Genome-Wide Association Analyses and Population Verification Highlight the Potential Genetic Basis of Horned Morphology during Polled Selection in Tibetan Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection and Whole-Genome Sequencing
2.2. Quality Control and Annotation
2.3. Genome-Wide Association Analysis
2.4. Enrichment Analysis of Candidate Genes
2.5. Group Validation of Significantly Correlated SNPs
3. Results
3.1. Summary Statistics of Phenotype Data and Quality Control
3.2. Population Structures and Genome-Wide Association Analysis
3.3. Enrichment Analysis
3.4. Population Verification of SNPs Significantly Correlated with Tibetan Sheep Horn Traits
3.5. Linkage Disequilibrium and Haplotype Block Association Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merilä, J.; Sheldon, B.C.; Kruuk, L.E.B. Explaining stasis: Microevolutionary studies in natural populations. Genetica 2001, 112, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Arnold, S.J. The measurement of selection on correlated characters. Evolution 1983, 37, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Coltman, D.W.; O’Donoghue, P.; Hogg, J.T.; Festa-Bianchet, M. Selection and genetic (co) variance in bighorn sheep. Evolution 2005, 59, 1372–1382. [Google Scholar] [PubMed]
- Allais-Bonnet, A.; Grohs, C.; Medugorac, I.; Krebs, S.; Djari, A.; Graf, A.; Fritz, S.; Seichter, D.; Baur, A.; Russ, I.; et al. Novel Insights into the Bovine Polled Phenotype and Horn Ontogenesis in Bovidae. PLoS ONE 2013, 8, e63512. [Google Scholar] [CrossRef] [PubMed]
- Koene, P. Breeding and Feeding For Animal Health and Welfare In Organic Livestock Systems-Animal Welfare and Genetics In Organic Farming of Layers: The Example Of Cannibalism. In Proceedings of the fourth NAHWOA Workshop, Wageningen, The Netherlands, 24–27 March 2001; pp. 62–85. [Google Scholar]
- Misch, L.J.; Duffield, T.F.; Millman, S.T.; Lissemore, K.D. An investigation into the practices of dairy producers and veterinarians in dehorning dairy calves in Ontario. Can. Vet. J. 2007, 48, 1249–1254. [Google Scholar] [PubMed]
- Graf, B.; Senn, M. Behavioural and physiological responses of calves to dehorning by heat cauterization with or without local anaesthesia. Appl. Anim. Behav. Sci. 1999, 62, 153–171. [Google Scholar] [CrossRef]
- Prayaga, K.C. Genetic options to replace dehorning in beef cattle—A review. Aust. J. Agric. Res. 2007, 58, 1–8. [Google Scholar] [CrossRef]
- Roman, A.V. L’élevage Bovin en Égypte Antique. Ph.D. Thesis, École Nationale Vétérinaire d’Alfort, Maisons-Alfort, France, 2004. [Google Scholar]
- Dolling, C. Hornedness and polledness in sheep. I. The inheritance of polledness in the Merino. Aust. J. Agric. Res. 1960, 11, 427–438. [Google Scholar] [CrossRef]
- Coltman, D.W.; Pemberton, J.M. Inheritance of Coat Colour and Horn Type in Hirta Soay Sheep; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Dominik, S.; Henshall, J.M.; Hayes, B.J. A single nucleotide polymorphism on chromosome 10 is highly predictive for the polled phenotype in Australian Merino sheep. Anim. Genet. 2012, 43, 468–470. [Google Scholar] [CrossRef]
- Montgomery, G.W.; Henry, H.M.; Dodds, K.G.; Beattie, A.E.; Wuliji, T.; Crawford, A.M. Mapping the Horns (Ho) locus in sheep: A fur-ther locus controlling horn development in domestic animals. J Hered. 1996, 87, 358–363. [Google Scholar] [CrossRef]
- Johnston, S.E.; Mcewan, J.C.; Pickering, N.K.; Kijas, J.W.; Beraldi, D.; Pilkington, J.G.; Pemberton, J.M.; Slate, J. Genome–wide association mapping identifies the genetic basis of discrete and quantitative variation in sexual weaponry in a wild sheep population. Mol. Ecol. 2011, 20, 2555–2566. [Google Scholar] [CrossRef]
- Wiedemar, N.; Drögemüller, C. A 1.8-kb insertion in the 3′-UTR of RXFP2 is associated with polledness in sheep. Anim. Genet. 2015, 46, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Lühken, G.; Krebs, S.; Rothammer, S.; Küpper, J.; Mioč, B.; Russ, I.; Medugorac, I. The 1.78-kb insertion in the 3′-untranslated region of RXFP2 does not segregate with horn status in sheep breeds with variable horn status. Genet. Sel. Evol. 2016, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, G.; Li, Q.; Zhao, D.; Chen, Y. Discovery of SNPs in RXFP2 related to horn types in sheep. Small Rumin. Res. 2014, 116, 133–136. [Google Scholar] [CrossRef]
- Medugorac, I.; Seichter, D.; Graf, A.; Russ, I.; Blum, H.; Göpel, K.H.; Rothammer, S.; Förster, M.; Krebs, S. Bovine Polledness—An Autosomal Dominant Trait with Allelic Heterogeneity. PLoS ONE 2012, 7, e39477. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows—Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Van Der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ Data to High-Confidence Variant Calls: The Genome Analysis Toolkit Best Practices Pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Mbatchou, J.; Barnard, L.; Backman, J.; Marcketta, A.; Kosmicki, J.A.; Ziyatdinov, A.; Benner, C.; O’dushlaine, C.; Barber, M.; Boutkov, B.; et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat. Genet. 2021, 53, 1097–1103. [Google Scholar] [CrossRef]
- Dey, R.; Schmidt, E.M.; Abecasis, G.R.; Lee, S. A Fast and Accurate Algorithm to Test for Binary Phenotypes and Its Application to PheWAS. Am. J. Hum. Genet. 2017, 101, 37–49. [Google Scholar] [CrossRef]
- Chen, H.; Huffman, J.E.; Brody, J.A.; Wang, C.; Lee, S.; Li, Z.; Gogarten, S.M.; Sofer, T.; Bielak, L.F.; Bis, J.C.; et al. Efficient Variant Set Mixed Model Association Tests for Continuous and Binary Traits in Large-Scale Whole-Genome Sequencing Studies. Am. J. Hum. Genet. 2019, 104, 260–274. [Google Scholar] [CrossRef]
- Wu, M.C.; Lee, S.; Cai, T.; Li, Y.; Boehnke, M.; Lin, X. Rare-Variant Association Testing for Sequencing Data with the Sequence Kernel Association Test. Am. J. Hum. Genet. 2011, 89, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Wu, M.C.; Lin, X. Optimal tests for rare variant effects in sequencing association studies. Biostatistics 2012, 13, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef]
- Sim, Z.; Coltman, D.W. Heritability of Horn Size in Thinhorn Sheep. Front. Genet. 2019, 10, 959. [Google Scholar] [CrossRef]
- Pan, Z.; Li, S.; Liu, Q.; Wang, Z.; Zhou, Z.; Di, R.; Miao, B.; Hu, W.; Wang, X.; Hu, X.; et al. Whole-genome sequences of 89 Chinese sheep suggest role of RXFP2 in the development of unique horn phenotype as response to semi-feralization. GigaScience 2018, 7, giy019. [Google Scholar] [CrossRef]
- Tian, D.; Han, B.; Li, X.; Liu, D.; Zhou, B.; Zhao, C.; Zhang, N.; Wang, L.; Pei, Q.; Zhao, K. Genetic diversity and selection of Tibetan sheep breeds revealed by whole-genome resequencing. Anim. Biosci. 2023, 36, 991–1002. [Google Scholar] [CrossRef]
- Kardos, M.; Luikart, G.; Bunch, R.; Dewey, S.; Edwards, W.; McWilliam, S.; Stephenson, J.; Allendorf, F.W.; Hogg, J.T.; Kijas, J. Whole-genome resequencing uncovers molecular signatures of natural and sexual selection in wild bighorn sheep. Mol. Ecol. 2015, 24, 5616–5632. [Google Scholar] [CrossRef] [PubMed]
- Duijvesteijn, N.; Bolormaa, S.; Daetwyler, H.D.; Van Der Werf, J.H.J. Genomic prediction of the polled and horned phenotypes in Merino sheep. Genet. Sel. Evol. 2018, 50, 28. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.F.; Huang, J.H.; Zhu, Q.H.; Luo, L.Y.; Lu, R.; Xie, X.L.; Salehian-Dehkordi, H.; Esmailizadeh, A.; Liu, G.E.; et al. Structural variant landscapes reveal convergent signatures of evolution in sheep and goats. Genome Biol. 2024, 25, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Salomon, B.; Komatsuda, T. The Domestication Syndrome Genes Responsible for the Major Changes in Plant Form in the Triticeae Crops. Plant Cell Physiol. 2011, 52, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.S.; Wrangham, R.W.; Fitch, W.T. The Domestication Syndrome in Mammals: A Unified Explanation Based on Neural Crest Cell Behavior and Genetics. Genetics 2014, 197, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Lord, K.A.; Larson, G.; Coppinger, R.P.; Karlsson, E.K. The History of Farm Foxes Undermines the Animal Domestication Syndrome. Trends Ecol. Evol. 2020, 35, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Poissant, J.; Davis, C.S.; Malenfant, R.M.; Hogg, J.T.; Coltman, D.W. QTL mapping for sexually dimorphic fitness-related traits in wild bighorn sheep. Heredity 2012, 108, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.E.; Beraldi, D.; McRae, A.F.; Pemberton, J.M.; Slate, J. Horn type and horn length genes map to the same chromosomal region in Soay sheep. Heredity 2010, 104, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G. Reproductive seasonality and maturation throughout the complete life-cycle in the mouflon ram (Ovis musimon). Anim. Reprod. Sci. 1998, 53, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.J. Deer Antlers: Regeneration, Function and Evolution; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Lincoln, G.A. Teeth, horns and antlers: The weapons of sex. In The Differences between the Sexes; Cambridge University Press: Cambridge, UK, 1994; pp. 131–158. [Google Scholar]
- Gao, Y.; Xi, S.; Cai, B.; Wu, T.; Wang, Q.; Kalds, P.; Huang, S.; Wang, Y.; Han, S.; Pan, M.; et al. Sheep with Partial RXFP2 Knockout Exhibit Normal Horn Phenotype but Unilateral Cryptorchidism. J. Integr. Agric. 2023. [Google Scholar] [CrossRef]
- Lincoln, G.A.; Klandorf, H.; Anderson, N. Photoperiodic Control of Thyroid Function and Wool and Horn Growth in Rams and the Effect of Cranial Sympathectomy. Endocrinology 1980, 107, 1543–1548. [Google Scholar] [CrossRef]
- Wood, T.B. The Inheritance of Horns and Face Colour in Sheep. J. Agric. Sci. 1909, 3, 145–154. [Google Scholar] [CrossRef]
- Lee, S.H.; Van Der Werf, J.H.J.; Hayes, B.J.; Goddard, M.E.; Visscher, P.M. Predicting Unobserved Phenotypes for Complex Traits from Whole-Genome SNP Data. PLoS Genet. 2008, 4, e1000231. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, C.M.; McGraw, D.W.; Stack, C.B.; Stephens, J.C.; Judson, R.S.; Nandabalan, K.; Arnold, K.; Ruano, G.; Liggett, S.B. Complex promoter and coding region β2 -adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc. Natl. Acad. Sci. USA 2000, 97, 10483–10488. [Google Scholar] [CrossRef]
- Stephens, J.C.; Schneider, J.A.; Tanguay, D.A.; Choi, J.; Acharya, T.; Stanley, S.E.; Jiang, R.; Messer, C.J.; Chew, A.; Han, J.H.; et al. Haplotype variation and linkage disequilibrium in 313 human genes. Science 2001, 293, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Akey, J.; Jin, L.; Xiong, M. Haplotypes vs single marker linkage disequilibrium tests: What do we gain? Eur. J. Hum. Genet. Nat. 2001, 9, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.R.; Lai, E.H.; Gilbert, J.R.; Rogala, A.R.; Afshari, A.J.; Riley, J.; Finch, K.L.; Stevens, J.F.; Livak, K.J.; Slotterbeck, B.D.; et al. SNPing away at complex diseases: Analysis of sin-gle-nucleotide polymorphisms around APOE in Alzheimer disease. Am. J. Hum. Genet. 2000, 67, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Windig, J.J.; Hoving-Bolink, R.A.; Veerkamp, R.F. Breeding for polledness in Holstein cattle. Livest. Sci. 2015, 179, 96–101. [Google Scholar] [CrossRef]
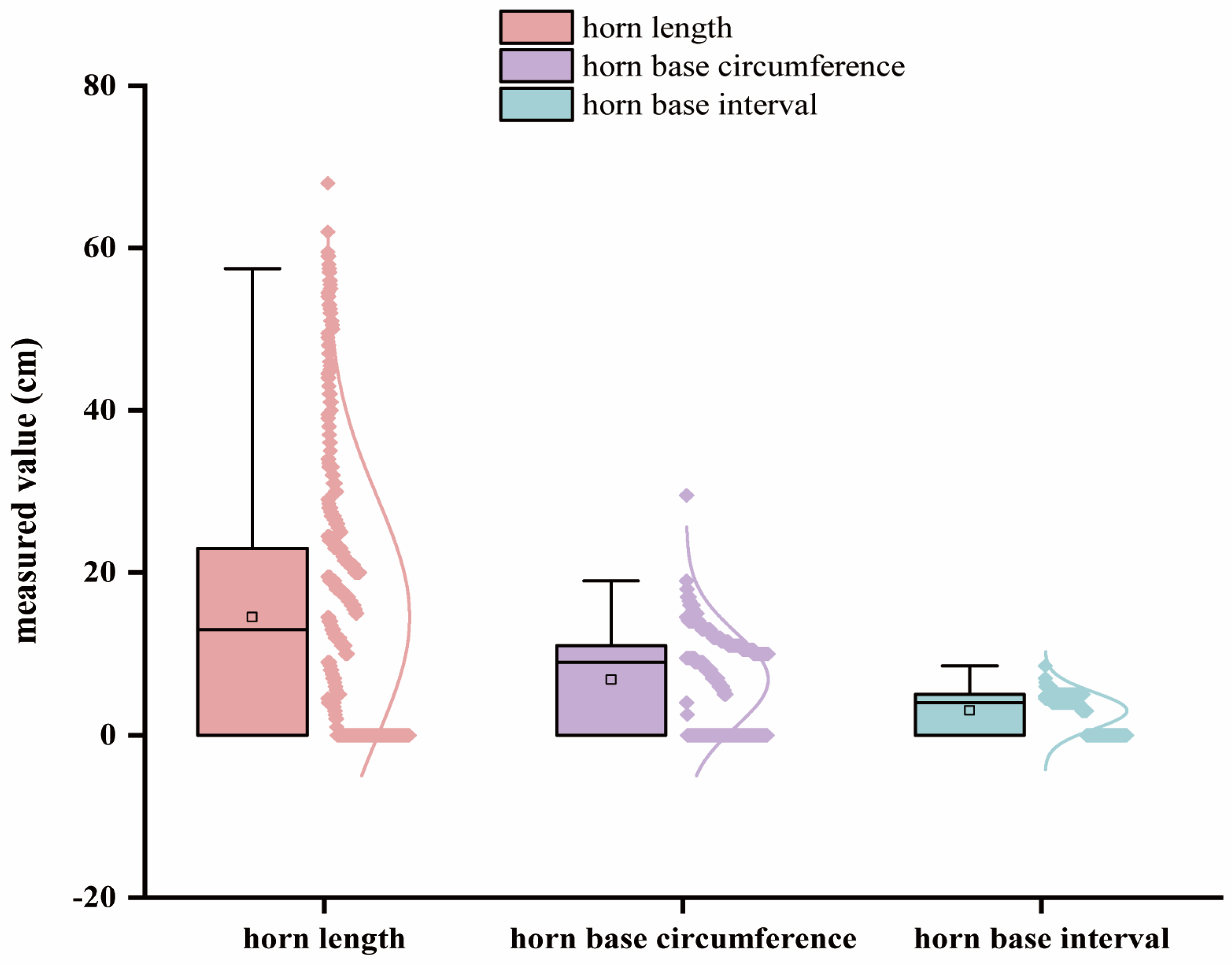
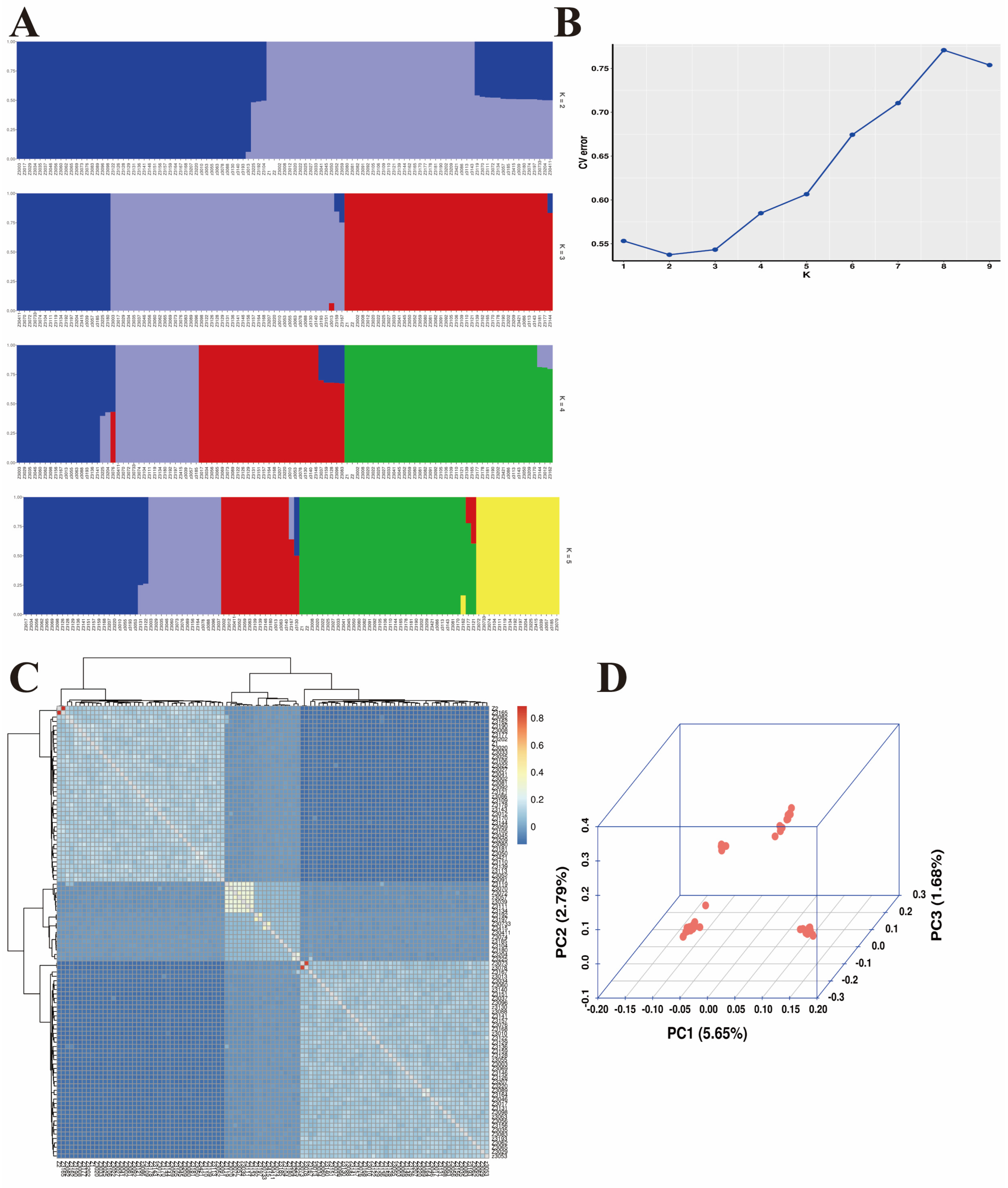
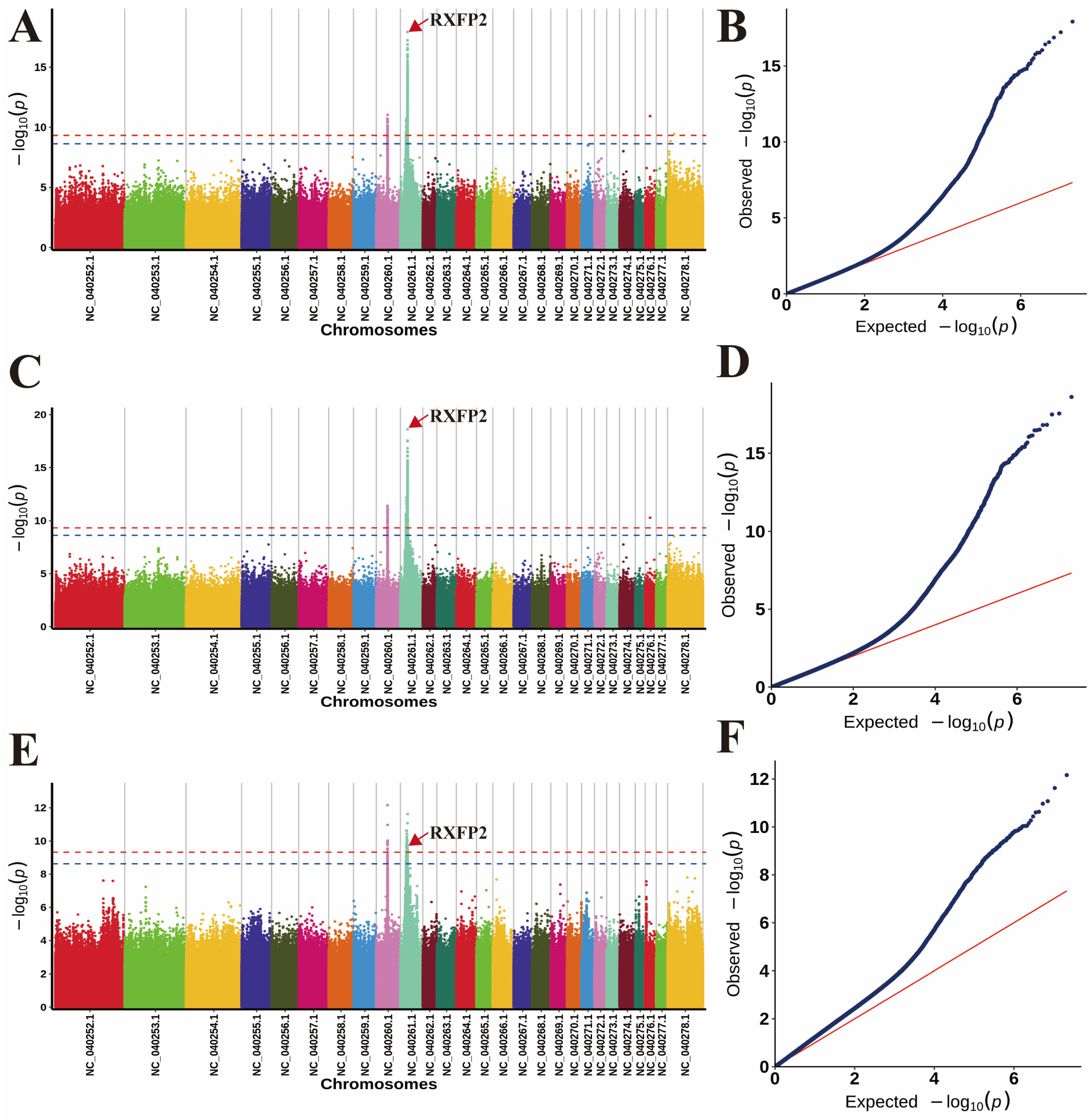
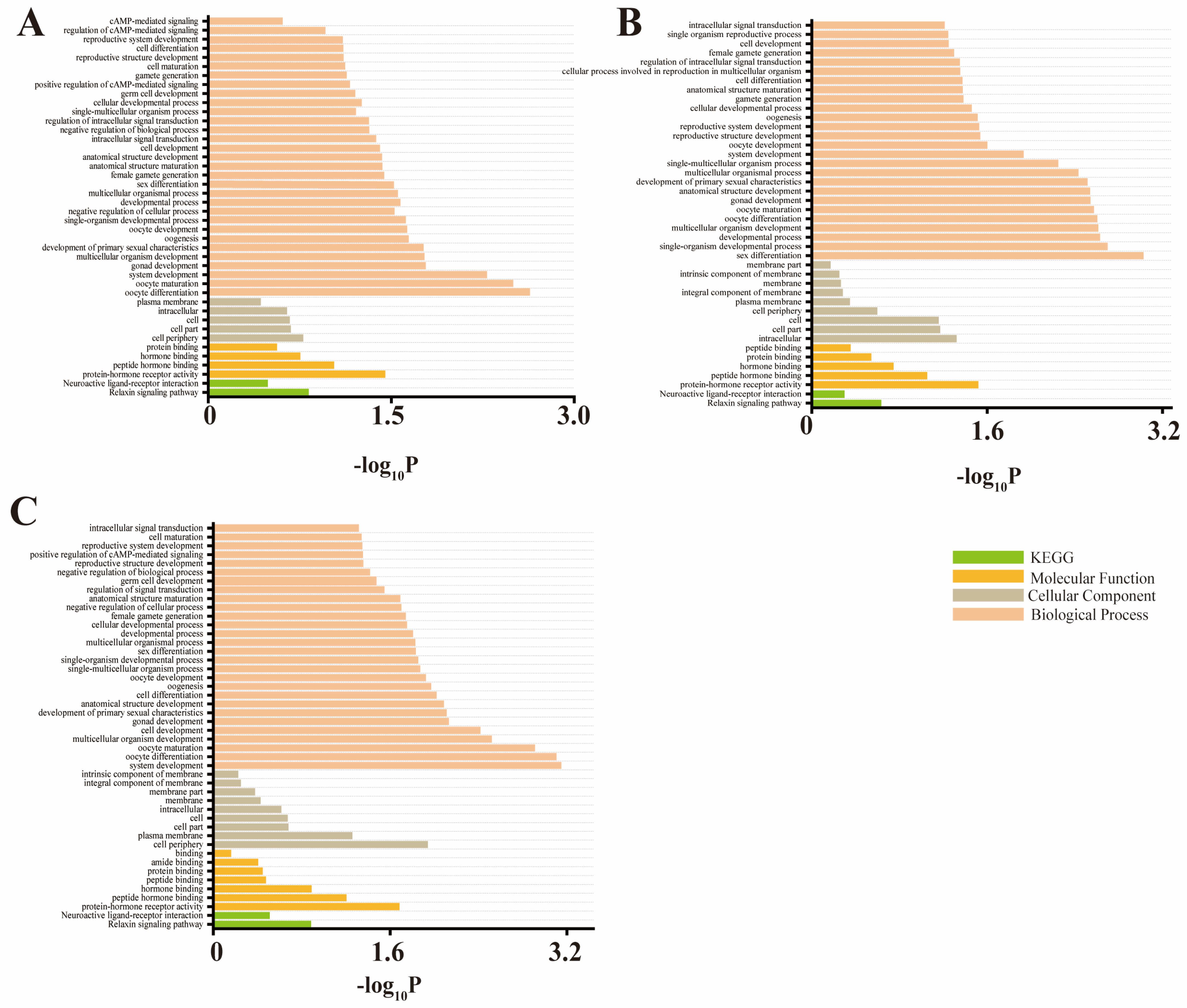

| Trait | Chr | Related SNPs | Position (bp) | p-Value | Candidate Gene |
|---|---|---|---|---|---|
| horn length | 10 | NC_040261.1 | 30,953,972 | 1.19 × 10−18 | RXFP2 within |
| 30,953,997 | 5.98 × 10−18 | ||||
| 30,957,852 | 3.76 × 10−17 | ||||
| 30,986,879 | 8.96 × 10−17 | ||||
| 30,953,070 | 1.72 × 10−16 | ||||
| horn base circumference | 10 | NC_040261.1 | 30,953,972 | 2.42 × 10−19 | |
| 30,953,997 | 3.24 × 10−18 | ||||
| 30,953,070 | 3.36 × 10−17 | ||||
| 30,986,879 | 8.84 × 10−17 | ||||
| horn base interval | 10 | NC_040261.1 | 30,935,807 | 6.24 × 10−10 |
| Traits | Loic | Current Name (Breed) | References |
|---|---|---|---|
| horn type | c.789C>T | Tan sheep | [17] |
| c.1117A>G | |||
| c.1125A>G | |||
| c.2059C>T | |||
| horn size | OAR10_29685536 | Thinhorn sheep | [29] |
| horn shape | OAR10 29461968 | 89 Chinese sheep | [30,31] |
| horn size | 29.45–29.55 M | Bighorn sheep | [32] |
| horn phenotype | OAR10_29415140 | Soay sheep | [14] |
| OAR10_29455959 | |||
| OAR10_29511510 | |||
| polled phenotype | OAR10_29389966 | Merino sheep | [12] |
| horn phenotype | OAR10_29448537 | Merino sheep | [33] |
| OAR10_29458450 | |||
| OAR10_29546872 | |||
| horn phenotype | OAR10_29481646 | Tibetan sheep | a, [31] |
| OAR10_29469024 | |||
| OAR10_30935807 | Tibetan sheep | a | |
| OAR10_30953070 | |||
| OAR10_30953972 | |||
| OAR10_30953997 | |||
| OAR10_30957852 | |||
| OAR10_30986879 |
| Sex | Horn Type | SNP | Genotype | Polled Frequency (%) | ||
|---|---|---|---|---|---|---|
| Wild | Mutant | Homozygous | ||||
| Male (n = 193) | Normal | g.30935807 | 85 | 1 | 0 | 43.88 |
| g.30953070 | 1 | 23 | 62 | |||
| g.30953972 | 0 | 24 | 62 | |||
| g.30953997 | 1 | 23 | 62 | |||
| g.30957852 | 1 | 25 | 60 | |||
| g.30986879 | 4 | 43 | 39 | |||
| Scurred | g.30935807 | 36 | 12 | 1 | 25.00 | |
| g.30953070 | 3 | 34 | 12 | |||
| g.30953972 | 1 | 36 | 12 | |||
| g.30953997 | 3 | 34 | 12 | |||
| g.30957852 | 3 | 34 | 12 | |||
| g.30986879 | 13 | 27 | 9 | |||
| Polled | g.30935807 | 41 | 19 | 1 | 31.12 | |
| g.30953070 | 21 | 40 | 0 | |||
| g.30953972 | 0 | 61 | 0 | |||
| g.30953997 | 21 | 40 | 0 | |||
| g.30957852 | 22 | 39 | 0 | |||
| g.30986879 | 27 | 34 | 0 | |||
| Female (n = 189) | Normal | g.30935807 | 57 | 2 | 0 | 31.55 |
| g.30953070 | 0 | 13 | 46 | |||
| g.30953972 | 46 | 13 | 0 | |||
| g.30953997 | 0 | 13 | 46 | |||
| g.30957852 | 0 | 15 | 44 | |||
| g.30986879 | 1 | 28 | 30 | |||
| Scurred | g.30935807 | 23 | 2 | 1 | 13.90 | |
| g.30953070 | 2 | 8 | 16 | |||
| g.30953972 | 0 | 10 | 16 | |||
| g.30953997 | 2 | 8 | 16 | |||
| g.30957852 | 2 | 8 | 16 | |||
| g.30986879 | 3 | 15 | 8 | |||
| Polled | g.30935807 | 77 | 25 | 0 | 54.55 | |
| g.30953070 | 17 | 74 | 11 | |||
| g.30953972 | 0 | 91 | 11 | |||
| g.30953997 | 17 | 74 | 11 | |||
| g.30957852 | 18 | 74 | 10 | |||
| g.30986879 | 39 | 59 | 4 | |||
| SNP | Genotype | Horn Length (cm) | Horn Base Circumference (cm) | Horn Base Interval (cm) |
|---|---|---|---|---|
| g.30935807 | AA (962) | 16.51 ± 14.77 Aa | 7.72 ± 5.57 Aa | 3.36 ± 2.09 Aa |
| GA (157) | 2.85 ± 7.13 Bb | 1.61 ± 3.92 Bb | 0.93 ± 1.90 Bb | |
| GG (6) | 3.50 ± 4.28 ABb | 3.00 ± 4.69 ABb | 1.50 ± 2.35 ABb | |
| p = 5.945 × 10−21 | p = 2.3087 × 10−17 | p = 0.008457 | ||
| g.30953070 | TT (72) | 1.98 ± 5.57 C | 1.10 ± 3.29 C | 0.97 ± 1.77 C |
| CT (419) | 8.65 ± 13.34 B | 4.14 ± 5.65 B | 2.13 ± 2.35 B | |
| CC (634) | 19.85 ± 13.95 A | 9.28 ± 4.73 A | 3.83 ± 1.79 A | |
| p = 2.3448 × 10−9 | p = 2.0722 × 10−23 | p = 1.0313 × 10−45 | ||
| g.30953972 | GG (28) | 2.88 ± 7.51 c | 1.55 ± 3.93 c | 0.57 ± 1.44 C |
| AG (465) | 8.12 ± 13.10 Bb | 3.86 ± 5.56 Bb | 2.06 ± 2.32 B | |
| AA (632) | 19.78 ± 13.91 Aa | 9.26 ± 4.73 Aa | 3.83 ± 1.79 A | |
| p = 0.012412 | p = 6.8297 × 10−15 | p = 7.1798 × 10−49 | ||
| g.30953997 | AA (73) | 2.25 ± 5.99 C | 1.22 ± 3.43 C | 1.01 ± 1.80 C |
| GA (422) | 8.86 ± 13.54 B | 4.21 ± 5.68 B | 2.15 ± 2.35 B | |
| GG (630) | 19.76 ± 13.92 A | 9.26 ± 4.73 A | 3.82 ± 1.80 A | |
| p = 1.0693 × 10−8 | p = 4.3225 × 10−23 | p = 1.0569 × 10−45 | ||
| g.30957852 | TT (77) | 1.85 ± 5.41 C | 1.03 ± 3.19 C | 0.96 ± 1.76 C |
| CT (424) | 8.81 ± 13.30 B | 4.26 ± 5.67 B | 2.17 ± 2.35 B | |
| CC (624) | 20.00 ± 13.97 A | 9.31 ± 4.71 A | 3.84 ± 1.79 A | |
| p = 1.7251 × 10−10 | p = 2.2845 × 10−26 | p = 1.267 × 10−47 | ||
| g.30986879 | TT (176) | 3.33 ± 8.47 C | 1.77 ± 4.15 C | 1.08 ± 1.99 C |
| CT (490) | 11.63 ± 13.25 B | 5.79 ± 5.73 B | 2.80 ± 2.32 B | |
| CC (459) | 21.94 ± 14.31 A | 9.90 ± 4.44 A | 3.98 ± 1.60 A | |
| p = 5.5701 × 10−14 | p = 1.2202 × 10−39 | p = 5.9519 × 10−57 | ||
| g.29469024 | AA (131) | 2.90 ± 8.96 C | 1.36 ± 3.78 C | 0.60 ± 1.57 C |
| GA (463) | 12.31 ± 14.61 B | 5.80 ± 5.82 B | 2.54 ± 2.34 B | |
| GG (531) | 19.36 ± 13.88 A | 9.10 ± 4.92 A | 4.02 ± 1.560 A | |
| p = 9.9762 × 10−15 | p = 51.2842 × 10−35 | p = 1.1715 × 10−73 | ||
| g.29481646 | TT (833) | 16.56 ± 14.47 A | 7.82 ± 5.47 A | 3.45 ± 2.04 A |
| CT (27) | 5.85 ± 11.51 B | 2.65 ± 5.12 B | 1.13 ± 1.98 B | |
| CC (265) | 9.05 ± 14.10 B | 4.20 ± 5.77 B | 1.85 ± 2.31 B | |
| p = 1.4899 × 10−14 | p = 0.01 | p = 5.599 × 10−11 | ||
| g.29461968 | GG (306) | 2.72 ± 8.85 C | 1.25 ± 3.64 C | 0.56 ± 1.53 C |
| AG (434) | 16.70 ± 14.65 B | 7.88 ± 5.54 B | 3.45 ± 2.07 B | |
| AA (385) | 21.49 ± 12.84 A | 10.11 ± 3.97 A | 4.47 ± 0.87 A | |
| p = 6.7264 × 10−34 | p = 2.828 × 10−86 | p = 75.0538 × 10−47 |
| Loci | Haplotypes | Numbers | Horn Length (cm) | Horn Base Circumference (cm) | Horn Base Interval (cm) |
|---|---|---|---|---|---|
| g.29481646 g.29469024 g.29461968 | AAGGCT | 11 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B |
| AAGGTT | 120 | 3.16 ± 9.32 B | 1.48 ± 3.92 B | 0.65 ± 1.63 B | |
| GAGACT | 9 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B | |
| GAGATT | 324 | 16.46 ± 14.55 A | 7.79 ± 5.46 A | 3.41 ± 2.08 Aa | |
| GAGGCC | 122 | 2.99 ± 9.73 B | 1.34 ± 3.77 B | 0.59 ± 1.60 B | |
| GAGGTT | 8 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B | |
| GGAACC | 3 | 23.83 ± 20.52 A | 10.33 ± 4.75 A | 4.50 ± 1.00 Aa | |
| GGAACT | 7 | 22.57 ± 11.58 A | 10.21 ± 4.84 A | 4.36 ± 0.75 Aa | |
| GGAATT | 375 | 21.45 ± 12.83 A | 10.11 ± 3.96 A | 4.48 ± 0.88 Aa | |
| GGGACC | 96 | 19.53 ± 14.65 A | 9.12 ± 5.32 A | 4.00 ± 1.72 Aa | |
| GGGATT | 5 | 8.30 ± 11.85 A | 4.40 ± 6.12 A | 1.80 ± 2.49 Ab | |
| GGGGCC | 44 | 2.00 ± 6.68 B | 0.98 ± 3.20 B | 0.48 ± 1.38 B |
| Loci | Haplotypes | Numbers | Horn Length (cm) | Horn Base Circumference (cm) | Horn Base Interval (cm) |
|---|---|---|---|---|---|
| g.30935807 g.30953070 g.30953972 g.30953997 g.30957852 g.30986879 | AACCAAGGCCCC | 363 | 22.17 ± 13.79 Aa | 10.04 ± 4.14 Aa | 4.07 ± 1.51 Aa |
| AACCAAGGCCCT | 222 | 17.49 ± 13.23 B | 8.68 ± 4.95 B | 3.73 ± 1.95 Ab | |
| AACCAAGGCCTT | 25 | 12.18 ± 15.80 B | 5.50 ± 6.68 B | 1.96 ± 2.34 B | |
| AACCAAGGCTCC | 11 | 10.68 ± 8.21 B | 6.91 ± 4.89 Ab | 3.41 ± 2.22 Aa | |
| AACCAAGGCTCT | 2 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B | |
| AACTGAGACCCC | 3 | 3.33 ± 5.77 B | 2.33 ± 4.04 B | 2.00 ± 3.46 Aa | |
| AACTGAGACTCC | 70 | 23.78 ± 16.71 Aa | 10.16 ± 5.28 Aa | 3.86 ± 1.70 Aa | |
| AACTGAGACTCT | 177 | 8.19 ± 11.83 B | 4.06 ± 5.42 B | 2.43 ± 2.38 B | |
| AACTGAGACTTT | 48 | 3.57 ± 8.35 B | 2.02 ± 4.40 B | 1.38 ± 2.09 B | |
| AATTGAAATTCT | 6 | 0.33 ± 0.82 B | 0.42 ± 1.02 B | 2.17 ± 2.40 Aa | |
| AATTGAAATTTT | 18 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 1.11 ± 1.84 B | |
| AATTGGAATTCT | 5 | 12.60 ± 12.80 Aa | 6.70 ± 6.32 Aa | 2.40 ± 2.27 Aa | |
| AATTGGAATTTT | 3 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B | |
| GACCAAGGCCCC | 6 | 16.33 ± 13.93 Aa | 8.08 ± 6.48 Aa | 2.83 ± 2.32 Aa | |
| GACTGAGACTCT | 66 | 3.85 ± 8.63 B | 2.07 ± 4.64 B | 1.07 ± 1.95 B | |
| GACTGAGACTTT | 47 | 1.48 ± 3.29 B | 1.20 ± 2.70 B | 0.90 ± 2.07 B | |
| GACTGAGATTCT | 4 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 2.00 ± 2.31 Ab | |
| GATTGAAATTTT | 16 | 1.63 ± 3.34 B | 0.69 ± 2.75 B | 0.50 ± 1.37 B | |
| GATTGGAATTCT | 2 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B | |
| GATTGGAATTTT | 15 | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B | |
| GGCTGAGACTCT | 2 | 3.50 ± 4.95 Ab | 4.00 ± 5.66 Aa | 2.00 ± 2.83 Aa | |
| GGTTGGAATTTT | 4 | 3.50 ± 4.73 B | 2.50 ± 5.00 B | 1.25 ± 2.50 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, D.; Zhang, Z.; Huang, B.; Han, B.; Li, X.; Zhao, K. Genome-Wide Association Analyses and Population Verification Highlight the Potential Genetic Basis of Horned Morphology during Polled Selection in Tibetan Sheep. Animals 2024, 14, 2152. https://doi.org/10.3390/ani14152152
Tian D, Zhang Z, Huang B, Han B, Li X, Zhao K. Genome-Wide Association Analyses and Population Verification Highlight the Potential Genetic Basis of Horned Morphology during Polled Selection in Tibetan Sheep. Animals. 2024; 14(15):2152. https://doi.org/10.3390/ani14152152
Chicago/Turabian StyleTian, Dehong, Zian Zhang, Bin Huang, Buying Han, Xue Li, and Kai Zhao. 2024. "Genome-Wide Association Analyses and Population Verification Highlight the Potential Genetic Basis of Horned Morphology during Polled Selection in Tibetan Sheep" Animals 14, no. 15: 2152. https://doi.org/10.3390/ani14152152
APA StyleTian, D., Zhang, Z., Huang, B., Han, B., Li, X., & Zhao, K. (2024). Genome-Wide Association Analyses and Population Verification Highlight the Potential Genetic Basis of Horned Morphology during Polled Selection in Tibetan Sheep. Animals, 14(15), 2152. https://doi.org/10.3390/ani14152152







