Temperature-Dependent Sex Determination in Crocodilians and Climate Challenges
Abstract
:Simple Summary
Abstract
1. Introduction
2. Sex Determination in Crocodiles
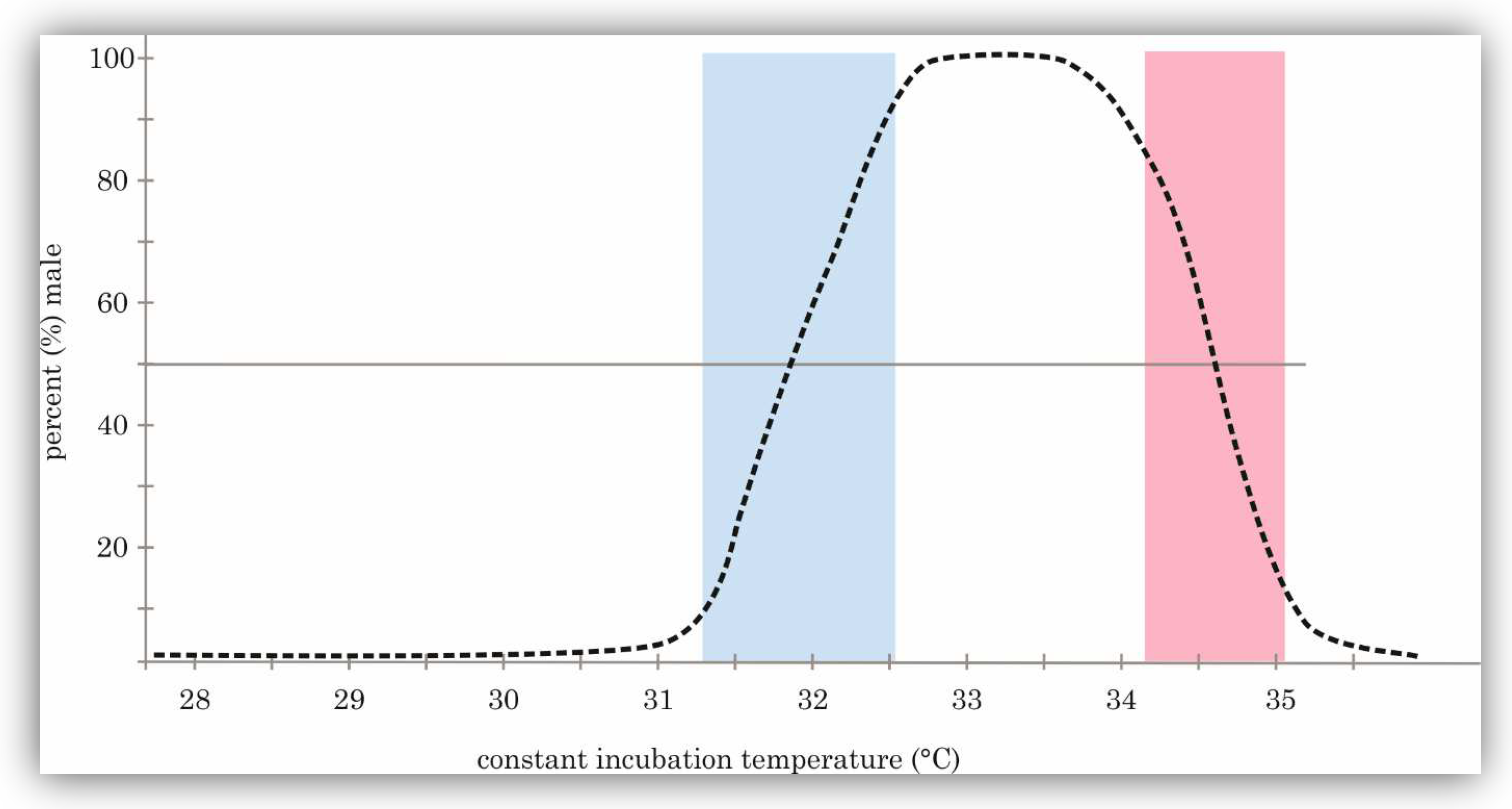
2.1. Patterns of Determination
2.2. Thermosensitive Period
2.3. Gonadal Differentiation
2.4. Selective Advantage of TSD
2.5. Mechanisms of Temperature-Dependent Sex Determination and the Influence of Genes, Hormones, and Epigenetic Factors
2.5.1. Genes and Proteins Involved in Temperature-Dependent Sex Determination
The Temperature-Dependent Calcium Channels: The Transient Receptor Potential Family
Signal Transduction Molecules: JARID2 and KDM6B
Transcription Factors as Regulatory Molecules: The Role of SOX9 and DMRT1
Nuclear Receptors as Differentiation Markers: SF-1
2.5.2. Hormonal Influence in Temperature-Dependent Sex Determination: Steroid Hormones and Aromatase
Steroid Hormones
The Role of Aromatase
Anti-Müllerian Hormone
2.5.3. Epigenetic Factors and miRNAs
3. The Role of Climate Change
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weber, C.; Capel, B. Sex determination without sex chromosomes. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200109. [Google Scholar] [CrossRef] [PubMed]
- Mittwoch, U. Sex determination in mythology and history. Arq. Bras. De Endocrinol. Metabol. 2005, 49, 7–13. [Google Scholar] [CrossRef]
- Stévant, I.; Papaioannou, M.D.; Nef, S. A brief history of sex determination. Mol. Cell Endocrinol. 2018, 468, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.J. Sex Determination in Reptiles. Q. Rev. Biol. 1980, 55, 3–21. [Google Scholar] [CrossRef]
- Janzen, F.J. Climate change and temperature-dependent sex determination in reptiles. Proc. Natl. Acad. Sci. USA 1994, 91, 7487–7490. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.M.; Barnett, M.; Sharpe, P.T. The molecular biology of temperature-dependent sex determination. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997, 350, 297–304. [Google Scholar]
- Deeming, D.C.; Ferguson, M.W.J. Effects of incubation temperature on growth and development of embryos ofAlligator mississippiensis. J. Comp. Physiol. B 1989, 159, 183–193. [Google Scholar] [CrossRef]
- Janzen, F.J.; Paukstis, G.L. Environmental Sex Determination in Reptiles: Ecology, Evolution, and Experimental Design. Q. Rev. Biol. 1991, 66, 149–179. [Google Scholar] [CrossRef]
- Janzen, F.J. Experimental evidence for the evolutionary significance of temperature-dependent sex determination. Evolution 1995, 49, 864–873. [Google Scholar]
- Kohno, S.; Parrott, B.B.; Yatsu, R.; Miyagawa, S.; Moore, B.C.; Iguchi, T.; Guillette, L., Jr. Gonadal differentiation in reptiles exhibiting environmental sex determination. Sex. Dev. 2014, 8, 208–226. [Google Scholar] [CrossRef]
- Pieau, C.; Dorizzi, M.; Richard-Mercier, N. Temperature-dependent sex determination and gonadal differentiation in reptiles. Genes Mech. Vertebr. Sex Determ. 2001, 91, 117–141. [Google Scholar]
- Lang, J.W.; Andrews, H.V. Temperature-dependent sex determination in crocodilians. J. Exp. Zool. 1994, 270, 28–44. [Google Scholar] [CrossRef]
- González, E.J.; Martínez-López, M.; Morales-Garduza, M.A.; García-Morales, R.; Charruau, P.; Gallardo-Cruz, J.A. The sex-determination pattern in crocodilians: A systematic review of three decades of research. J. Anim. Ecol. 2019, 88, 1417–1427. [Google Scholar] [CrossRef]
- Dorizzi, M.; Mignot, T.-M.; Guichard, A.; Desvages, G.; Pieau, C. Involvement of oestrogens in sexual differentiation of gonads as a function of temperature in turtles. Differentiation 1991, 47, 9–17. [Google Scholar] [CrossRef]
- Valenzuela, N. Temperature-Dependent Sex Determination in Vertebrates; Lance, V., Ed.; Smithsonian Books: Washington, DC, USA, 2004. [Google Scholar]
- Deeming, D.C.; Ferguson, M.W.J. Environmental regulation of sex determination in reptiles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997, 322, 19–39. [Google Scholar]
- Lance, V. Reproductive Biology of the Crocodylia, 1st ed.; Elsevier: Waltham, MA, USA, 2021. [Google Scholar]
- Deeming, D.C.; Ferguson, M.W.J. The Mechanism of Temperature Dependent Sex Determination in Crocodilians: A Hypothesis. Am. Zool. 1989, 29, 973–985. [Google Scholar] [CrossRef]
- Ferguson, M.W.J.; Joanen, T. Temperature of egg incubation determines sex in Alligator mississippiensis. Nature 1982, 296, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Hutton, J.M. Incubation temperatures, sex ratios and sex determination in a population of Nile crocodiles (Crocodylus niloticus). J. Zool. 1987, 211, 143–155. [Google Scholar] [CrossRef]
- Webb, G.J.W.; Cooper-Preston, H. Effects of Incubation Temperature on Crocodiles and the Evolution of Reptilian Oviparity1. Am. Zool. 1989, 29, 953–971. [Google Scholar] [CrossRef]
- Ewert, M.A.; Jackson, D.R.; Nelson, C.E. Patterns of temperature-dependent sex determination in turtles. J. Exp. Zool. 1994, 270, 3–15. [Google Scholar] [CrossRef]
- Mrosovsky, N.; Pieau, C. Transitional range of temperature, pivotal temperatures and thermosensitive stages for sex determination in reptiles. Amphib. Reptil. 1991, 12, 169–179. [Google Scholar] [CrossRef]
- Ferguson, M.W.J.; Joanen, T. Temperature-dependent sex determination in Alligator mississippiensis. J. Zool. 1983, 200, 143–177. [Google Scholar] [CrossRef]
- Escobedo-Galván, A.H.; López-Luna, M.A.; Cupul-Magaña, F.G. Thermal fluctuation within nests and predicted sex ratio of Morelet’s Crocodile. J. Therm. Biol. 2016, 58, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.J. Temperature-sensitive periods of sex determination in a lizard: Similarities with turtles and crocodilians. J. Exp. Zool. 1987, 241, 143–148. [Google Scholar] [CrossRef]
- Akashi, H.; Hasui, D.; Ueda, K.; Ishikawa, M.; Takeda, M.; Miyagawa, S. Understanding the role of environmental temperature on sex determination through comparative studies in reptiles and amphibians. J. Exp. Zool. Part. Ecol. Integr. Physiol. 2024, 341, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Mark, W.J. Reproductive Biology and Embryology of the Crocodilians. In Biology of the Reptilia; Gans, Billett and Maderson; 1985; Available online: https://www.semanticscholar.org/paper/Reproductive-biology-and-embryology-of-the-IN%3A-and-Ferguson/592ddbe544fa5d9c2968f842898e4f1f59978504 (accessed on 25 September 2023).
- Merchant-Larios, H.; Díaz-Hernández, V. Environmental Sex Determination Mechanisms in Reptiles. Sex. Dev. 2013, 7, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Pieau, C.; Dorizzi, M. Oestrogens and temperature-dependent sex determination in reptiles: All is in the gonads. J. Endocrinol. 2004, 181, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, A.; Volarevic, V.; Armstrong, L.; Lako, M.; Stojkovic, M. Primordial Germ Cells: Current Knowledge and Perspectives. Stem. Cells Int. 2016, 2016, 1741072. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.A.; Shine, R. The adaptive significance of temperature-dependent sex determination: Experimental tests with a short-lived lizard. Evolution 2005, 59, 2209–2221. [Google Scholar]
- Charnov, E.L.; Bull, J. When is sex environmentally determined? Nature 1977, 266, 828–830. [Google Scholar] [CrossRef]
- Coriat, A.M.; Valleley, E.; Ferguson, M.W.; Sharpe, P.T. Chromosomal and temperature-dependent sex determination: The search for a conserved mechanism. J. Exp. Zool. 1994, 270, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Korpelainen, H. Sex Ratios and Conditions Required for Environmental Sex Determination in Animals. Biol. Rev. 1990, 65, 147–184. [Google Scholar] [CrossRef] [PubMed]
- Lakin, R.J.; Barrett, P.M.; Stevenson, C.; Thomas, R.J.; Wills, M.A. First evidence for a latitudinal body mass effect in extant Crocodylia and the relationships of their reproductive characters. Biol. J. Linn. Soc. 2020, 129, 875–887. [Google Scholar] [CrossRef]
- Steele, A.L.; Warner, D.A. Sex-specific effects of developmental temperature on morphology, growth and survival of offspring in a lizard with temperature-dependent sex determination. Biol. J. Linn. Soc. 2020, 130, 320–335. [Google Scholar] [CrossRef]
- Weber, C.; Zhou, Y.; Lee, J.G.; Looger, L.L.; Qian, G.; Ge, C.; Capel, B. Temperature-dependent sex determination is mediated by pSTAT3 repression of Kdm6b. Science 2020, 368, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, W.N.; Blumberg, B.; Sutton, S.; Place, A.R.; Lance, V.A. Alligator aromatase cDNA sequence and its expression in embryos at male and female incubation temperatures. J. Exp. Zool. 2001, 290, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Buemio, A.; Chu, R.; Vafaee, M.; Crews, D. Epigenetic Control of Gonadal Aromatase (cyp19a1) in Temperature-Dependent Sex Determination of Red-Eared Slider Turtles. PLoS ONE 2013, 8, e63599. [Google Scholar] [CrossRef] [PubMed]
- Urushitani, H.; Katsu, Y.; Miyagawa, S.; Kohno, S.; Ohta, Y.; Guillette, L.J., Jr.; Iguchi, T. Molecular cloning of anti-Müllerian hormone from the American alligator, Alligator mississippiensis. Mol. Cell Endocrinol. 2011, 333, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Western, P.S.; Harry, J.L.; Graves, J.A.M.; Sinclair, A.H. Temperature-dependent sex determination in the american alligator: AMH precedes SOX9 expression. Dev. Dyn. 1999, 216, 411–419. [Google Scholar] [CrossRef]
- Yatsu, R.; Miyagawa, S.; Kohno, S.; Saito, S.; Lowers, R.H.; Ogino, Y.; Fukuta, N.; Katsu, Y.; Ohta, Y.; Tominaga, M.; et al. TRPV4 associates environmental temperature and sex determination in the American alligator. Sci. Rep. 2015, 5, 18581. [Google Scholar] [CrossRef]
- Güler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-Evoked Activation of the Ion Channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Juárez, A.; Moreno-Mendoza, N. Mechanisms related to sexual determination by temperature in reptiles. J. Therm. Biol. 2019, 85, 102400. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Ye, J.; Zhang, H.; Zhang, Y.; Sun, W.; Sang, Y.; Qian, G. Dmrt1 induces the male pathway in a turtle species with temperature-dependent sex determination. Development 2017, 144, 2222–2233. [Google Scholar] [PubMed]
- Ge, C.; Ye, J.; Weber, C.; Sun, W.; Zhang, H.; Zhou, Y.; Capel, B. The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science 2018, 360, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Q.; Zhou, Q.; Yang, H.Q.; Fang, L.M.; Tang, K.Y.; Sun, L.; Fang, S.G. Molecular mechanism of temperature-dependent sex determination and differentiation in Chinese alligator revealed by developmental transcriptome profiling. Sci. Bull. 2018, 63, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.L.; Lowers, R.H.; Rainwater, T.R.; Stolen, E.; Drake, J.M.; Wilkinson, P.M.; Weiss, S.; Back, B.; Guillette, L., Jr.; Parrott, B.B. Spatial and temporal variation in nest temperatures forecasts sex ratio skews in a crocodilian with environmental sex determination. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200210. [Google Scholar] [CrossRef] [PubMed]
- de Santa Barbara, P.; Moniot, B.; Poulat, F.; Berta, P. Expression and subcellular localization of SF-1, SOX9, WT1, and AMH proteins during early human testicular development. Dev. Dyn. 2000, 217, 293–298. [Google Scholar] [CrossRef]
- Kent, J.; Wheatley, S.C.; Andrews, J.E.; Sinclair, A.H.; Koopman, P. A male-specific role for SOX9 in vertebrate sex determination. Development 1996, 122, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Harley, V.R.; Clarkson, M.J.; Argentaro, A. The Molecular Action and Regulation of the Testis-Determining Factors, SRY (Sex-Determining Region on the Y Chromosome) and SOX9 [SRY-Related High-Mobility Group (HMG) Box 9]. Endocr. Rev. 2003, 24, 466–487. [Google Scholar] [CrossRef]
- Agrawal, R.; Wessely, O.; Anand, A.; Singh, L.; Aggarwal, R.K. Male-specific expression of Sox9 during gonad development of crocodile and mouse is mediated by alternative splicing of its proline-glutamine-alanine rich domain. FEBS J. 2009, 276, 4184–4196. [Google Scholar] [CrossRef]
- Vining, B.; Ming, Z.; Bagheri-Fam, S.; Harley, V. Diverse Regulation but Conserved Function: SOX9 in Vertebrate Sex Determination. Genes 2021, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y. SF-1: A key regulator of development and function in the mammalian reproductive system. Pediatr. Int. 1996, 38, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Crews, D. Estradiol and Incubation Temperature Modulate Regulation of Steroidogenic Factor 1 in the Developing Gonad of the Red-Eared Slider Turtle. Endocrinology 2001, 142, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Western, P.S.; Harry, J.L.; Marshall Graves, J.A.; Sinclair, A.H. Temperature-dependent sex determination in the American alligator: Expression of SF1, WT1 and DAX1 during gonadogenesis. Gene 2000, 241, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, C.; Ramsey, M.; Queen, J.; Crews, D. Expression of Sox9, Mis, and Dmrt1 in the gonad of a species with temperature-dependent sex determination. Dev. Dyn. 2007, 236, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, H.; Yuan, Y.-C.; Chen, S. Molecular Characterization of Aromatase. Ann. N. Y. Acad. Sci. 2009, 1155, 112–120. [Google Scholar] [CrossRef]
- Conley, A.J.; Elf, P.; Corbin, C.J.; Dubowsky, S.; Fivizzani, A.; Lang, J.W. Yolk Steroids Decline during Sexual Differentiation in the Alligator. Gen. Comp. Endocrinol. 1997, 107, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Joss, J.M.P. Uptake of 3H-estradiol by embryonic crocodile gonads during the period of sexual differentiation. J. Exp. Zool. 1994, 270, 219–224. [Google Scholar] [CrossRef]
- Smith, C.A.; Joss, J.M.P. Steroidogenic Enzyme Activity and Ovarian Differentiation in the Saltwater Crocodile, Crocodylus porosus. Gen. Comp. Endocrinol. 1994, 93, 232–245. [Google Scholar] [CrossRef]
- Wibbels, T.; Bull, J.J.; Crews, D. Temperature-dependent sex determination: A mechanistic approach. J. Exp. Zool. 1994, 270, 71–78. [Google Scholar] [CrossRef]
- Lance, V.A.; Bogart, M.H. Disruption of ovarian development in alligator embryos treated with an aromatase inhibitor. Gen. Comp. Endocrinol. 1992, 86, 59–71. [Google Scholar] [CrossRef]
- Crews, D.; Wibbels, T.; Gutzke, W.H.N. Action of sex steroid hormones on temperature-induced sex determination in the snapping turtle (Chelydra serpentina). Gen. Comp. Endocrinol. 1989, 76, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Clyne, C.; Rubin, G.; Boon, W.C.; Robertson, K.; Britt, K.; Jones, M. Aromatase–a brief overview. Annu. Rev. Physiol. 2002, 64, 93–127. [Google Scholar] [CrossRef] [PubMed]
- Milnes, M.R.; Roberts, R.N.; Guillette, L.J. Effects of incubation temperature and estrogen exposure on aromatase activity in the brain and gonads of embryonic alligators. Environ. Health Perspect. 2002, 110 (Suppl. S3), 393–396. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Elf, P.K.; Lang, J.W.; Joss, J.M.P. Aromatase enzyme activity during gonadal sex differentiation in alligator embryos. Differentiation 1995, 58, 281–290. [Google Scholar] [CrossRef]
- Callard, G.V.; Petro, Z.; Ryan, K.J. Identification of Aromatase in the Reptilian Brain1,2. Endocrinology 1977, 100, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Rey, R. Anti-Müllerian hormone is a specific marker of Sertoli- and granulosa-cell origin in gonadal tumors. Hum. Pathol. 2000, 31, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Quinn, A.; Koopman, P. The molecular genetics of sex determination and sex reversal in mammals. Semin. Reprod. Med. 2012, 30, 351–363. [Google Scholar] [CrossRef]
- Pieau, C.; Girondot, M.; Richard-Mercier, N.; Desvages, G.; Dorizzi, M.; Zaborski, P. Temperature sensitivity of sexual differentiation of gonads in the European pond turtle: Hormonal involvement. J. Exp. Zool. 1994, 270, 86–94. [Google Scholar] [CrossRef]
- Josso, N.; Racine, C.; di Clemente, N.; Rodolfo Rey Xavier, F. The role of anti-Müllerian hormone in gonadal development. Mol. Cell Endocrinol. 1998, 145, 3–7. [Google Scholar] [CrossRef]
- Vigier, B.; Forest, M.G.; Eychenne, B.; Bézard, J.; Garrigou, O.; Robel, P.; Josso, N. Anti-Müllerian hormone produces endocrine sex reversal of fetal ovaries. Proc. Natl. Acad. Sci. USA 1989, 86, 3684–3688. [Google Scholar] [CrossRef]
- Nagahama, Y.; Chakraborty, T.; Paul-Prasanth, B.; Ohta, K.; Nakamura, M. Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol. Rev. 2021, 101, 1237–1308. [Google Scholar] [CrossRef] [PubMed]
- Parrott, B.B.; Kohno, S.; Cloy-McCoy, J.A.; Guillette, L.J. Differential Incubation Temperatures Result in Dimorphic DNA Methylation Patterning of the SOX9 and Aromatase Promoters in Gonads of Alligator (Alligator mississippiensis) Embryos1. Biol. Reprod. 2014; ahead of print. [Google Scholar] [CrossRef]
- McCoy, J.A.; Parrott, B.B.; Rainwater, T.R.; Wilkinson, P.M.; Guillette, L.J. Incubation history prior to the canonical thermosensitive period determines sex in the American alligator. Reproduction 2015, 150, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Summers, J.; Silber, S. Environmental versus genetic sex determination: A possible factor in dinosaur extinction? Fertil. Steril. 2004, 81, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Brochu, C. Phylogenetic Approaches Toward Crocodylian History. Annu. Rev. Earth Planet. Sci. 2003, 31, 357–397. [Google Scholar] [CrossRef]
- Benton, M.J.; Clark James, M. Archosaur phylogeny and the relationships of the Crocodylia. Phylogeny Classif. Tetrapods 1988, 1, 295–338. [Google Scholar]
- World Meteorological Organization. Global Temperatures Set to Reach New Records in Next Five Years; World Meteorological Organization: Geneva, Switzerland, 2023; Available online: https://wmo.int/news/media-centre/global-temperatures-set-reach-new-records-next-five-years (accessed on 9 September 2023).
- Mitchell, N.J.; Janzen, F.J. Temperature-dependent sex determination and contemporary climate change. Sex. Dev. 2010, 4, 129–140. [Google Scholar] [CrossRef]
- Eversole, C.; Henke, S. The Conservation History of the American Alligator. In American Alligators: Habitats, Behaviors, and Threats; Nova Science Publisher: New York, NY, USA, 2018; pp. 1–14. [Google Scholar]
- Pearlstine, L.G.; Pearlstine, E.V.; Aumen, N.G. A review of the ecological consequences and management implications of climate change for the Everglades. J. N. Am. Benthol. Soc. 2010, 29, 1510–1526. [Google Scholar] [CrossRef]
- Fukuda, Y.; McDonald, P.J.; Crase, B. Lost to the Sea: Predicted Climate Change Threats to Saltwater Crocodile Nesting Habitat. Front. Ecol. Evol. 2022; ahead of print. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schilling-Tóth, B.M.; Belcher, S.M.; Knotz, J.; Ondrašovičová, S.; Bartha, T.; Tóth, I.; Zsarnovszky, A.; Kiss, D.S. Temperature-Dependent Sex Determination in Crocodilians and Climate Challenges. Animals 2024, 14, 2015. https://doi.org/10.3390/ani14132015
Schilling-Tóth BM, Belcher SM, Knotz J, Ondrašovičová S, Bartha T, Tóth I, Zsarnovszky A, Kiss DS. Temperature-Dependent Sex Determination in Crocodilians and Climate Challenges. Animals. 2024; 14(13):2015. https://doi.org/10.3390/ani14132015
Chicago/Turabian StyleSchilling-Tóth, Boglárka Mária, Scott M. Belcher, Josefine Knotz, Silvia Ondrašovičová, Tibor Bartha, István Tóth, Attila Zsarnovszky, and Dávid Sándor Kiss. 2024. "Temperature-Dependent Sex Determination in Crocodilians and Climate Challenges" Animals 14, no. 13: 2015. https://doi.org/10.3390/ani14132015




