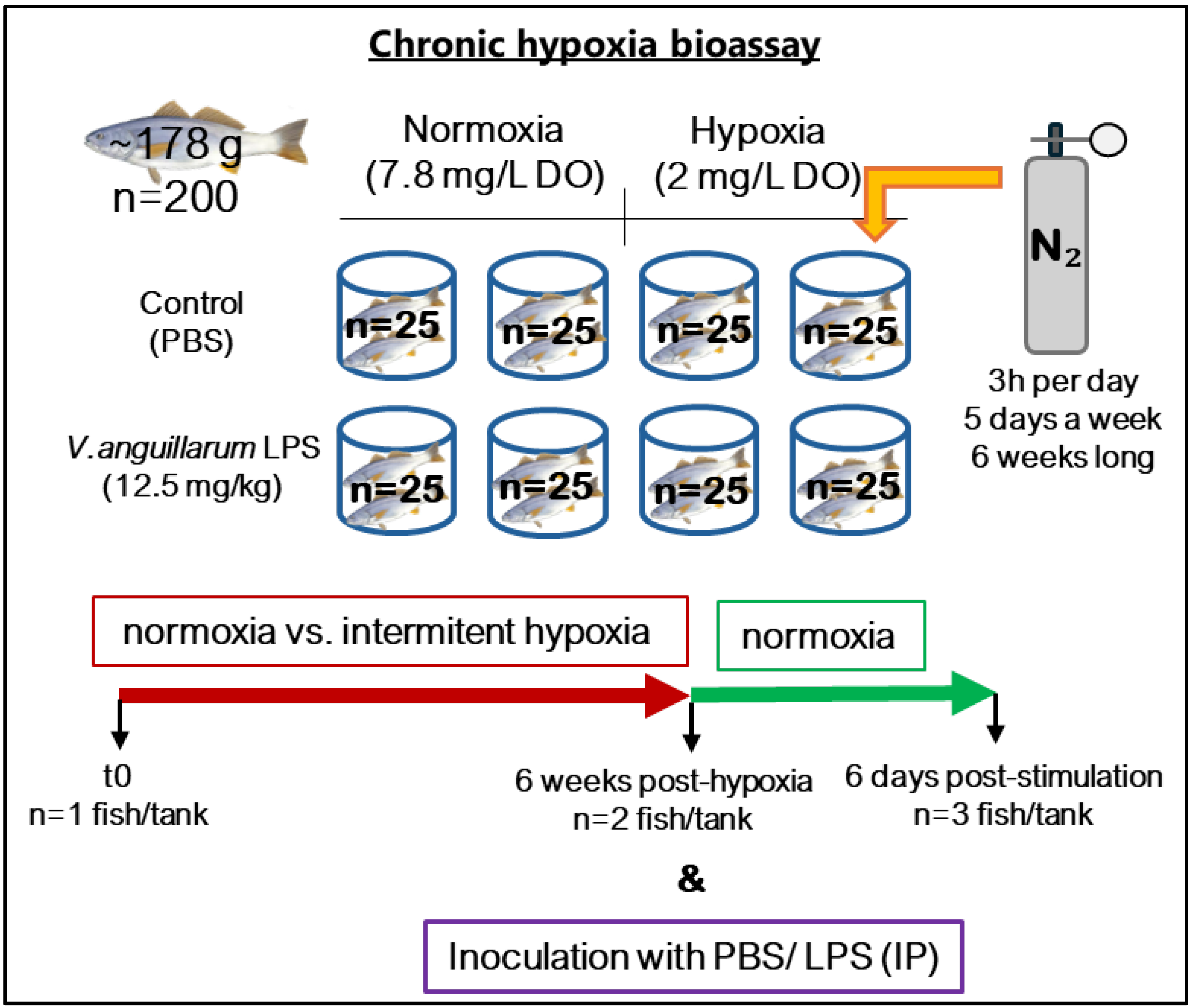
2.3. Chronic Hypoxia and LPS Challenge
2.4. Mucus Sample Recovery and Total Protein Extraction
2.5. Antibacterial Activity
2.6. Lysozyme Activity
2.7. Peroxidase Activity
2.8. Statistical Analysis
3. Results
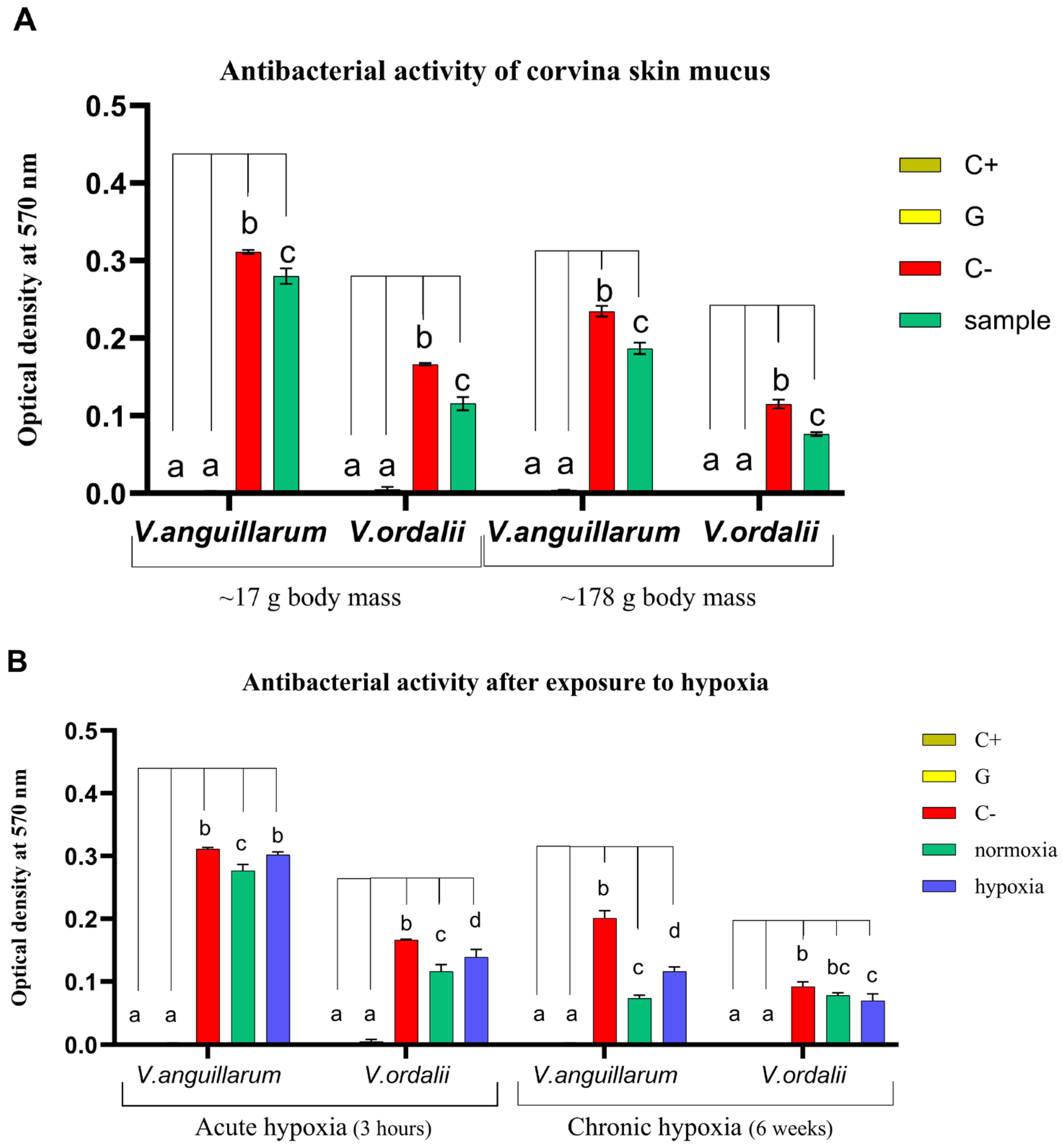
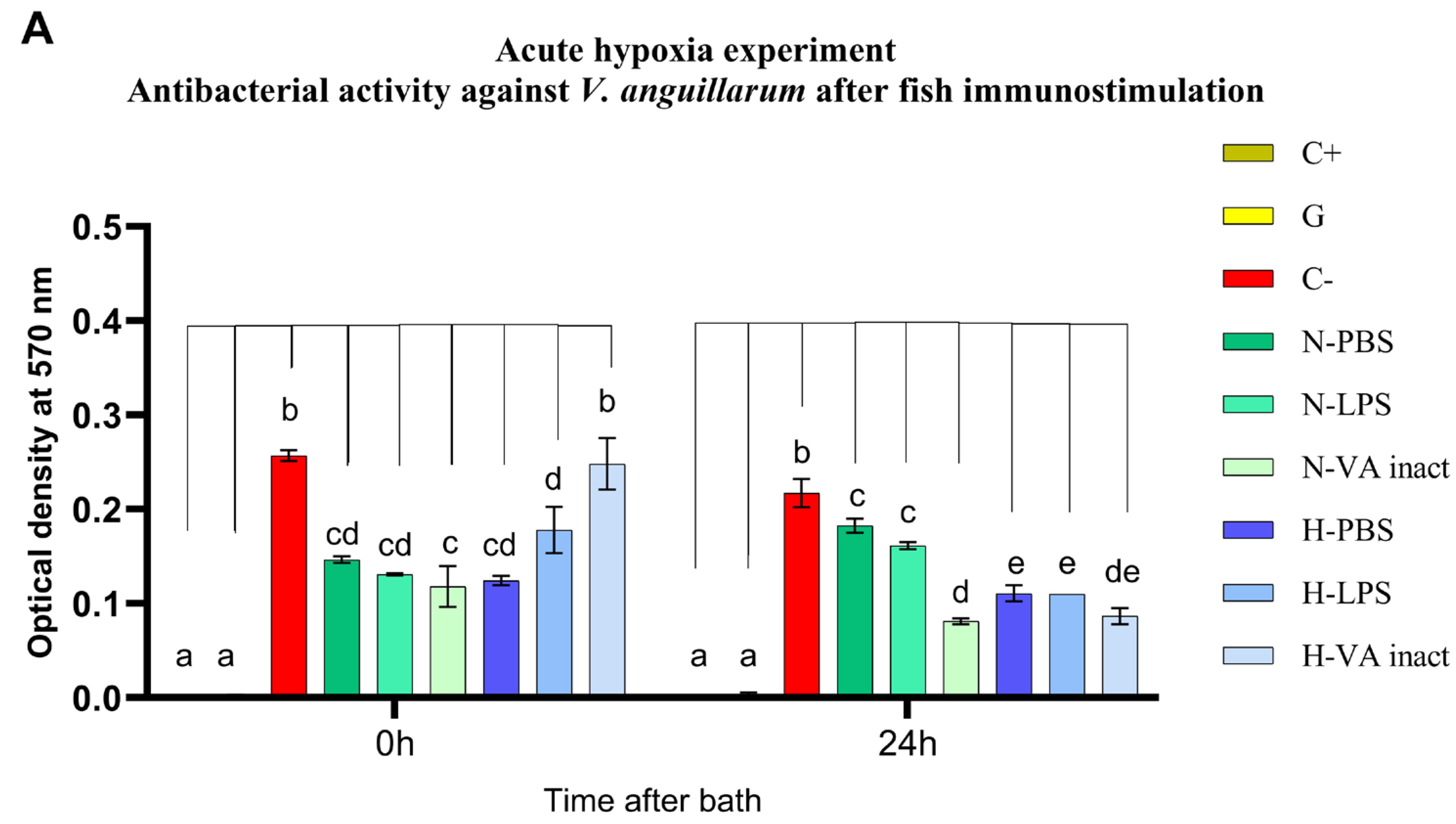
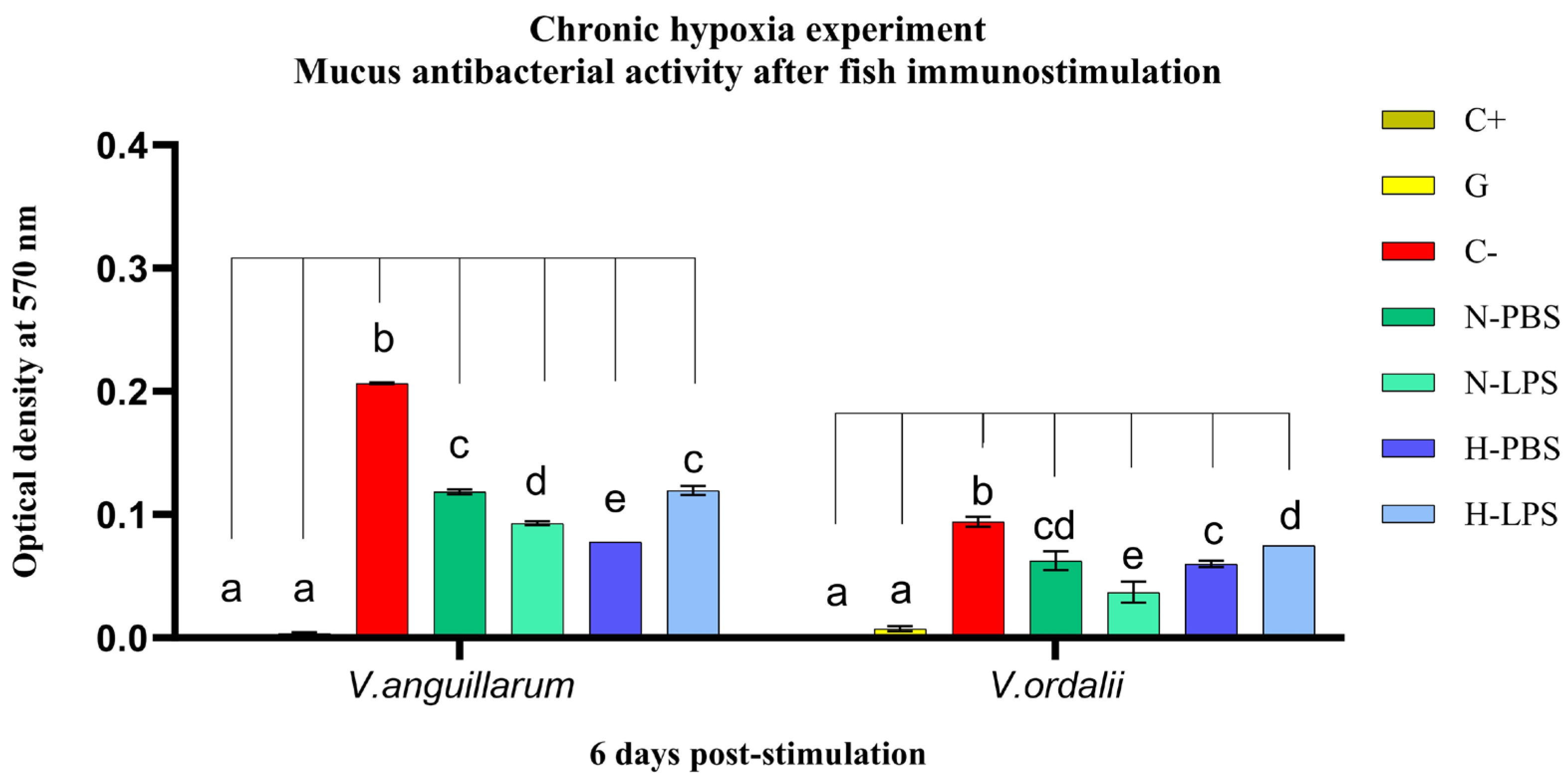
3.1. Antibacterial Activity
3.2. Lysozyme Activity
3.3. Peroxidase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chirichigno, N.F. Clave para identificar los peces marinos del Perú. Inf. Inst. Mar. Perú 1974, 44, 1–387. [Google Scholar]
- Álvarez, C.A.; Alvarado, J.F.; Farías, M.; Cárcamo, C.B.; Flores, H.; Guzmán, F.; Martín, S.S.; Varas, J.; Messina, S.; Acosta, F.; et al. First Insights about Orexigenic Activity and Gastrointestinal Tissue Localization of Ghrelin from Corvina Drum (Cilus gilberti). Aquaculture 2023, 571, 739468. [Google Scholar] [CrossRef]
- Snieszko, S.F. Recent advances in scientific knowledge and developments pertaining to diseases of fishes. Adv. Vet. Sci. Comp. Med. 1973, 17, 291–314. [Google Scholar]
- Hegde, A.; Kabra, S.; Basawa, R.M.; Khile, D.A.; Abbu, R.U.F.; Thomas, N.A.; Manickam, N.B.; Raval, R. Bacterial Diseases in Marine Fish Species: Current Trends and Future Prospects in Disease Management. World J. Microbiol. Biotechnol. 2023, 39, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.; Roque, A.; Gavaia, P.J. Review of the principal diseases affecting cultured meagre (Argyrosomus regius). Aquac. Res. 2018, 49, 1373–1382. [Google Scholar] [CrossRef]
- Yang, A.; Li, W.; Tao, Z.; Ye, H.; Xu, Z.; Li, Y.; Gao, Y.; Yan, X. Vibrio harveyi isolated from marine aquaculture species in eastern China and virulence to the large yellow croaker (Larimichthys crocea). J. Appl. Microbiol. 2021, 131, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish. Dis. 2011, 34, 643–661. [Google Scholar] [CrossRef]
- Manchanayake, T.; Salleh, A.; Amal, M.N.A.; Yasin, I.S.M.; Zamri-Saad, M. Pathology and pathogenesis of Vibrio infection in fish: A review. Aquac. Rep. 2023, 28, 101459. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enríquez, R.; Moran, G.J.V.M. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I.; Magadán, S. Omics in fish mucosal immunity. Dev. Comp. Immunol. 2017, 75, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Godoy, M. Inmunología de los peces óseos: Revisión. Rev. Mex. Cienc. Pecu. 2010, 1, 47–57. [Google Scholar]
- Concha, K.; Olivares, P.; Fonseca-Salamanca, F.; Sanchez, R.; Serrano, F.; Parodi, J. Aditivos Mucogénicos para el Control de Caligus rogercresseyi en Salmón del Atlántico (Salmo salar). Rev. Investig. Veter Del Peru 2017, 28, 477. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Abellán, E.; Meseguer, J.; Esteban, M.A. Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immunol. 2014, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Tapissier-Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Biological and ecological roles of external fish mucus: A review. Fishes 2018, 3, 41. [Google Scholar] [CrossRef]
- Dash, S.; Das, S.K.; Samal, J.; Thatoi, H.N. Epidermal mucus, a major determinant in fish health: A review. Iran. J. Vet. Res. 2018, 19, 72–81. [Google Scholar] [PubMed]
- Olabuenaga, S.E. Fish Immune System. Gayana (Concepc.) 2000, 64, 205–215. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ingle, H.; Prasad, D.V.R.; Kumar, H. Recognition of bacterial infection by innate immune sensors. Crit. Rev. Microbiol. 2013, 39, 229–246. [Google Scholar] [CrossRef]
- Mulero, I.; Sepulcre, M.P.; Roca, F.J.; Meseguer, J.; García-Ayala, A.; Mulero, V. Characterization of macrophages from the bony fish gilthead seabream using an antibody against the macrophage colony-stimulating factor receptor. Dev. Comp. Immunol. 2008, 32, 1151–1159. [Google Scholar] [CrossRef]
- Salinas, I. The mucosal immune system of teleost fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.L.; Dixon, B. Salmonid antibacterial immunity: An aquaculture perspective. Biology 2020, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef]
- Boletín CEAZA. Mar–Abril 2022. Análisis de las Condiciones Atmosféricas y Oceanográficas de la Región de Coquimbo (Diciembre 2021–Febrero 2022). Available online: www.ceazamar.cl/boletines/pdfjs/viewer.php?file=/boletines/abril_2022.pdf (accessed on 1 April 2022). [CrossRef]
- Boletín CEAZA. Mar–Octubre 2023. Análisis de las Condiciones Atmosféricas y Oceanográficas de la Región de Coquimbo (Junio–Agosto 2023). Available online: www.ceazamar.cl/boletines/pdfjs/viewer.php?file=/boletines/octubre_2023.pdf (accessed on 1 October 2023). [CrossRef]
- Lardies, M.A.; Benitez, S.; Osores, S.; Vargas, C.A.; Duarte, C.; Lagos, N.A.; Lohrmann, K.B. Physiological and Histopathological Impacts of Increased Carbon Dioxide and Temperature on the Scallops Argopecten Purpuratus Cultured under Upwelling Influences in Northern Chile. Aquaculture 2017, 479, 455–466. [Google Scholar] [CrossRef]
- Helly, J.J.; Levin, L.A. Global distribution of naturally occurring marine hypoxia on continental margins. Deep. Sea Res. I Oceanogr. Res. Pap. 2004, 51, 1159–1168. [Google Scholar] [CrossRef]
- Fuenzalida, R.; Schneider, W.; Garcés-Vargas, J.; Bravo, L.; Lange, C. Vertical and horizontal extension of the oxygen minimum zone in the eastern South Pacific Ocean. Deep. Sea Res. II Top. Stud. Oceanogr. 2009, 56, 992–1003. [Google Scholar] [CrossRef]
- Paulmier, A.; Ruiz-Pino, D. Oxygen minimum zones (OMZs) in the modern ocean. Prog. Oceanogr. 2009, 80, 113–128. [Google Scholar] [CrossRef]
- FAO. Impactos Del Cambio Climático en la Pesca Y la Acuicultura: Síntesis de Los Conocimientos Y Las Opciones de Adaptación Y Mitigación Actuales. Resumen Del Documento Técnico de Pesca Y Acuicultura de La; FAO: Rome, Italy, 2018; 48p. [Google Scholar]
- Vega Ramirez, M.; López-Santiago, R.; Moreno, M.; Flores, V. Respuesta Inmune En Peces. In Inmunología Veterinaria, 1st ed.; El Manual Moderno: México City, Mexico, 2010; Chapter 24; pp. 309–314. ISBN 978-607-448-057-3. [Google Scholar]
- Wang, Z.; Pu, D.; Zheng, J.; Li, P.; Lü, H.; Wei, X.; Li, M.; Li, D.; Gao, L. Hypoxia-induced physiological responses in fish: From organism to tissue to molecular levels. Ecotoxicol. Environ. Saf. 2023, 267, 115609. [Google Scholar] [CrossRef]
- Machado, M.; Arenas, F.; Svendsen, J.C.; Azeredo, R.; Pfeifer, L.J.; Wilson, J.M.; Costas, B. Effects of water acidification on Senegalese sole (Solea senegalensis) health status and metabolic rate: Implications for immune responses and energy use. Front. Physiol. 2020, 11, 26. [Google Scholar] [CrossRef]
- Hilles, A.R.; Mahmood, S.; Hashim, R. Evaluation of the antibacterial activities of skin mucus from Asian swamp eel (Monopterusalbus). Indian J. Mar. Sci. 2019, 48, 1855–1859. [Google Scholar]
- Parry, R.M.; Chandan, R.C.; Shahani, K.M. A rapid and sensitive assay of muramidase. Proc. Soc. Exp. Biol. Med. 1965, 119, 384–386. [Google Scholar] [CrossRef]
- Lee, Y.C.; Yang, D. Determination of lysozyme activities in a microplate format. Anal. Biochem. 2002, 310, 223–224. [Google Scholar] [CrossRef]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Veter. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Ross, N.W.; MacKinnon, S.L. Comparison of antimicrobial activity in the epidermal mucus extracts of fish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 150, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Sanahuja, I.; Fernández-Alacid, L.; Ordóñez-Grande, B.; Sánchez-Nuño, S.; Ramos, A.; Araujo, R.M.; Ibarz, A. Comparison of several non-specific skin mucus immune defences in three piscine species of aquaculture interest. Fish Shellfish Immunol. 2019, 89, 428–436. [Google Scholar] [CrossRef]
- Ellis, A. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Bohle, H.; Kjetil, F.; Bustos, P.; Riofrío, A.; Peters, C. Fenotipo atípico de Vibrio ordalii, bacteria altamente patogénica aislada desde salmón del Atlántico cultivado en las costas marinas del sur de Chile. Arch. Med. Veter. 2007, 39, 43–52. [Google Scholar] [CrossRef]
- Silva-Rubio, A.; Avendaño-Herrera, R.; Jaureguiberry, B.; Toranzo, A.E.; Magariños, B. First description of serotype O3 in Vibrio anguillarum strains isolated from salmonids in Chile. J. Fish Dis. 2008, 31, 235–239. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T. The innate and adaptive immune system of fish. In Infectious Disease in Aquaculture: Prevention and Control; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 3–68. [Google Scholar] [CrossRef]
- Yavuzcan, H. Plasma lysozyme levels and secondary stress response in rainbow trout, Oncorhynchus mykiss (Walbaum) after exposure to Leteux-Meyer mixture. Turk. J. Vet. Anim. Sci. 2006, 30, 265–269. [Google Scholar]
- Campoverde, C.; Milne, D.J.; Estévez, A.; Duncan, N.; Secombes, C.J.; Andree, K.B. Ontogeny and modulation after PAMPs stimulation of β-defensin, hepcidin, and piscidin antimicrobial peptides in meagre (Argyrosomus regius). Fish Shellfish Immunol. 2017, 69, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, R.; Heidari, B.; Aghamaali, M. Evaluation of lysozyme, complement C3, and total protein in different developmental stages of Caspian kutum (Rutilus frisii kutum K.). Arch. Pol. Fish. 2016, 24, 15–22. [Google Scholar] [CrossRef]
- Katzenback, B.A. Antimicrobial Peptides as Mediators of Innate Immunity in Teleosts. Biology 2015, 4, 607–639. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Scapigliati, G.; Scalia, D.; Marras, A.; Meloni, S.; Mazzini, M. Immunoglobulin levels in the teleost sea bass Dicentrarchus labrax (L.) in relation to age, season, and water oxygenation. Aquaculture 1999, 174, 207–212. [Google Scholar] [CrossRef]
- Takeshi, N.; Junji, I.; Moritsugu, I.; Norihisa, K.; Yasuhiro, T.; Yoshio, K.; Hisaki, N.; Kazuhiro, F.; Miki, N.; Tomoki, Y. Distribution of glutathione peroxidase activity in fish. Fish Sci. 2008, 65, 665–666. [Google Scholar] [CrossRef]
- Kumari, S.; Tyor, A.K.; Bhatnagar, A. Evaluation of the antibacterial activity of skin mucus of three carp species. Int. Aquat. Res. 2019, 11, 225–239. [Google Scholar] [CrossRef]
- Salinas, I.; Fernandez-Montero, A.; Ding, Y.; Sunyer, J.O. Mucosal immunoglobulins of teleost fish: A decade of advances. Dev. Comp. Immunol. 2021, 121, 104079. [Google Scholar] [CrossRef]
- Paulsen, S.M.; Lunde, H.; Engstad, R.E.; Robertsen, B. In vivo effects of beta-glucan and LPS on regulation of lysozyme activity and mRNA expression in Atlantic salmon (Salmo salar L.). Fish Shellfish Immunol. 2003, 14, 39–54. [Google Scholar] [CrossRef]
- Selvaraj, V.; Sampath, K.; Sekar, V. Administration of lipopolysaccharide increases specific and non-specific immune parameters and survival in carp (Cyprinus carpio) infected with Aeromonas hydrophila. Aquaculture 2009, 286, 176–183. [Google Scholar] [CrossRef]
- Silva, B.C.; Martins, M.L.; Jatobá, A.; Neto, C.C.B.; Vieira, F.N.; Pereira, G.V.; Jerônimo, G.T.; Seiffert, W.Q.; Mouriño, J.L.P. Hematological and immunological responses of Nile tilapia after polyvalent vaccine administration by different routes. Pesqui. Agropecu. Bras. 2009, 29, 874–880. [Google Scholar] [CrossRef]
- Velji, M.I.; Albright, L.J.; Evelyn, T.P. Protective immunity in juvenile coho salmo Oncorhynchus kisutch following immunization with Vibrio ordalii lipopolysaccharide or from exposure to live V. ordalii cells. Dis. Aquat. Org. 1990, 9, 25–29. [Google Scholar] [CrossRef]
- Nayak, S.; Jahan, N.; Pattnaik, S. Effect of parenteral endotoxin administration on the immuno-haematological responses of catfish, Heteropneustes fossilis. Fish Shellfish. Immunol. Rep. 2021, 2, 100022. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Sampath, K.; Sekar, V. Extraction and Characterization of Lipopolysaccharide from Aeromonas hydrophila and Its Effects on Survival and Hematology of the Carp, Cyprinus carpio. Asian Fish. Sci. 2004, 17, 163–173. [Google Scholar] [CrossRef]
- Nayak, S.; Swain, P.; Nanda, P.; Dash, S.; Shukla, S.; Meher, P.; Maiti, N. Effect of endotoxin on the immunity of Indian major carp, Labeo rohita. Fish Shellfish. Immunol. 2008, 24, 394–399. [Google Scholar] [CrossRef]
- Wang, D.; Cao, Q.; Zhu, W.; Hu, Y.; Zhang, X.; Yin, S.; Wang, T. Individual and combined effects of salinity and lipopolysaccharides on the immune response of juvenile Takifugu fasciatus. Fish Physiol. Biochem. 2019, 45, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Rappolt, M.; Schromm, A.B.; Howe, J.; Lohner, K.; Andrä, J.; Koch, M.H.; Brandenburg, K. Divalent cations affect chain mobility and aggregate structure of lipopolysaccharide from Salmonella minnesota reflected in a decrease of its biological activity. Biochim Biophys Acta (BBA) 2005, 1715, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Snieszko, S.F. The effects of environmental stress on outbreaks of infectious diseases of fishes. J. Fish Biol. 1974, 6, 197–208. [Google Scholar] [CrossRef]
- Cabillon, N.A.R.; Lazado, C.C. Mucosal barrier functions of fish under changing environmental conditions. Fishes 2019, 4, 2. [Google Scholar] [CrossRef]
- Wedemeyer, G.A. Physiological Response of juvenile coho salmon (Oncorhynchus kisutch) and rainbow trout (Salmo gairdneri) to handling and crowding stress in intensive fish culture. J. Fish. Res. Board Can. 1976, 33, 2699–2702. [Google Scholar] [CrossRef]
- Gattuso, A.; Garofalo, F.; Cerra, M.C.; Imbrogno, S. Hypoxia Tolerance in Teleosts: Implications of Cardiac Nitrosative Signals. Front. Physiol. 2018, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Ruiz, M.; García-Beltrán, J.M.; Cerezo, I.M.; Balebona, M.C.; Moriñigo, M.; Esteban, M. Immunomodulation and skin microbiota perturbations during an episode of chronic stress in gilthead seabream. Fish Shellfish. Immunol. 2022, 122, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Mandic, M.; Joyce, W.; Perry, S.F. The evolutionary and physiological significance of the Hif pathway in teleost fishes. J. Exp. Biol. 2021, 224, jeb231936. [Google Scholar] [CrossRef]
- Luo, S.-Y.; Wang, J.-Q.; Liu, C.; Gao, X.-M.; Zhang, Y.-B.; Ding, J.; Hou, C.-C.; Zhu, J.-Q.; Lou, B.; Shen, W.-L.; et al. Hif-1 α/Hsf1/Hsp70 signaling pathway regulates redox homeostasis and apoptosis in large yellow croaker (Larimichthys crocea) under environmental hypoxia. Zool. Res. 2021, 42, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Beemelmanns, A.; Miest, J.J. Are fish immunocompetent enough to face climate change? Biol. Lett. 2024, 20, 20230346. [Google Scholar] [CrossRef] [PubMed]
- Gallage, S.; Katagiri, T.; Endo, M.; Futami, K.; Endo, M.; Maita, M. Influence of moderate hypoxia on vaccine efficacy against Vibrio anguillarum in Oreochromis niloticus (Nile tilapia). Fish Shellfish Immunol. 2016, 51, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.A.; Jerez-Cepa, I.; Cárcamo, C.B.; Toledo, P.; Flores, H.; Brokordt, K. Growth performance, physiological responses to hypoxia and flesh quality of Chilean croaker (Cilus gilberti) stocked at different densities. Aquaculture 2020, 525, 735316. [Google Scholar] [CrossRef]

| Bacterial Strain | Optical Density at 600 nm | UFC mL−1 |
|---|---|---|
| Vibrio anguillarum 507 | 0.380 | 1.00 × 109 |
| Vibrio ordalii DSM 19621 | 0.100 | 1.00 × 108 |
| Experiment | Time Point | Treatment | Lysozyme Activity (U mL−1) | Equivalent Amount of Egg Lysozyme (µg per 100 µg of Sample) |
|---|---|---|---|---|
| Acute hypoxia | After hypoxia (3 h) | Normoxia | ND | - |
| Hypoxia | 4.00 ± 5.66 | 0.09 ± 0.13 | ||
| After immunostimulation | N-PBS | 10.00 ± 2.83 | 0.19 ± 0.01 | |
| N-VA | ND | - | ||
| H-PBS | ND | - | ||
| H-VA | ND | - | ||
| 24 h post- immunostimulation | N-PBS | 4.00 ± 0.00 a | 0.16 ± 0.00 | |
| N-LPS | 2.00 ± 2.83 a | 0.08 ± 0.12 | ||
| N-VA | ND a | - | ||
| H-PBS | 32.00 ± 0.00 b | 0.29 ± 0.00 | ||
| H-LPS | ND a | - | ||
| H-VA | 40.00 ± 5.66 b | 0.33 ± 0.03 | ||
| Chronic hypoxia | After intermittent hypoxia (6 weeks) | Normoxia | 60.00 ± 11.31 | 0.42 ± 0.05 |
| Hypoxia | 30.00 ± 2.83 | 0.28 ± 0.01 | ||
| 6 days post- immunostimulation | N-PBS | 54.00 ± 2.83 | 0.39 ± 0.01 | |
| N-LPS | 146.00 ± 8.49 | 0.81 ± 0.04 | ||
| H-PBS | 102.00 ± 36.77 | 0.61 ± 0.17 | ||
| H-LPS | 70.00 ± 8.49 | 0.47 ± 0.04 |
| Experiment | Time Point | Treatment | Peroxidase Activity (U mg−1) | Amount of Peroxidase per 100 µg of Total Protein Extracts (µg) |
|---|---|---|---|---|
| Acute hypoxia | After hypoxia (3 h) | Normoxia | 0.73 ± 0.42 | 0.07 ± 0.03 |
| Hypoxia | 0.97 ± 0.26 | 0.09 ± 0.02 | ||
| After immunostimulation | N-PBS | 0.20 ± 0.06 a | 0.04 ± 0.00 | |
| N-VA | 0.46 ± 0.01 bc | 0.05 ± 0.00 | ||
| H-PBS | 0.33 ± 0.01 ac | 0.05 ± 0.00 | ||
| H-VA | 0.60 ± 0.01 b | 0.06 ± 0.00 | ||
| 24 h post- immunostimulation | N-PBS | 0.30 ± 0.05 ab | 0.04 ± 0.00 | |
| N-LPS | 0.85 ± 0.03 c | 0.08 ± 0.00 | ||
| N-VA | 0.29 ± 0.06 ab | 0.04 ± 0.00 | ||
| H-PBS | 0.34 ± 0.02 a | 0.05 ± 0.00 | ||
| H-LPS | 0.14 ± 0.01 bd | 0.03 ± 0.00 | ||
| H-VA | 0.10 ± 0.01 d | 0.03 ± 0.00 | ||
| Chronic hypoxia | After intermittent hypoxia (6 weeks) | Normoxia | 3.26 ± 0.00 | 0.52 ± 0.00 |
| Hypoxia | 3.32 ± 0.03 | 0.53 ± 0.00 | ||
| 6 days post- immunostimulation | N-PBS | 0.05 ± 0.01 a | 0.03 ± 0.00 | |
| N-LPS | 2.57 ± 0.01 b | 0.42 ± 0.00 | ||
| H-PBS | 0.65 ± 0.04 c | 0.12 ± 0.01 | ||
| H-LPS | 0.74 ± 0.06 c | 0.13 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega, B.; Toro-Araneda, T.; Alvarado, J.F.; Cárcamo, C.B.; Guzmán, F.; Acosta, F.; Oliva, M.; Serrano, E.; Galarza, J.I.; Álvarez, C.A. Effects of Hypoxia on the Antibacterial Activity of Epidermal Mucus from Chilean Meagre (Cilus gilberti). Animals 2024, 14, 2014. https://doi.org/10.3390/ani14132014
Vega B, Toro-Araneda T, Alvarado JF, Cárcamo CB, Guzmán F, Acosta F, Oliva M, Serrano E, Galarza JI, Álvarez CA. Effects of Hypoxia on the Antibacterial Activity of Epidermal Mucus from Chilean Meagre (Cilus gilberti). Animals. 2024; 14(13):2014. https://doi.org/10.3390/ani14132014
Chicago/Turabian StyleVega, Belinda, Teresa Toro-Araneda, Juan F. Alvarado, Claudia B. Cárcamo, Fanny Guzmán, Félix Acosta, Marcia Oliva, Edison Serrano, Janeth I. Galarza, and Claudio A. Álvarez. 2024. "Effects of Hypoxia on the Antibacterial Activity of Epidermal Mucus from Chilean Meagre (Cilus gilberti)" Animals 14, no. 13: 2014. https://doi.org/10.3390/ani14132014






