Index Development to Comprehensive Assess Liver Function during the Dairy Cows’ Transition Period in Low-Tropic Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Animals and Sampling Scheme
2.3. Statistical Analysis
| RMSE | |
| MAE | |
| R2 | |
| RMSE: root mean square error. MAE: mean absolute error. R2: coefficient of determination. : observed value. : predicted value and n: number of observations. : index mean value | |
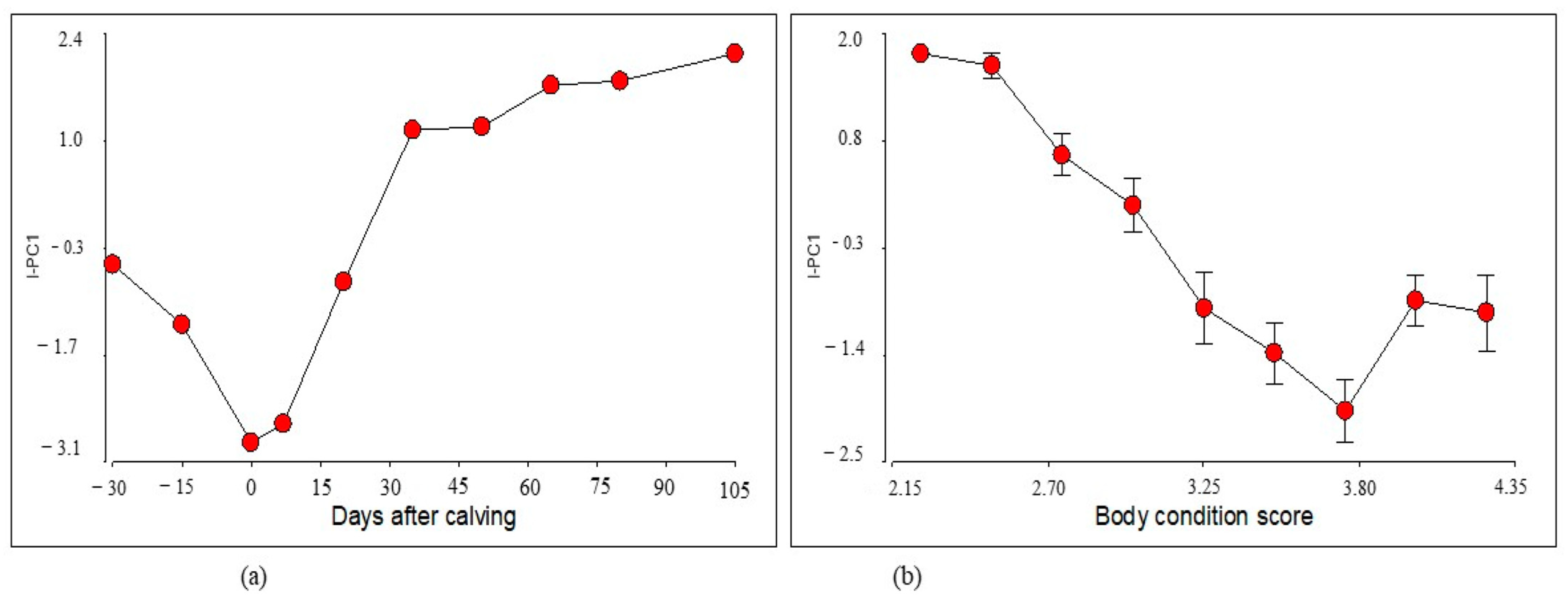
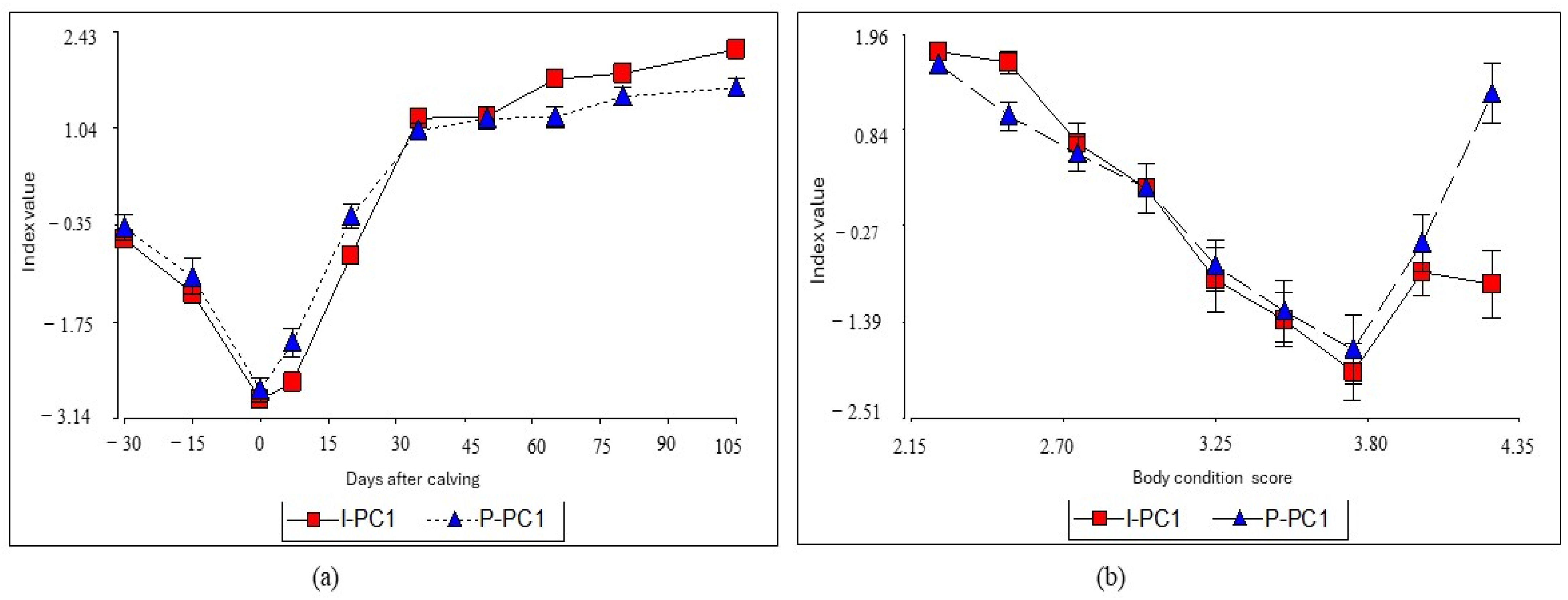
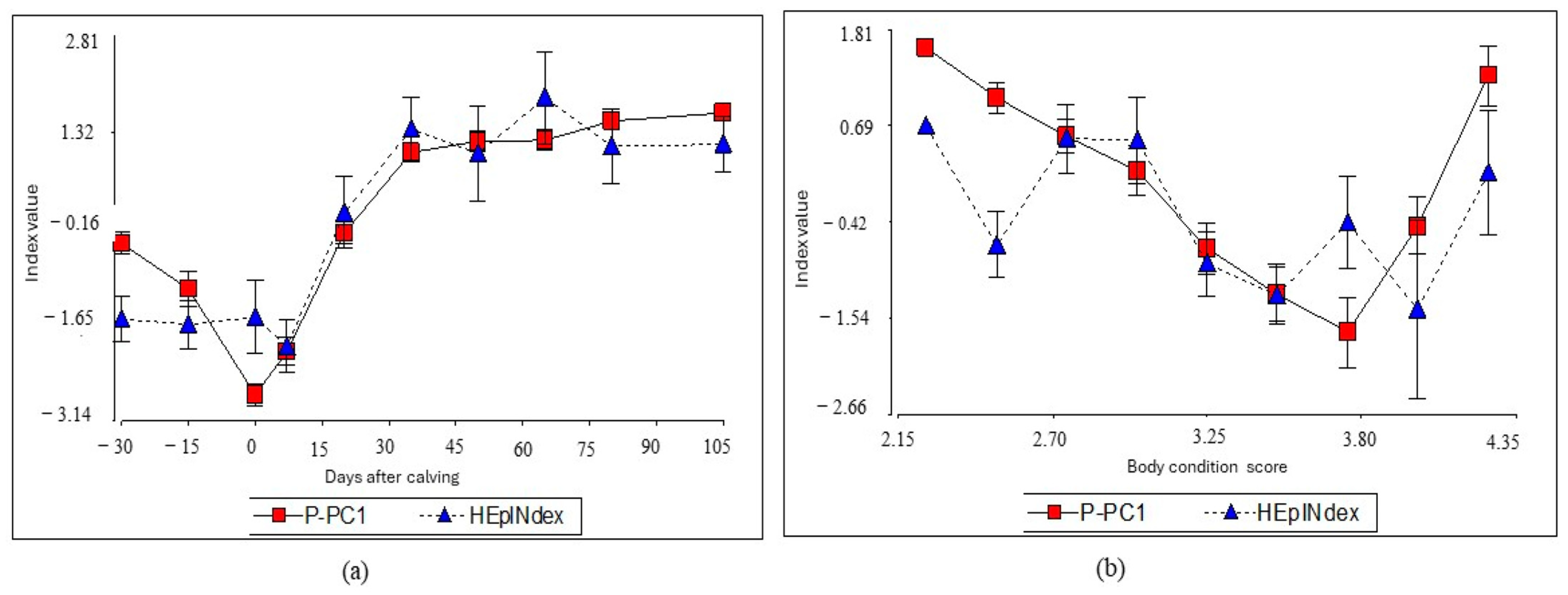
3. Results
4. Discussion
4.1. I-CP1 Index Performance
4.2. Index Clustering and Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, H.M. ADSA Foundation Scholar Award: Influencing hepatic metabolism: Can nutrient partitioning be modulated to optimize metabolic health in the transition dairy cow? J. Dairy Sci. 2020, 103, 6741–6750. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.J.; Boerman, J.P. Invited Review: Quantifying protein mobilization in dairy cows during the transition period. Appl. Anim. Sci. 2020, 36, 389–396. [Google Scholar] [CrossRef]
- Baldacim, V.A.P.; Madureira, K.M.; Ramos, J.S.; Costa, C.P.; Mori, C.S.; Dias, M.R.B.; Gomes, V. Dynamic of metabolic indicators, insulin like-growth factor I (IGF-I) and cortisol in Holstein cows during the transition period. Acta Sci. Vet. 2018, 46, 8. [Google Scholar]
- Schmitt, R.; Pieper, L.; Gonzalez-Grajales, L.A.; Swinkels, J.; Gelfert, C.C.; Staufenbiel, R. Evaluation of different acute-phase proteins for herd health diagnostics in early postpartum Holstein Friesian dairy cows. J. Dairy Res. 2021, 88, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Banuelos, S.; Mendonça, L.G. Transition dairy cow health is associated with first postpartum ovulation risk, metabolic status, milk production, rumination, and physical activity. J. Dairy Sci. 2020, 103, 9573–9586. [Google Scholar] [CrossRef] [PubMed]
- Banuelos, S.; Stevenson, J.S. Transition cow metabolites and physical traits influence days to first postpartum ovulation in dairy cows. Theriogenology 2021, 173, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Putman, A.K.; Brown, J.L.; Gandy, J.C.; Wisnieski, L.; Sordillo, L.M. Changes in biomarkers of nutrient metabolism, inflammation. and oxidative stress in dairy cows during the transition into the early dry period. J. Dairy Sci. 2018, 101, 9350–9359. [Google Scholar] [CrossRef]
- Andjelić, B.; Djoković, R.; Cincović, M.; Bogosavljević-Bošković, S.; Petrović, M.; Mladenović, J.; Čukić, A. Relationships between milk and blood biochemical parameters and metabolic status in dairy cows during lactation. Metabolites 2022, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- González, F.D.; Muiño, R.; Pereira, V.; Campos, R.; Benedito, J.L. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high-yielding dairy cows. J. Vet. Sci. 2011, 12, 251–255. [Google Scholar] [CrossRef]
- Stojević, Z.; Piršljin, J.; Milinković-Tur, S.; Zdelar-Tuk, M.; Beer Ljubić, B. Activities of AST, ALT and GGT in clinically healthy dairy cows during lactation and in the dry period. Vet. Arh. 2005, 75, 67–73. [Google Scholar]
- Allen, M.S. Control of feed intake by hepatic oxidation in ruminant animals: Integration of homeostasis and homeorhesis. Animals 2020, 14, s55–s64. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S.; Bradford, B.J.; Oba, M. Board-invited review: The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 2009, 87, 3317–3334. [Google Scholar] [CrossRef] [PubMed]
- Mordak, R.; Kupczyński, R.; Kuczaj, M.; Niżański, W. Analysis of correlations between selected blood markers of liver function and milk composition in cows during late lactation period. Ann. Anim. Sci. 2020, 20, 871–886. [Google Scholar] [CrossRef]
- Arshad, U.; Santos, J.E.P. Hepatic triacylglycerol associations with production and health in dairy cows. J. Dairy Sci. 2022, 105, 5393–5409. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, G.; Trevisi, E. Use of the liver activity index and other metabolic variables in the assessment of metabolic health in dairy herds. Vet. Clin. Food Anim. Pract. 2013, 29, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, K. Transition cow index. In Proceedings of the 39th Proceedings American Association of Bovine Practitioners, St. Paul, MN, USA, 20–24 September 2006; pp. 139–143. [Google Scholar]
- Lukas, J.M.; Reneau, J.K.; Wallace, R.L.; De Vries, A. A study of methods for evaluating the success of the transition period in early-lactation dairy cows. J. Dairy Sci. 2015, 98, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Soonberg, M.; Kass, M.; Kaart, T.; Barraclough, R.; Haskell, M.J.; Arney, D.R. Effect of grouping on behaviour of dairy heifers and cows in the transition period. J. Dairy Res. 2021, 88, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Khan, M.Z.; Abbas, Z.; Hu, L.; Ullah, Q.; Wang, Y.; Wang, Y. Major nutritional metabolic alterations influencing the reproductive system of postpartum dairy cows. Metabolites 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Gábor, G.; Balogh, O.G.; Kern, L.; Gábor, P.R.; Fébel, H. Nutrition. Metabolic Status and Reproductive Efficiency in Dairy Herds. Open J. Anim. Sci. 2016, 6, 75–84. [Google Scholar] [CrossRef]
- Batista, C.P.; Castro Ruiz, S.M.; Correa Cardona, H.J.; Gonçalves, R.S.; Valle, S.D.F.; Diaz Gonzalez, F.H. Relation between liver lipid content and plasma biochemical indicators in dairy cows. Acta Sci. Vet. 2020, 48, 1–9. [Google Scholar] [CrossRef]
- Paiano, R.B.; Birgel, D.B.; Bonilla, J.; Birgel Junior, E.H. Evaluation of biochemical profile of dairy cows with metabolic diseases in tropical conditions. Reprod. Dom. Anim. 2020, 55, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Holdridge, L.R. Ecología Basada em Zonas de Vida; Instituto Interamericano de Cooperación para la Agricultura: San José de Costa Rica, Costa Rica, 2000; p. 216. [Google Scholar]
- Payne, J.M. The Compton metabolic profile test. Proc. R. Soc. Med. 1972, 64, 181–183. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.A.; Tablada, E.M. InfoStat, Versión 2020. Universidad Nacional de Córdoba, Argentina. 2024. Available online: http://www.infostat.com.ar/index.php?mod=page&id=34 (accessed on 3 June 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 3 June 2024).
- Štolcová, D.; Řehák, D.; Bartoň, L.; Rajmon, R. Štolcová. Blood biochemical parameters measured during the periparturient period in cows of Holstein and fleckvieh breeds differing in production purpose. Czech J. Anim. Sci. 2020, 65, 172–181. [Google Scholar] [CrossRef]
- Djokovic, R.; Cincovic, M.; Ilic, Z.; Kurcubic, V.; Andjelic, B.; Petrovic, M.; Jasovic, B. Estimation metabolic status in high yielding dairy cows during transition period and full lactation. Acta Sci. Vet. 2019, 47, 1–6. [Google Scholar] [CrossRef]
- Puppel, K.; Kuczyńska, B. Metabolic profiles of cow’s blood; a review. J. Sci. Food Agric. 2016, 96, 4321–4328. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.F.; Cao, Y.C.; Cai, C.J.; Chao, Y.U.; Li, S.X.; Yao, J.H. Temporal dynamics of nutrient balance, plasma biochemical and immune traits, and liver function in transition dairy cows. J. Integr. Agric. 2020, 19, 820–837. [Google Scholar] [CrossRef]
- García-Casillas, A.C.G.; Montiel-Ramos, L.A.M. Integración bioquímica para modelar las respuestas metabólicas en la producción láctea de bovinos lecheros. Soc. Rural. Prod. Medio Ambiente 2014, 12, 71–194. [Google Scholar]
- Gerspach, C.; Imhasly, S.; Gubler, M.; Naegeli, H.; Ruetten, M.; Laczkó, E. Altered plasma lipidome profile of dairy cows with fatty liver disease. Res. Vet. Sci. 2017, 110, 47–59. [Google Scholar] [CrossRef]
- Batista, C.P.; Gonçalves, R.S.; Contreras, L.V.Q.; de Faria Valle, S.; González, F. Correlation between liver lipidosis, body condition score variation. and hepatic analytes in dairy cows. Braz. J. Vet. Med. 2022, 44, e005121. [Google Scholar] [CrossRef]
- Mezzetti, M.; Cattaneo, L.; Passamonti, M.M.; Lopreiato, V.; Minuti, A.; Trevisi, E. The transition period updated: A review of the new insights into the adaptation of dairy cows to the new lactation. Dairy 2021, 2, 617–636. [Google Scholar] [CrossRef]
- Caixeta, L.S.; Omontese, B.O. Monitoring and improving the metabolic health of dairy cows during the transition period. Animals 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]

| Cluster | Days after Calving | n | Mean Index | TP g/dL | Urea mmol/L | COL mmol/L | NEFA mmol/L | ALT U/I |
| 1 | −30, −15 and 20 | 47 | −0.56 | 74.78 ± 6.67 | 5.57 ± 1.55 | 3.3 ± 0.88 | 0.66 ± 0.26 | 23.15 ± 4.37 |
| 2 | 0 and 7 | 31 | −2.38 | 69.3± 4.52 | 5.8± 1.38 | 2.45± 0.56 | 0.92± 0.29 | 19.7± 3.19 |
| 3 | 35 to 105 | 80 | 1.30 | 77.2± 6.67 | 6.47± 2.15 | 5.17± 1.23 | 0.56± 0.18 | 27.64± 8.04 |
| Cluster | BCS | n | Mean index | TP g/dL | Urea mmol/L | COL mmol/L | NEFA mmol/L | ALT U/I |
| 1 | 2.25; 2.50 and 4.25 | 14 | 1.09 | 72.52 ± 4.26 | 6.53 ± 1.82 | 4.47 ± 0.86 | 0.48 ± 0.07 | 25.12 ± 4.87 |
| 2 | 2.75 and 3.0 | 89 | 0.40 | 76.19 ± 7.22 | 6.31 ± 2.16 | 4.45 ± 1.63 | 0.64 ± 0.25 | 25.23 ± 8.3 |
| 3 | 3.25; 3.50; 3.75 and 4.0 | 50 | −1.04 | 73.06 ± 6.51 | 5.54 ± 1.04 | 3.17 ± 1.06 | 0.74 ± 0.3 | 23.71 ± 5.31 |
| P-PC1 | HEpINdex | ||||||
|---|---|---|---|---|---|---|---|
| Cluster | Days after Calving | Mean | Min | Max | Mean | Min | Max |
| 1 | −30, −15 and 20 | −0.56 | −2.37 | 1.62 | −1.03 | −4.43 | 4.8 |
| 2 | 0 and 7 | −2.38 | −4.29 | −0.74 | −1.76 | −5.62 | 2.3 |
| 3 | 35 to 105 | 1.3 | −0.42 | 2.45 | 1.3 | −3.42 | 7.06 |
| Cluster | BCS | Mean | Min | Max | Mean | Min | Max |
| 1 | 2.25; 2.50 y 4.25 | 1.09 | 0.16 | 2.17 | −0.47 | −2.62 | 1.29 |
| 2 | 2.75 and 3.0 | 0.4 | −4.29 | 2.45 | 0.53 | −5.62 | 7.06 |
| 3 | 3.25; 3.50; 3.75 and 4.0 | −1.04 | −4.09 | 1.47 | −0.98 | −4.07 | 2.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Gaona, R.; Correa-Orozco, A.; Salamanca-Carreño, A.; Vélez-Terranova, M. Index Development to Comprehensive Assess Liver Function during the Dairy Cows’ Transition Period in Low-Tropic Conditions. Animals 2024, 14, 2056. https://doi.org/10.3390/ani14142056
Campos-Gaona R, Correa-Orozco A, Salamanca-Carreño A, Vélez-Terranova M. Index Development to Comprehensive Assess Liver Function during the Dairy Cows’ Transition Period in Low-Tropic Conditions. Animals. 2024; 14(14):2056. https://doi.org/10.3390/ani14142056
Chicago/Turabian StyleCampos-Gaona, Rómulo, Adriana Correa-Orozco, Arcesio Salamanca-Carreño, and Mauricio Vélez-Terranova. 2024. "Index Development to Comprehensive Assess Liver Function during the Dairy Cows’ Transition Period in Low-Tropic Conditions" Animals 14, no. 14: 2056. https://doi.org/10.3390/ani14142056
APA StyleCampos-Gaona, R., Correa-Orozco, A., Salamanca-Carreño, A., & Vélez-Terranova, M. (2024). Index Development to Comprehensive Assess Liver Function during the Dairy Cows’ Transition Period in Low-Tropic Conditions. Animals, 14(14), 2056. https://doi.org/10.3390/ani14142056






