Dietary Crude Protein and Lysine Levels Affect Meat Quality and Myofiber Characteristic of Slow-Growing Chicken
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Birds
2.2. Feeding and Management
2.3. Measurement
2.4. Statistical Analyses
3. Results
3.1. Growth Performance
3.2. Slaughter Performance
3.3. Meat Quality
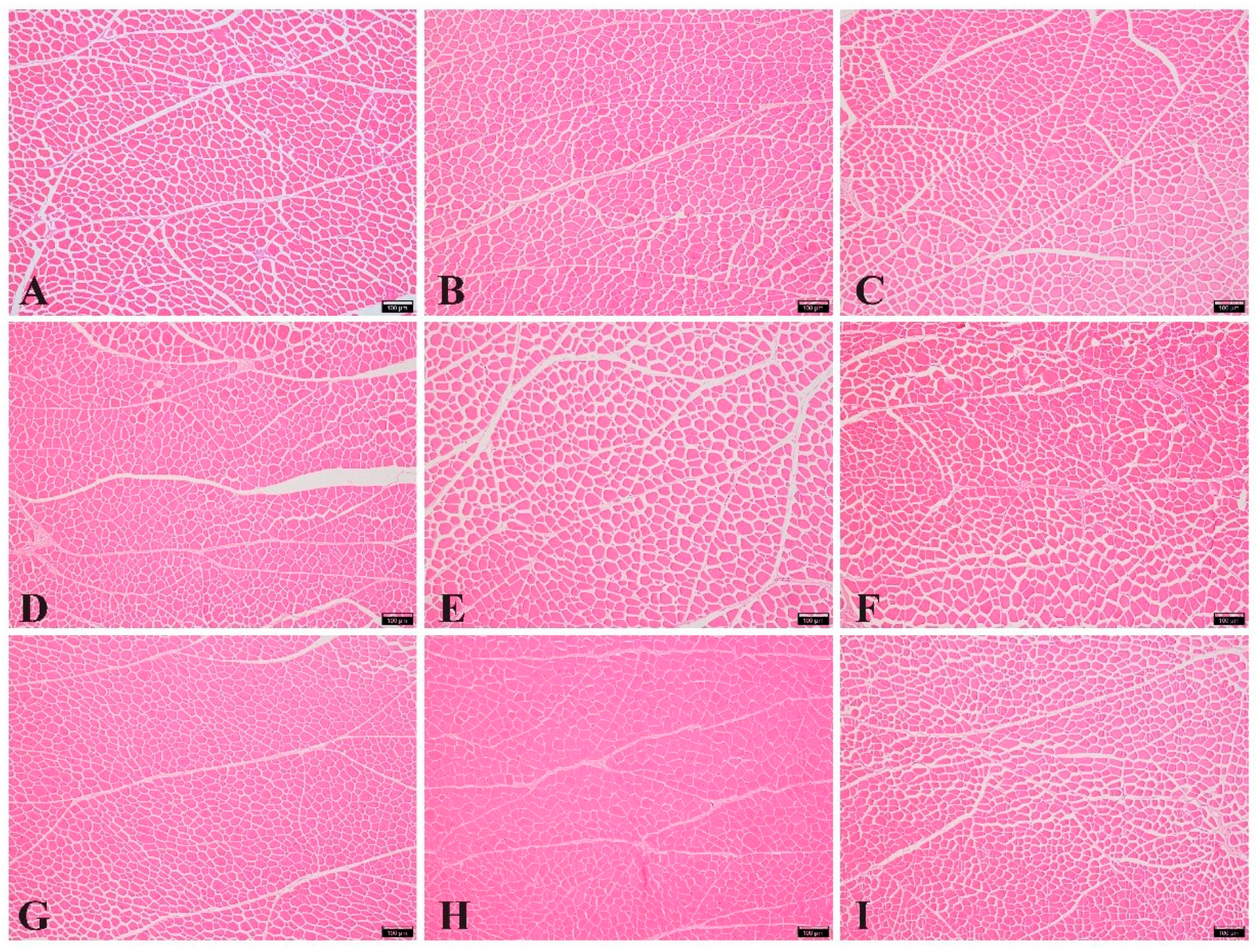
3.4. Myofiber Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chodova, D.; Tumova, E.; Volek, Z.; Skrivanova, V.; Vlckova, J. The effect of one-week intensive feed restriction and age on the carcass composition and meat quality of growing rabbits. Czech J. Anim. Sci. 2016, 61, 151–158. [Google Scholar] [CrossRef]
- Powell, D.J.; McFarland, D.C.; Cowieson, A.J.; Muir, W.I.; Velleman, S.G. The effect of nutritional status on myogenic gene expression of satellite cells derived from different muscle types. Poult. Sci. 2014, 93, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Dairo, F.A.S.; Adesehinwa, A.O.K.; Oluwasola, T.A.; Oluyemi, J.A. High and low dietary energy and protein levels for broiler chickens. Afr. J. Agric. Res. 2010, 5, 2030–2038. [Google Scholar]
- Gheisari, H.R.; Asasi, K.; Mostafa, I.; Mohsenifard, E. Effect of different levels of dietary crude protein on growth performance, body composition of broiler chicken and low protein diet in broiler chicken. Int. J. Poult. Sci. 2015, 14, 285–292. [Google Scholar] [CrossRef]
- Ndazigaruye, G.; Kim, D.H.; Kang, C.W.; Kang, K.R.; Joo, Y.J.; Lee, S.R.; Lee, K.W. Effects of low-protein diets and exogenous protease on growth performance, carcass traits, intestinal morphology, cecal volatile fatty acids and serum parameters in broilers. Animals 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.Y.; Choi, J.; Yadav, S.; Tompkins, Y.H.; Kim, W.K. Effects of low-crude protein diets supplemented with arginine, glutamine, threonine, and methionine on regulating nutrient absorption, intestinal health, and growth performance of Eimeria-infected chickens. Poult. Sci. 2021, 100, 101427. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.; Janssens, G.P.; Demeyer, P.; Bağci, Ö.; Delezie, E. Reduction of dietary crude protein and feed form: Impact on broiler litter quality, ammonia concentrations, excreta composition, performance, welfare, and meat quality. Anim. Nutr. 2022, 9, 291–303. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.M.; Callan, J.J.; O’Doherty, J.V. The effect of dietary crude protein level, cereal type and exogenous enzyme supplementation on nutrient digestibility, nitrogen excretion, faecal volatile fatty acid concentration and ammonia emissions from pigs. Anim. Feed Sci. Technol. 2006, 127, 73–88. [Google Scholar] [CrossRef]
- Barekatain, R.; Romero, L.F.; Sorbara, J.O.B.; Cowieson, A.J. Balanced nutrient density for broiler chickens using a range of digestible lysine-to-metabolizable energy ratios and nutrient density: Growth performance, nutrient utilization and apparent metabolizable energy. Anim. Nutr. 2021, 7, 430–439. [Google Scholar] [CrossRef]
- Sharma, N.K.; Choct, M.; Toghyani, M.; Laurenson, Y.C.; Girish, C.K.; Swick, R.A. Dietary energy, digestible lysine, and available phosphorus levels affect growth performance, carcass traits, and amino acid digestibility of broilers. Poult. Sci. 2018, 97, 1189–1198. [Google Scholar] [CrossRef]
- Wen, Z.G.; Rasolofomanana, T.J.; Tang, J.; Jiang, Y.; Xie, M.; Yang, P.L.; Hou, S.S. Effects of dietary energy and lysine levels on growth performance and carcass yields of Pekin ducks from hatch to 21 days of age. Poult. Sci. 2017, 96, 3361–3366. [Google Scholar] [CrossRef]
- Gaylord, T.G.; Barrows, F.T. Multiple amino acid supplementations to reduce dietary protein in plant-based rainbow trout, Oncorhynchus mykiss, feeds. Aquaculture 2009, 287, 180–184. [Google Scholar] [CrossRef]
- Liao, S.F.; Wang, T.; Regmi, N. Lysine nutrition in swine and the related monogastric animals: Muscle protein biosynthesis and beyond. SpringerPlus 2015, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- Palma-Granados, P.; Seiquer, I.; Benítez, R.; Ovilo, C.; Nieto, R. Effects of lysine deficiency on carcass composition and activity and gene expression of lipogenic enzymes in muscles and backfat adipose tissue of fatty and lean piglets. Animal 2019, 13, 2406–2418. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, S.; Özkul, H.; Özkan, S.; Gous, R.; Yaşa, İ.; Babacanoğlu, E. Effect of dietary protein regime on meat quality traits and carcase nutrient content of broilers from two commercial genotypes. Br. Poult. Sci. 2010, 51, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Pesti, G.M. Impact of dietary amino acid and crude protein levels in broiler feeds on biological performance. J. Appl. Poult. Res. 2009, 18, 477–486. [Google Scholar] [CrossRef]
- Urdaneta-Rincon, M.; Leeson, S. Muscle (pectoralis major) protein turnover in young broiler chickens fed graded levels of lysine and crude protein. Poult. Sci. 2004, 83, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.A.; Khan, E.U.; Qaisrani, S.N.; Rashid, M.A.; Shaheen, M.S.; Nazir, A.; Talib, H.; Ahmad, S. Interactive effect of amino acids balanced at ideal lysine ratio and exogenous protease supplemented to low CP diet on growth performance, carcass traits, gut morphology, and serum metabolites in broiler chicken. Trop. Anim. Health Prod. 2022, 54, 186. [Google Scholar] [CrossRef] [PubMed]
- Ng’ambi, J.W.; Maoba, S.M.; Norris, D.; Malatje, M.S.; Mbajiorgu, C.A. Effect of dietary lysine to crude protein ratio on performance of male Ross 308 broiler chickens. Trop. Anim. Health Prod. 2009, 41, 11–16. [Google Scholar] [CrossRef]
- Rezaei, M.; Moghaddam, H.N.; Reza, J.P.; Kermanshahi, H. The effects of dietary protein and lysine levels on broiler performance, carcass characteristics and N excretion. Int. J. Poult. Sci. 2004, 3, 148–152. [Google Scholar]
- Faridi, A.; Gitoee, A.; France, J. Evaluation of the effects of crude protein and lysine on the growth performance of two commercial strains of broilers using meta-analysis. Livest. Sci. 2015, 181, 77–84. [Google Scholar] [CrossRef]
- Sterling, K.G.; Vedenov, D.V.; Pesti, G.M.; Bakalli, R.I. Economically optimal dietary crude protein and lysine levels for starting broiler chicks. Poult. Sci. 2005, 84, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sterling, K.G.; Pesti, G.M.; Bakalli, R.I. Performance of broiler chicks fed various levels of dietary lysine and crude protein. Poult. Sci. 2003, 82, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Chrystal, P.V.; Moss, A.F.; Liu, S.Y.; Yuan, J.; Selle, P.H. Effects of reducing dietary crude protein and whole grain feeding on performance and amino acid metabolism in broiler chickens offered wheat-based diets. Anim. Feed Sci. Technol. 2020, 260, 114386. [Google Scholar] [CrossRef]
- Geng, A.L.; Zhang, Y.; Zhang, J.; Wang, H.H.; Chu, Q.; Liu, H.G. Effects of lighting pattern and photoperiod on egg production and egg quality of a native chicken under free-range condition. Poult. Sci. 2018, 97, 2378–2384. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, Q.Q.; Wang, H.H.; Chu, Q.; Zhang, J.; Yan, Z.X.; Liu, H.G.; Geng, A.L. Dietary metabolizable energy and crude protein levels affect pectoral muscle composition and gut microbiota in native growing chickens. Poult. Sci. 2023, 102, 102353. [Google Scholar] [CrossRef] [PubMed]
- DB11/T 1378-2023; Technical Code of Practice of Feeding and Management of Beijing-You Chicken. Beijing Bureau of Market Supervision: Beijing, China, 2023.
- GB/T 18246-2000; Determination of Amino Acids in Feeds. National Municipal Bureau of Quality and Technical Supervision: Beijing, China, 2000.
- GB/T 6432-1994; Method for the Determination of Crude Protein in Feedstuffs. National Municipal Bureau of Quality and Technical Supervision: Beijing, China, 1994.
- NY/T 823-2020; Performance Terminology and Measurements for Poultry. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2020.
- Chen, X.; Cao, J.; Chang, C.; Geng, A.; Wang, H.; Chu, Q.; Yan, Z.; Zhang, X.; Zhang, Y.; Liu, H. Effects of Age on Compounds, Metabolites and Meat Quality in Beijing-You Chicken Breast Meat. Animals 2023, 13, 3419. [Google Scholar] [CrossRef]
- Abdel-Maksoud, A.; Yan, F.; Cerrate, S.; Coto, C.; Wang, Z.; Waldroup, P.W. Effect of dietary crude protein, lysine level and amino acid balance on performance of broilers 0 to 18 days of age. Int. J. Poult. Sci. 2010, 9, 21–27. [Google Scholar] [CrossRef]
- Dozier, W.A., III; Corzo, A.; Kidd, M.T.; Schilling, M.W. Dietary digestible lysine requirements of male and female broilers from forty-nine to sixty-three days of age. Poult. Sci. 2008, 87, 1385–1391. [Google Scholar] [CrossRef]
- Cemin, H.S.; Vieira, S.L.; Stefanello, C.; Kipper, M.; Kindlein, L.; Helmbrecht, A. Digestible lysine requirements of male broilers from 1 to 42 days of age reassessed. PLoS ONE 2017, 12, e0179665. [Google Scholar] [CrossRef]
- Ciftci, I.; Ceylan, N. Effects of dietary threonine and crude protein on growth performance, carcase and meat composition of broiler chickens. Br. Poult. Sci. 2004, 45, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.H.; Pesti, G.M.; Bakalli, R.; Lee, J.; Toledo, R.T.; Eitenmiller, R.R.; Phillips, R.D. The performance of broiler chicks fed diets containing extruded cottonseed meal supplemented with lysine. Poult. Sci. 2001, 80, 762–768. [Google Scholar] [CrossRef]
- Kendall, D.C.; Gaines, A.M.; Allee, G.L.; Usry, J.L. Commercial validation of the true ileal digestible lysine requirement for eleven-to twenty-seven-kilogram pigs. J. Anim. Sci. 2008, 86, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Kuźniacka, J.; Biesek, J.; Maiorano, G.; Adamski, M. Meat quality traits and fatty acid composition of breast muscles from ducks fed with yellow lupin. Animal 2020, 14, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.W.; Moss, A.F.; Groves, P.J.; Wilkinson, S.J.; Stuetz, R.M.; Selle, P.H. The multidimensional causal factors of ‘wet litter’ in chicken-meat production. Sci. Total Environ. 2016, 562, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Van Harn, J.; Dijkslag, M.A.; Van-Krimpen, M.M. Effect of low protein diets supplemented with free amino acids on growth performance, slaughter yield, litter quality, and footpad lesions of male broilers. Poult. Sci. 2019, 98, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Berri, C.; Besnard, J.; Relandeau, C. Increasing dietary lysine increases final pH and decreases drip loss of broiler breast meat. Poult. Sci. 2008, 87, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Peebles, E.D.; Schilling, M.W.; Mercier, Y. Effects of dietary lysine and methionine supplementation on Ross 708 male broilers from 21 to 42 d of age (I): Growth performance, meat yield, and cost effectiveness. J. Appl. Poult. Res. 2016, 25, 197–211. [Google Scholar] [CrossRef]
- Chodová, D.; Tůmová, E.; Ketta, M.; Skřivanová, V. Breast meat quality in males and females of fast-, medium- and slow-growing chickens fed diets of 2 protein levels. Poult. Sci. 2021, 100, 100997. [Google Scholar] [CrossRef]
- Bregendahl, K.; Sell, J.L.; Zimmerman, D.R. Effect of low-protein diets on growth performance and body composition of broiler chicks. Poult. Sci. 2002, 81, 1156–1167. [Google Scholar] [CrossRef]
- Law, F.L.; Zulkifli, I.; Soleimani, A.F.; Liang, J.B.; Awad, E.A. The effects of low-protein diets and protease supplementation on broiler chickens in a hot and humid tropical environment. Asian-Australas. J. Anim. Sci. 2018, 31, 1291. [Google Scholar] [CrossRef] [PubMed]
- Aletor, V.A.; Hamid, I.I.; Niess, E.; Pfeffer, E. Low-protein amino acid-supplemented diets in broiler chickens: Effects on performance, carcass characteristics, whole-body composition and efficiencies of nutrient utilization. J. Sci. Food Agric. 2000, 80, 547–554. [Google Scholar] [CrossRef]
- Awad, E.A.; Zulkifli, I.; Soleimani, A.F.; Aljuobori, A. Effects of feeding male and female broiler chickens on low-protein diets fortified with different dietary glycine levels under the hot and humid tropical climate. Ital. J. Anim. Sci. 2017, 16, 453–461. [Google Scholar] [CrossRef]
- Ray, E.C.; Avissar, N.E.; Sax, H.C. Growth factor regulation of enterocyte nutrient transport during intestinal adaptation. Am. J. Surg. 2002, 183, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Chrystal, P.V.; Moss, A.F.; Khoddami, A.; Naranjo, V.D.; Selle, P.H.; Liu, S.Y. Impacts of reduced-crude protein diets on key parameters in male broiler chickens offered maize-based diets. Poult. Sci. 2020, 99, 505–516. [Google Scholar] [CrossRef] [PubMed]
- De-Cesare, A.; do-Valle, I.F.; Sala, C.; Sirri, F.; Astolfi, A.; Castellani, G.; Manfreda, G. Effect of a low protein diet on chicken ceca microbiome and productive performances. Poult. Sci. 2019, 98, 3963–3976. [Google Scholar] [CrossRef]
- Swennen, Q.; Janssens, G.P.J.; Millet, S.; Vansant, G.; Decuypere, E.; Buyse, J. Effects of substitution between fat and protein on feed intake and its regulatory mechanisms in broiler chickens: Endocrine functioning and intermediary metabolism. Poult. Sci. 2005, 84, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, O.; Darcel, N.; Fromentin, G.; Tome, D. Control of protein and energy intake-brain mechanisms. Eur. J. Clin. Nutr. 2013, 67, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Rojas, I.C.; Murakami, A.E.; Duarte, C.R.A.; Eyng, C.; Oliveira, C.A.L.; Janeiro, V. Valine, isoleucine, arginine and glycine supplementation of low-protein diets for broiler chickens during the starter and grower phases. Br. Poult. Sci. 2014, 55, 766–773. [Google Scholar] [CrossRef]
- Darsi, E.; Shivazad, M.; Zaghari, M.; Namroud, N.F.; Mohammadi, R. Effect of reduced dietary crude protein levels on growth performance, plasma uric acid and electrolyte concentration of male broiler chicks. J. Agr. Sci. Tech. 2012, 14, 789–797. [Google Scholar]
- Van-Emous, R.A.; Kwakkel, R.P.; Van-Krimpen, M.M.; Hendriks, W.H. Effects of growth patterns and dietary crude protein levels during rearing on body composition and performance in broiler breeder females during the rearing and laying period. Poult. Sci. 2013, 92, 2091–2100. [Google Scholar] [CrossRef]
- Mousa, M.A.; Asman, A.S.; Ali, R.M.; Sayed, R.K.; Majrashi, K.A.; Fakiha, K.G.; Alhotan, R.A.; Selim, S. Impacts of Dietary Lysine and Crude Protein on Performance, Hepatic and Renal Functions, Biochemical Parameters, and Histomorphology of Small Intestine, Liver, and Kidney in Broiler Chickens. Vet. Sci. 2023, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Song, A.A.L.; Loh, T.C.; Rahim, R.A. Effects of lysine and methionine in a low crude protein diet on the growth performance and gene expression of immunity genes in broilers. Poult. Sci. 2020, 99, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, V.; Passantino, L.; Perillo, A.; Lopresti, G.; Passantino, A.; Khan, R.U.; Tufarelli, V. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult. Sci. 2012, 91, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, C.; Vallée, C.; Constantin, P.; Chagneau, A.M.; Lessire, M.; Lescoat, P.; Berri, C.; Baéza, E.; Bizeray, D.; Bouvarel, I. Sequential feeding with variations in energy and protein levels improves gait score in meat-type chickens. Animal 2008, 2, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.P.; Zhao, G.P.; Jiang, R.R.; Zheng, M.Q.; Chen, J.L.; Liu, R.R.; Wen, J. Effects of diet-induced differences in growth rate on metabolic, histological, and meat-quality properties of 2 muscles in male chickens of 2 distinct broiler breeds. Poult. Sci. 2012, 91, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Nakashima, K.; Ishida, A.; Ashihara, A.; Katsumata, M. Effects of low protein diet and low protein diet supplemented with synthetic essential amino acids on meat quality of broiler chickens. Anim. Sci. J. 2013, 84, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, L.; Wu, P.; Feng, L.; Jiang, W.; Liu, Y.; Kuang, S.Y.; Li, S.W.; Mi, H.F.; Tang, L.; et al. Dietary protein levels changed the hardness of muscle by acting on muscle fiber growth and the metabolism of collagen in sub-adult grass carp (Ctenopharyngodon idella). J. Anim. Sci. Biotechnol. 2022, 13, 109. [Google Scholar] [CrossRef]
- Yousefi, N.; Abbasi, S. Food Proteins: Solubility & Thermal Stability Improvement Techniques. Food Chem. Adv. 2022, 1, 100090. [Google Scholar]
- Gan, Z.; Fu, T.; Kelly, D.P.; Vega, R.B. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res. 2018, 28, 969–980. [Google Scholar] [CrossRef]
- Murgia, M.; Tan, J.; Geyer, P.E.; Doll, S.; Mann, M.; Klopstock, T. Proteomics of cytochrome oxidase-negative versus-positive muscle fiber sections in mitochondrial myopathy. Cell Rep. 2019, 29, 3825–3834. [Google Scholar] [CrossRef] [PubMed]
- Bastianelli, D.; Quentin, M.; Bouvarel, I.; Relandeau, C.; Lescoat, P.; Picard, M.; Tesseraud, S. Early lysine deficiency in young broiler chicks. Animal 2007, 1, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Schilling, M.W.; Jackson, V.; Peebles, E.D.; Mercier, Y. Effects of dietary lysine and methionine supplementation on Ross 708 male broilers from 21 to 42 days of age (II): Breast meat quality. J. Appl. Poult. Res. 2016, 25, 212–222. [Google Scholar] [CrossRef]
- Liu, N.; Deng, X.J.; Wang, J.P.; Deng, Q.Q.; Ting-Sheng, X.U. Research progress on regulation factors of myogenic regulatory factors and myogenesis. China Anim. Husb. Vet. Med. 2015, 42, 2644–2649. [Google Scholar]
- Jin, C.L.; Ye, J.L.; Yang, J.; Gao, C.Q.; Yan, H.; Li, H.C.; Wang, X.Q. mTORC1 mediates lysine-induced satellite cell activation to promote skeletal muscle growth. Cells 2019, 8, 1549. [Google Scholar] [CrossRef] [PubMed]
- Srikanchai, T.; Murani, E.; Wimmers, K.; Ponsuksili, S. Four loci differentially expressed in muscle tissue depending on water-holding capacity are associated with meat quality in commercial pig herds. Mol. Biol. Rep. 2010, 37, 595–601. [Google Scholar] [CrossRef]
- Langley, B.; Thomas, M.; Bishop, A.; Sharma, M.; Gilmour, S.; Kambadur, R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 2002, 277, 49831–49840. [Google Scholar] [CrossRef] [PubMed]
- Blackstock, C.D.; Higashi, Y.; Sukhanov, S.; Shai, S.Y.; Stefanovic, B.; Tabony, A.M.; Yoshida, T.; Delafontaine, P. Insulin-like growth factor-1 increases synthesis of collagen type I via induction of the mRNA-binding protein LARP6 expression and binding to the 5′ stem-loop of COL1a1 and COL1a2 mRNA. J. Biol. Chem. 2014, 289, 7264–7274. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.C.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Tang, L.; Liu, X.A.; Zhou, X.Q. Effects of dietary methionine on growth performance, muscle nutritive deposition, muscle fibre growth and type I collagen synthesis of on-growing grass carp (Ctenopharyngodon idella). Br. J. Nutr. 2021, 126, 321–336. [Google Scholar] [CrossRef]
- Stefanovic, L.; Longo, L.; Zhang, Y.; Stefanovic, B. Characterization of binding of LARP6 to the 5′ stem-loop of collagen mRNAs: Implications for synthesis of type I collagen. RNA Biol. 2004, 11, 1386–1401. [Google Scholar] [CrossRef]
- Sato, T.; Ito, Y.; Nedachi, T.; Nagasawa, T. Lysine suppresses protein degradation through autophagic–lysosomal system in C2C12 myotubes. Mol. Cell. Biochem. 2014, 391, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.D.; Jung, E.Y.; Lim, H.J.; Yang, H.S.; Joo, S.T.; Jeong, J.Y. Influence of meat exudates on the quality characteristics of fresh and freeze-thawed pork. Meat Sci. 2013, 95, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Traore, S.; Aubry, L.; Gatellier, P.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Sante-Lhoutellier, V. Higher drip loss is associated with protein oxidation. Meat Sci. 2012, 90, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Kim, G.D.; Yang, H.S.; Joo, S.T. Effect of freeze–thaw cycles on physicochemical properties and color stability of beef semimembranosus muscle. Food Res. Int. 2011, 44, 3222–3228. [Google Scholar] [CrossRef]
- Olsson, G.B.; Olsen, R.L.; Ofstad, R. Post-mortem structural characteristics and water-holding capacity in Atlantic halibut muscle. LWT-Food Sci. Technol. 2003, 36, 125–133. [Google Scholar] [CrossRef]
- Lepetit, J. Collagen contribution to meat toughness: Theoretical aspects. Meat Sci. 2008, 80, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ha, M.; Warner, R.D.; Dunshea, F.R. Meta-analysis of the relationship between collagen characteristics and meat tenderness. Meat Sci. 2022, 185, 108717. [Google Scholar] [CrossRef]
| CP, % | 16.0 | 16.0 | 16.0 | 17.0 | 17.0 | 17.0 | 18.0 | 18.0 | 18.0 |
|---|---|---|---|---|---|---|---|---|---|
| Lysine, % | 0.69 | 0.84 | 0.99 | 0.69 | 0.84 | 0.99 | 0.69 | 0.84 | 0.99 |
| Ingredients, % | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Corn | 64.05 | 65.30 | 65.80 | 58.20 | 62.60 | 63.10 | 56.37 | 59.90 | 60.50 |
| Wheat bran | 12.80 | 9.80 | 9.60 | 16.50 | 11.00 | 10.90 | 17.00 | 12.20 | 12.00 |
| Soybean meal | 14.00 | 19.00 | 19.00 | 13.00 | 18.10 | 18.00 | 12.00 | 17.10 | 17.10 |
| Corn gluten meal | 4.15 | 0.90 | 0.60 | 6.40 | 3.30 | 3.00 | 9.00 | 5.80 | 5.40 |
| Soybean oil | 0.00 | 0.00 | 0.00 | 0.90 | 0.00 | 0.00 | 0.63 | 0.00 | 0.00 |
| Vitamin premix 1 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| 50% Choline chloride | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| DL-Methionine | 0.12 | 0.14 | 0.15 | 0.07 | 0.09 | 0.10 | 0.02 | 0.04 | 0.05 |
| 70% Lysine | 0.00 | 0.09 | 0.37 | 0.00 | 0.09 | 0.37 | 0.00 | 0.09 | 0.37 |
| 98% Threonine | 0.08 | 0.06 | 0.07 | 0.04 | 0.03 | 0.04 | 0.01 | 0.00 | 0.00 |
| Mineral premix 2 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Zeolite powder | 0.94 | 0.88 | 0.58 | 1.03 | 0.96 | 0.66 | 1.11 | 1.04 | 0.75 |
| Limestone | 1.67 | 1.64 | 1.64 | 1.67 | 1.64 | 1.64 | 1.67 | 1.64 | 1.64 |
| Calcium bisphosphate | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 |
| NaCl | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Rice hull powder | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutritional levels 3 | |||||||||
| Metabolizable energy MJ/kg | 11.51 | 11.51 | 11.52 | 11.51 | 11.51 | 11.51 | 11.51 | 11.51 | 11.52 |
| Crude protein, % | 16.01 | 16.05 | 16.07 | 16.99 | 17.03 | 17.01 | 18.00 | 18.02 | 17.98 |
| Lysine, % | 0.68 | 0.85 | 0.98 | 0.70 | 0.83 | 0.97 | 0.67 | 0.85 | 0.98 |
| Digestible lysine | 0.61 | 0.76 | 0.91 | 0.62 | 0.74 | 0.90 | 0.61 | 0.75 | 0.90 |
| Methionine, % | 0.41 | 0.40 | 0.41 | 0.39 | 0.38 | 0.39 | 0.37 | 0.37 | 0.37 |
| Digestible methionine | 0.38 | 0.37 | 0.38 | 0.36 | 0.36 | 0.36 | 0.34 | 0.34 | 0.34 |
| Methionine + Cystine, % | 0.68 | 0.67 | 0.68 | 0.68 | 0.69 | 0.68 | 0.68 | 0.68 | 0.68 |
| Digestible Methionine + Cystine | 0.63 | 0.62 | 0.62 | 0.61 | 0.64 | 0.62 | 0.62 | 0.61 | 0.62 |
| Calcium, % | 0.85 | 0.86 | 0.85 | 0.85 | 0.85 | 0.86 | 0.84 | 0.85 | 0.85 |
| Total phosphorus, % | 0.66 | 0.65 | 0.65 | 0.68 | 0.66 | 0.65 | 0.68 | 0.67 | 0.65 |
| Non-phytate phosphorus, % | 0.40 | 0.40 | 0.40 | 0.41 | 0.40 | 0.40 | 0.41 | 0.41 | 0.40 |
| Threonine, % | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 |
| Digestible Threonine | 0.59 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 |
| CP, % | Lysine, % | AFI, g | BWG, g | F/G (g:g) | Mortality Rate, % |
|---|---|---|---|---|---|
| 16.0 | 0.69 | 480.80 ± 6.55 a | 87.28 ± 3.47 | 5.50 | 1.67 ± 3.73 |
| 16.0 | 0.84 | 470.34 ± 7.47 ab | 89.3 ± 7.65 | 5.27 | 3.33 ± 4.56 |
| 16.0 | 0.99 | 468.57 ± 9.22 abc | 88.92 ± 4.85 | 5.27 | 0 |
| 17.0 | 0.69 | 463.67 ± 9.63 bcd | 90.15 ± 4.46 | 5.14 | 6.67 ± 6.97 |
| 17.0 | 0.84 | 465.92 ± 5.24 bcd | 90.31 ± 8.42 | 5.16 | 0 |
| 17.0 | 0.99 | 480.25 ± 15.34 a | 89.87 ± 3.89 | 5.34 | 6.67 ± 3.73 |
| 18.0 | 0.69 | 453.08 ± 14.25 d | 90.85 ± 3.97 | 4.98 | 3.33 ± 4.56 |
| 18.0 | 0.84 | 462.81 ± 11.60 bcd | 94.85 ± 4.57 | 4.88 | 0 |
| 18.0 | 0.99 | 454.72 ± 3.52 cd | 96.27 ± 7.31 | 4.72 | 1.67 ± 3.73 |
| Main effects | |||||
| CP, % | 16.0 | 473.24 ± 9.15 a | 88.50 ± 5.26 b | 5.34 | 1.67 ± 3.45 |
| 17.0 | 469.94 ± 12.63 a | 90.11 ± 5.50 ab | 5.22 | 4.44 ± 5.33 | |
| 18.0 | 456.87 ± 10.93 b | 93.99 ± 5.60 a | 4.86 | 1.67 ± 3.45 | |
| p-value | <0.010 | 0.034 | 0.325 | 0.086 | |
| Lysine, % | 0.69 | 465.85 ± 15.38 | 89.42 ± 4.02 | 5.21 | 3.89 ± 5.33 |
| 0.84 | 466.36 ± 8.51 | 91.49 ± 7.01 | 5.09 | 1.11 ± 2.93 | |
| 0.99 | 467.85 ± 14.55 | 91.69 ± 6.14 | 5.10 | 2.78 ± 4.07 | |
| p-value | 0.850 | 0.488 | 0.329 | 0.150 | |
| CP × lysine | p-value | 0.012 | 0.848 | 0.618 | 0.062 |
| CP, % | Lysine, % | Dressed Percentage, % | Percentage of Half-Eviscerated Weight with Giblet, % | Percentage of Eviscerated Weight, % | Percentage of Breast Muscle Yield, % | Percentage of Leg Muscle Yield, % |
|---|---|---|---|---|---|---|
| 16.0 | 0.69 | 90.51 ± 1.94 | 79.33 ± 1.64 | 68.88 ± 1.82 | 10.05 ± 0.68 | 10.89 ± 1.09 b |
| 16.0 | 0.84 | 89.63 ± 3.42 | 79.45 ± 1.90 | 70.38 ± 1.73 | 10.38 ± 1.53 | 12.66 ± 1.13 b |
| 16.0 | 0.99 | 90.51 ± 2.38 | 78.47 ± 2.06 | 68.73 ± 1.73 | 10.08 ± 0.50 | 11.77 ± 1.68 b |
| 17.0 | 0.69 | 90.05 ± 1.56 | 81.39 ± 2.30 | 72.81 ± 1.95 | 10.72 ± 0.39 | 13.46 ± 1.31 ab |
| 17.0 | 0.84 | 91.21 ± 3.19 | 81.63 ± 3.63 | 71.14 ± 3.79 | 10.41 ± 0.91 | 14.18 ± 1.30 a |
| 17.0 | 0.99 | 92.02 ± 1.10 | 80.59 ± 2.58 | 70.87 ± 3.22 | 10.85 ± 1.28 | 13.19 ± 1.53 ab |
| 18.0 | 0.69 | 92.19 ± 1.70 | 80.84 ± 1.92 | 72.27 ± 1.64 | 10.34 ± 0.62 | 13.99 ± 0.82 ab |
| 18.0 | 0.84 | 89.56 ± 2.97 | 78.15 ± 2.81 | 68.81 ± 2.85 | 10.36 ± 0.67 | 13.83 ± 1.02 ab |
| 18.0 | 0.99 | 91.88 ± 3.35 | 79.44 ± 2.93 | 70.58 ± 3.68 | 10.96 ± 0.56 | 13.74 ± 1.38 ab |
| Main effects | ||||||
| CP, % | 16.0 | 90.22 ± 2.44 | 79.08 ± 1.76 | 69.33 ± 1.77 | 10.17 ± 0.96 | 11.77 ± 1.45 b |
| 17.0 | 91.09 ± 2.11 | 81.2 ± 2.66 | 71.61 ± 2.93 | 10.66 ± 0.87 | 13.61 ± 1.33 a | |
| 18.0 | 91.21 ± 2.79 | 79.48 ± 2.61 | 70.55 ± 2.97 | 10.55 ± 0.81 | 13.86 ± 1.00 a | |
| p-value | 0.585 | 0.105 | 0.124 | 0.266 | <0.01 | |
| Lysine, % | 0.69 | 90.92 ± 1.84 | 80.52 ± 2.00 | 71.32 ± 2.44 | 10.37 ± 0.73 | 12.78 ± 1.91 |
| 0.84 | 90.13 ± 3.00 | 79.74 ± 3.00 | 70.11 ± 2.83 | 10.38 ± 1.01 | 13.56 ± 1.35 | |
| 0.99 | 91.47 ± 2.33 | 79.5 ± 2.48 | 70.06 ± 2.88 | 10.63 ± 0.87 | 12.90 ± 1.64 | |
| p-value | 0.441 | 0.584 | 0.424 | 0.641 | 0.266 | |
| CP × lysine | p-value | 0.663 | 0.739 | 0.433 | 0.733 | 0.595 |
| CP, % | Lysine, % | Drip Loss, % | Cooking Loss, % | pH24h | Shear Force, N |
|---|---|---|---|---|---|
| 16.0 | 0.69 | 5.73 ± 1.24 | 16.17 ± 5.57 | 6.28 ± 0.19 | 20.84 ± 5.77 |
| 16.0 | 0.84 | 3.47 ± 0.55 | 15.32 ± 4.44 | 6.31 ± 0.11 | 21.64 ± 3.44 |
| 16.0 | 0.99 | 6.71 ± 2.95 | 17.44 ± 4.39 | 6.27 ± 0.06 | 19.91 ± 3.26 |
| 17.0 | 0.69 | 6.04 ± 1.62 | 16.70 ± 2.44 | 6.20 ± 0.17 | 22.73 ± 4.45 |
| 17.0 | 0.84 | 6.66 ± 2.11 | 17.36 ± 1.27 | 6.32 ± 0.13 | 24.48 ± 5.38 |
| 17.0 | 0.99 | 7.22 ± 1.10 | 16.83 ± 3.13 | 6.14 ± 0.08 | 23.41 ± 5.13 |
| 18.0 | 0.69 | 5.28 ± 1.20 | 15.63 ± 4.02 | 6.30 ± 0.12 | 28.66 ± 5.37 |
| 18.0 | 0.84 | 5.88 ± 5.37 | 14.27 ± 2.64 | 6.27 ± 0.21 | 25.74 ± 4.76 |
| 18.0 | 0.99 | 5.69 ± 1.74 | 16.05 ± 3.31 | 6.24 ± 0.10 | 34.26 ± 11.06 |
| Main effects | |||||
| CP, % | 16.0 | 5.30 ± 2.21 | 16.31 ± 4.46 | 6.28 ± 0.12 | 20.80 ± 4.28 b |
| 17.0 | 6.64 ± 1.58 | 16.96 ± 2.20 | 6.22 ± 0.14 | 23.54 ± 4.95 b | |
| 18.0 | 5.62 ± 3.03 | 15.32 ± 3.15 | 6.27 ± 0.14 | 29.55 ± 8.26 a | |
| p-value | 0.378 | 0.550 | 0.502 | <0.01 | |
| Lysine, % | 0.69 | 5.68 ± 1.28 | 16.17 ± 3.84 | 6.26 ± 0.16 | 24.08 ± 6.12 |
| 0.84 | 5.34 ± 3.34 | 15.65 ± 3.08 | 6.3 ± 0.14 | 23.96 ± 4.82 | |
| 0.99 | 6.54 ± 1.99 | 16.77 ± 3.36 | 6.22 ± 0.09 | 25.86 ± 9.43 | |
| p-value | 0.464 | 0.758 | 0.350 | 0.617 | |
| CP × lysine | p-value | 0.592 | 0.949 | 0.757 | 0.329 |
| CP, % | Lysine, % | Myofiber Density, (P·mm−2) | Myofiber Diameter, μm | Number of Fibers in the Muscle Bundle | Endomysium Thickness, μm | Perimysium Thickness, μm |
|---|---|---|---|---|---|---|
| 16.0 | 0.69 | 862.13 ± 190.91 cd | 29.72 ± 7.02 d | 111.09 ± 49.58 ab | 5.03 ± 4.11 ab | 8.67 ± 5.48 bc |
| 16.0 | 0.84 | 1020.39 ± 103.47 ab | 27.28 ± 5.98 g | 109.88 ± 24.91 ab | 3.81 ± 1.46 d | 6.77 ± 3.12 de |
| 16.0 | 0.99 | 1092.6 ± 191.56 a | 24.29 ± 5.54 i | 100.64 ± 22.18 ab | 3.66 ± 1.41 d | 8.80 ± 3.41 bc |
| 17.0 | 0.69 | 1010.41 ± 116.69 ab | 25.99 ± 6.39 h | 91.52 ± 26.16 ab | 4.43 ± 1.54 c | 9.03 ± 3.40 b |
| 17.0 | 0.84 | 832.01 ± 193.59 cd | 27.96 ± 7.11 f | 105.64 ± 34.55 ab | 4.76 ± 1.71 b | 8.31 ± 3.34 c |
| 17.0 | 0.99 | 756.30 ± 111.71 d | 30.36 ± 6.90 c | 113.48 ± 25.11 a | 5.29 ± 1.71 a | 12.56 ± 3.96 a |
| 18.0 | 0.69 | 832.00 ± 187.93 cd | 30.75 ± 7.61 b | 109.45 ± 32.31 ab | 4.27 ± 1.35 c | 7.13 ± 2.41 d |
| 18.0 | 0.84 | 768.88 ± 159.58 d | 32.62 ± 8.15 a | 88.56 ± 14.59 b | 3.72 ± 0.96 d | 5.64 ± 2.39 f |
| 18.0 | 0.99 | 938.18 ± 120.55 bc | 29.40 ± 7.43 e | 107.92 ± 22.46 ab | 3.53 ± 1.21 d | 6.42 ± 3.86 e |
| Main effects | ||||||
| CP, % | 16.0 | 991.70 ± 193.66 a | 27.10 ± 6.51 c | 107.20 | 4.16 ± 2.5 b | 8.08 ± 3.98 b |
| 17.0 | 866.24 ± 176.76 b | 28.10 ± 7.04 b | 103.55 | 4.83 ± 1.7 a | 9.97 ± 4.05 a | |
| 18.0 | 846.35 ± 169.05 b | 30.92 ± 7.75 a | 101.98 | 3.84 ± 1.29 c | 6.40 ± 3.09 c | |
| p-value | <0.01 | <0.01 | 0.609 | <0.01 | <0.01 | |
| Lysine, % | 0.69 | 901.51 | 28.82 ± 7.33 b | 104.02 | 4.58 ± 2.81 a | 8.28 ± 4.07 b |
| 0.84 | 873.76 | 29.29 ± 7.17 a | 101.36 | 4.09 ± 1.58 c | 6.91 ± 3.25 c | |
| 0.99 | 929.03 | 28.02 ± 7.15 c | 107.35 | 4.16 ± 1.66 b | 9.26 ± 4.37 a | |
| p-value | 0.247 | <0.01 | 0.569 | <0.01 | <0.01 | |
| CP × lysine | p-value | <0.01 | <0.01 | 0.026 | <0.01 | <0.01 |
| Myofiber Density | Myofiber Diameter | Number of Fibers in the Muscle Bundle | Endomysium Thickness | Perimysium Thickness | Drip Loss | pH24h | Cooking Loss | Shear Force | |
|---|---|---|---|---|---|---|---|---|---|
| Myofiber density | 1.000 | −0.883 ** | −0.149 | −0.523 ** | −0.143 | 0.465 | 0.374 | −0.057 | −0.401 |
| Myofiber diameter | 1.000 | 0.084 | 0.448 ** | −0.437 * | −0.384 | −0.206 | −0.262 | 0.455 | |
| Number of fibers in the muscle bundle | 1.000 | 0.410 | −0.353 * | 0.379 | 0.238 | −0.006 | −0.066 | ||
| Endomysium thickness | 1.000 | 0.764 * | −0.123 | −0.283 | 0.409 | −0.306 | |||
| Perimysium thickness | 1.000 | 0.075 | −0.272 | 0.553 | −0.682 * | ||||
| Drip loss | 1.000 | −0.527 ** | 0.546 ** | 0.078 | |||||
| pH24h | 1.000 | −0.834 ** | −0.205 | ||||||
| Cooking loss | 1.000 | 0.380 * | |||||||
| Shear force | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.; Zhao, W.; Zhang, Q.; Wang, X.; Zhang, J.; Yan, Z.; Cao, J.; Liu, H.; Geng, A. Dietary Crude Protein and Lysine Levels Affect Meat Quality and Myofiber Characteristic of Slow-Growing Chicken. Animals 2024, 14, 2068. https://doi.org/10.3390/ani14142068
Chang C, Zhao W, Zhang Q, Wang X, Zhang J, Yan Z, Cao J, Liu H, Geng A. Dietary Crude Protein and Lysine Levels Affect Meat Quality and Myofiber Characteristic of Slow-Growing Chicken. Animals. 2024; 14(14):2068. https://doi.org/10.3390/ani14142068
Chicago/Turabian StyleChang, Cheng, Weiyu Zhao, Qianqian Zhang, Xuan Wang, Jian Zhang, Zhixun Yan, Jing Cao, Huagui Liu, and Ailian Geng. 2024. "Dietary Crude Protein and Lysine Levels Affect Meat Quality and Myofiber Characteristic of Slow-Growing Chicken" Animals 14, no. 14: 2068. https://doi.org/10.3390/ani14142068
APA StyleChang, C., Zhao, W., Zhang, Q., Wang, X., Zhang, J., Yan, Z., Cao, J., Liu, H., & Geng, A. (2024). Dietary Crude Protein and Lysine Levels Affect Meat Quality and Myofiber Characteristic of Slow-Growing Chicken. Animals, 14(14), 2068. https://doi.org/10.3390/ani14142068






