Sustainable Fish Meal-Free Diets for Gilthead Sea Bream (Sparus aurata): Integrated Biomarker Response to Assess the Effects on Growth Performance, Lipid Metabolism, Antioxidant Defense and Immunological Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Diets
2.3. Experimental Setup and Sampling
2.4. Zootechnical Measurements
2.5. Humoral Immune Parameters
2.6. Oxidative Status
2.7. PCR-Array
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Humoral Parameters and Oxidative Status
3.3. Liver and Head Kidney Gene Expression Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Oliva-Teles, A.; Enes, P.; Peres, H. Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. In Feed and Feeding Practices in Aquaculture; Woodhead Publishing: Sawston, UK, 2015; pp. 203–233. [Google Scholar]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.; Metian, M. Feed matters: Satisfying the feed demand of aquaculture. Reviews in Fisheries. Sci. Aquac. 2015, 23, 1–10. [Google Scholar]
- Ahmed, N.; Thompson, S. The blue dimensions of aquaculture: A global synthesis. Sci. Total Environ. 2019, 652, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Eroldoğan, O.T.; Glencross, B.; Novoveska, L.; Gaudêncio, S.P.; Rinkevich, B.; Varese, G.C.; Carvalho, M.F.; Tasdemir, D.; Safarik, I.; Nielsen, S.L.; et al. From the sea to aquafeed: A perspective overview. Rev. Aquac. 2023, 15, 1028–1057. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Enes, P.; Couto, A.; Peres, H. Replacing fish meal and fish oil in industrial fish feeds. In Feed and Feeding Practices in Aquaculture; Woodhead Publishing: Sawston, UK, 2022; pp. 231–268. [Google Scholar]
- Colombo, S.M.; Roy, K.; Mraz, J.; Wan, A.H.L.; Davies, S.J.; Tibbetts, S.M.; Øverland, M.; Francis, D.S.; Rocker, M.M.; Gasco, L.; et al. Towards achieving circularity and sustainability in feeds for farmed blue foods. Rev. Aquac. 2023, 15, 1115–1141. [Google Scholar] [CrossRef]
- Glencross, B.; Fracalossi, D.M.; Hua, K.; Izquierdo, M.; Mai, K.; Øverland, M.; Robb, D.; Roubach, R.; Schrama, J.; Small, B.; et al. Harvesting the benefits of nutritional research to address global challenges in the 21st century. J. World Aquac. Soc. 2023, 54, 343–363. [Google Scholar] [CrossRef]
- Peres, H.; Lim, C. Utilization of soybean products in diets of nonsalmonid marine finfish. In Alternative Protein Sources in Aquaculture Diets; CRC Press: Boca Raton, FL, USA, 2023; pp. 281–312. [Google Scholar]
- Kokou, F.; Sarropoulou, E.; Cotou, E.; Kentouri, M.; Alexis, M.; Rigos, G. Effects of graded dietary levels of soy protein concentrate supplemented with methionine and phosphate on the immune and antioxidant responses of gilthead sea bream (Sparus aurata L.). Fish Shellfish Immunol. 2017, 64, 111–121. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Mozanzadeh, M.T.; Marammazi, J.G.; Safari, O.; Gisbert, E. Dietary replacement of fish meal by soy products (soybean meal and isolated soy protein) in silvery-black porgy juveniles (Sparidentex hasta). Aquaculture 2016, 464, 50–59. [Google Scholar] [CrossRef]
- Collins, S.A.; Øverland, M.; Skrede, A.; Drew, M.D. Effect of plant protein sources on growth rate in salmonids: Meta-analysis of dietary inclusion of soybean, pea and canola/rapeseed meals and protein concentrates. Aquaculture 2013, 400, 85–100. [Google Scholar] [CrossRef]
- Cavrois-Rogacki, T.; Leeming, D.; Lopez, P.M.; Davie, A.; Migaud, H. Plant-based protein ingredients can successfully replace fish meal in the diet of ballan wrasse (LABRUS BERGYLTA) juveniles. Aquaculture 2022, 546, 737419. [Google Scholar] [CrossRef]
- Qian, Y.F.; Limbu, S.M.; Qiao, F.; Luo, Y.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Seeking the best alternatives: A systematic review and meta-analysis on replacing fishmeal with plant protein sources in carnivorous fish species. Rev. Aquac. 2024, 16, 1099–1126. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Conceição, L.E.C.; Aragão, C.; Dias, J.; Costas, B.; Terova, G.; Martins, C.; Tort, L. Dietary nitrogen and fish welfare. Fish Physiol. Biochem. 2012, 38, 119–141. [Google Scholar] [CrossRef]
- Costas, B.; Couto, A.; Azeredo, R.; Machado, M.; Krogdahl, Å.; Oliva-Teles, A. Gilthead seabream (Sparus aurata) immune responses are modulated after feeding with purified antinutrients. Fish Shellfish Immunol. 2014, 41, 70–79. [Google Scholar] [CrossRef]
- Estensoro, I.; Ballester-Lozano, G.F.; Benedito-Palos, L.; Grammes, F.; Martos-Sitcha, J.A.; Mydland, L.-T.; Calduch-Giner, J.A.; Fuentes, J.; Karalazos, V.; Ortiz, A.; et al. Dietary butyrate helps to restore the intestinal status of a marine teleost (Sparus aurata) fed extreme diets low in fish meal and fish oil. PLoS ONE 2016, 11, e0166564. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Calduch-Giner, J.A.; Fouz, B.; Estensoro, I.; Simó-Mirabet, P.; Puyalto, M.; Karalazos, V.; Palenzuela, O.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Under Control: How a dietary additive can restore the gut microbiome and proteomic profile, and improve disease resilience in a marine teleostean fish fed vegetable diets. Microbiome 2017, 5, 164. [Google Scholar] [CrossRef] [PubMed]
- Egerton, S.; Wan, A.; Murphy, K.; Collins, F.; Ahern, G.; Sugrue, I.; Busca, K.; Egan, F.; Muller, N.; Whooley, J.; et al. Replacing fishmeal with plant protein in Atlantic salmon (Salmo salar) diets by supplementation with fish protein hydrolysate. Sci. Rep. 2020, 10, 4194. [Google Scholar] [CrossRef] [PubMed]
- Balbuena-Pecino, S.; Montblanch, M.; García-Meilán, I.; Fontanillas, R.; Gallardo, Á.; Gutiérrez, J.; Navarro, I.; Capilla, E. Hydroxytyrosol-rich extract from olive juice as an additive in gilthead sea bream juveniles fed a high-fat diet: Regulation of somatic growth. Front. Physiol. 2022, 13, 966175. [Google Scholar] [CrossRef]
- Rimoldi, S.; Montero, D.; Torrecillas, S.; Serradell, A.; Acosta, F.; Haffray, P.; Hostins, B.; Fontanillas, R.; Allal, F.; Bajek, A.; et al. Genetically superior European sea bass (Dicentrarchus labrax) and nutritional innovations: Effects of functional feeds on fish immune response, disease resistance, and gut microbiota. Aquac. Rep. 2023, 33, 101747. [Google Scholar] [CrossRef]
- Naya-Català, F.; Torrecillas, S.; Piazzon, M.C.; Sarih, S.; Calduch-Giner, J.; Fontanillas, R.; Hostins, B.; Sitjà-Bobadilla, A.; Acosta, F.; Pérez-Sánchez, J.; et al. Can the genetic background modulate the effects of feed additives? Answers from gut microbiome and transcriptome interactions in farmed gilthead sea bream (Sparus aurata) fed with a mix of phytogenics, organic acids or probiotics. Aquaculture 2024, 586, 740770. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Naya-Català, F.; Pereira, G.V.; Estensoro, I.; Del Pozo, R.; Calduch-Giner, J.A.; Nuez-Ortín, W.G.; Palenzuela, O.; Sitjà-Bobadilla, A.; Dias, J.; et al. A novel fish meal-free diet formulation supports proper growth and does not impair intestinal parasite susceptibility in gilthead sea bream (Sparus aurata) with a reshape of gut microbiota and tissue-specific gene expression patterns. Aquaculture 2022, 558, 738362. [Google Scholar] [CrossRef]
- Alfiko, Y.; Xie, D.; Astuti, R.T.; Wong, J.; Wang, L. Insects as a feed ingredient for fish culture: Status and trends. Aquac. Fish. 2022, 7, 166–178. [Google Scholar] [CrossRef]
- Gasco, L.; Acuti, G.; Bani, P.; Dalle Zotte, A.; Danieli, P.P.; de Angelis, A.; Fortina, R.; Marino, R.; Parisi, G.; Piccolo, G.; et al. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef]
- Aragão, C.; Cabano, M.; Colen, R.; Fuentes, J.; Dias, J. Alternative formulations for gilthead seabream diets: Towards a more sustainable production. Aquac. Nutr. 2020, 26, 444–455. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Ghetas, H.A.; Khallaf, M.A. Black soldier fly (Hermetia illucens) larvae meal in diets of European seabass: Effects on antioxidative capacity, non-specific immunity, transcriptomic responses, and resistance to the challenge with Vibrio alginolyticus. Fish Shellfish Immunol. 2021, 111, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Basto, A.; Calduch-Giner, J.; Oliveira, B.; Petit, L.; Sá, T.; Maia, M.R.; Fonseca, S.C.; Matos, E.; Pérez-Sánchez, J.; Valente, L.M. The use of defatted Tenebrio molitor larvae meal as a main protein source is supported in European sea bass (Dicentrarchus labrax) by data on growth performance, lipid metabolism and flesh quality. Front. Physiol. 2021, 12, 659567. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, B.; Zarantoniello, M.; Cardinaletti, G.; Cerri, R.; Giorgini, E.; Belloni, A.; Contò, M.; Tibaldi, E.; Olivotto, I. Hermetia illucens and poultry by-product meals as alternatives to plant protein sources in gilthead seabream (Sparus aurata) diet: A multidisciplinary study on fish gut status. Animals 2021, 11, 677. [Google Scholar]
- Glencross, B.D.; Huyben, D.; Schrama, J.W. The application of single-cell ingredients in aquaculture feeds—A review. Fishes 2020, 5, 22. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Carter, C.G.; Codabaccus, M.B. Assessing the value of single-cell ingredients in aquafeeds. Curr. Opin. Biotechnol. 2022, 76, 102734. [Google Scholar] [CrossRef]
- Solé-Jiménez, P.; Naya-Català, F.; Piazzon, M.C.; Estensoro, I.; Calduch-Giner, J.A.; Sitjà-Bobadilla, A.; Van Mullem, D.; Pérez-Sánchez, J. Reshaping of gut microbiota in gilthead sea bream fed microbial and processed animal proteins as the main dietary protein source. Front. Mar. Sci. 2021, 8, 705041. [Google Scholar] [CrossRef]
- Woodgate, S.L.; Wan, A.H.; Hartnett, F.; Wilkinson, R.G.; Davies, S.J. The utilisation of European processed animal proteins as safe, sustainable and circular ingredients for global aquafeeds. Rev. Aquac. 2022, 14, 1572–1596. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Fishmeal alternative protein sources for aquaculture feeds. In Chemistry of Foods: Feeds for the Aquaculture Sector—Current Situation and Alternative Sources; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1–20. [Google Scholar]
- Karapanagiotidis, I.T.; Psofakis, P.; Mente, E.; Malandrakis, E.; Golomazou, E. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquac. Nutr. 2019, 25, 3–14. [Google Scholar] [CrossRef]
- Sabbagh, M.; Schiavone, R.; Brizzi, G.; Sicuro, B.; Zilli, L.; Vilella, S. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture 2019, 511, 734220. [Google Scholar] [CrossRef]
- Irm, M.; Taj, S.; Jin, M.; Luo, J.; Andriamialinirina, H.J.T.; Zhou, Q. Effects of replacement of fish meal by poultry by-product meal on growth performance and gene expression involved in protein metabolism for juvenile black sea bream (Acanthoparus schlegelii). Aquaculture 2020, 528, 735544. [Google Scholar] [CrossRef]
- Santigosa, E.; Brambilla, F.; Milanese, L. Microalgae oil as an effective alternative source of EPA and DHA for gilthead seabream (Sparus aurata) aquaculture. Animals 2021, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.N.; Tester, J.W.; Beal, C.M.; Huntley, M.E.; Sills, D.L. Target cultivation and financing parameters for sustainable production of fuel and feed from microalgae. Environ. Sci. Technol. 2016, 50, 3333–3341. [Google Scholar] [CrossRef]
- Sprague, M.; Betancor, M.B.; Tocher, D.R. Microbial and genetically engineered oils as replacements for fish oil in aquaculture feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef]
- Malcorps, W.; Newton, R.W.; Sprague, M.; Glencross, B.D.; Little, D.C. Nutritional characterisation of European aquaculture processing by-products to facilitate strategic utilisation. Front. Sustain. Food Syst. 2021, 5, 720595. [Google Scholar] [CrossRef]
- Marques, L.; Calado, R.; Lillebø, A.I. Potential of ascidians as extractive species and their added value in marine integrated multitrophic aquaculture systems–From pests to valuable blue bioresources. Front. Mar. Sci. 2022, 9, 849870. [Google Scholar] [CrossRef]
- Gao, P.; Khong, H.Y.; Mao, W.; Chen, X.; Bao, L.; Wen, X.; Xu, Y. Tunicates as sources of high-quality nutrients and bioactive compounds for food/feed and pharmaceutical applications: A review. Foods 2023, 12, 3684. [Google Scholar] [CrossRef] [PubMed]
- Ytrestøyl, T.; Aas, T.S.; Åsgård, T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture 2015, 448, 365–374. [Google Scholar] [CrossRef]
- Shepherd, C.J.; Monroig, O.; Tocher, D.R. Future availability of raw materials for salmon feeds and supply chain implications: The case of Scottish farmed salmon. Aquaculture 2017, 467, 49–62. [Google Scholar] [CrossRef]
- Salin, K.R.; Arun, V.V.; Mohanakumaran Nair, C.; Tidwell, J.H. Sustainable aquafeed. In Sustainable Aquaculture; Springer: Cham, Switzerland, 2018; pp. 123–151. [Google Scholar]
- Gómez-Requeni, P.; Mingarro, M.; Calduch-Giner, J.; Medale, F.; Martín, S.A.M.; Houlihan, D.F.; Kaushik, S.; Pérez-Sánchez, J. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata). Aquaculture 2004, 232, 493–510. [Google Scholar] [CrossRef]
- Benedito-Palos, L.; Ballester-Lozano, G.F.; Simó, P.; Karalazos, V.; Ortiz, A.; Calduch-Giner, J.A.; Pérez-Sánchez, J. Lasting effects of butyrate and low FM/FO diets on growth performance, blood haematology/biochemistry and molecular growth-related markers in gilthead sea bream (Sparus aurata). Aquaculture 2016, 454, 8–18. [Google Scholar] [CrossRef]
- Simó-Mirabet, P.; Perera, E.; Calduch-Giner, J.A.; Afonso, J.M.; Pérez-Sánchez, J. Co-expression analysis of sirtuins and related metabolic biomarkers in juveniles of gilthead sea bream (Sparus aurata) with differences in growth performance. Front. Physiol. 2018, 9, 608. [Google Scholar] [CrossRef] [PubMed]
- Uyeh, D.D.; Pamulapati, T.; Mallipeddi, R.; Park, T.; Asem-Hiablie, S.; Woo, S.; Kim, J.; Kim, Y.; Ha, Y. Precision animal feed formulation: An evolutionary multi-objective approach. Anim. Feed Sci. Technol. 2019, 256, 114211. [Google Scholar] [CrossRef]
- Naya-Català, F.; do Vale Pereira, G.; Piazzon, M.C.; Fernandes, A.M.; Calduch-Giner, J.A.; Sitjà-Bobadilla, A.; Conceição, L.E.C.; Pérez-Sánchez, J. Cross-talk between intestinal microbiota and host gene expression in gilthead sea bream (Sparus aurata) juveniles: Insights in fish feeds for increased circularity and resource utilization. Front. Physiol. 2021, 12, 748265. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, N.; Alexis, M.N.; Henderson, R.J. Effects of dietary soybean and cod-liver oil levels on growth and body composition of gilthead bream (Sparus aurata). Aquaculture 1992, 104, 293–308. [Google Scholar] [CrossRef]
- Halver, J.E.; Hardy, R.W. Fish Nutrition, 3rd ed.; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Guardiola, F.A.; Cuesta, A.; Arizcun, M.; Meseguer, J.; Esteban, M.A. Comparative skin mucus and serum humoral defence mechanisms in the teleost gilthead seabream (Sparus aurata). Fish Shellfish Immunol. 2014, 36, 545–551. [Google Scholar] [CrossRef]
- Ellis, A.E. Serum antiproteases in fish. In Techniques in Fish Immunology; SOS Publications: Fair Haven, NJ, USA, 1990; pp. 95–99. [Google Scholar]
- Machado, M.; Azeredo, R.; Díaz-Rosales, P.; Afonso, A.; Peres, H.; Oliva-Teles, A.; Costas, B. Dietary tryptophan and methionine as modulators of European seabass (Dicentrarchus labrax) immune status and inflammatory response. Fish Shellfish Immunol. 2015, 42, 353–362. [Google Scholar] [CrossRef]
- Graham, S.; Secombes, C.J. The production of a macrophage-activating factor from rainbow trout Salmo gairdneri leucocytes. Immunology 1988, 65, 293–297. [Google Scholar]
- Cuesta, A.; Meseguer, J.; Esteban, M.A. Total serum immunoglobulin M levels are affected by immunomodulators in seabream (Sparus aurata L.). Vet. Immunol. Immunopathol. 2004, 101, 203–210. [Google Scholar] [CrossRef]
- Bird, R.P.; Draper, H.H. Comparative studies on different methods of malonaldehyde determination. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 299–305. [Google Scholar]
- Claiborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1984; pp. 283–284. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sturn, A.; Quackenbush, J.; Trajanoski, Z. GENESIS: Cluster analysis of microarray data. Bioinformatics 2002, 18, 207–208. [Google Scholar] [CrossRef]
- Kasumyan, A.O.; Døving, K.B. Taste preferences in fishes. Fish Fish. 2003, 4, 289–347. [Google Scholar] [CrossRef]
- Gatlin III, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, Å.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Weber, G.M.; Blemings, K.P.; Silverstein, J.T. Insulin-like growth factor-I and genetic effects on indexes of protein degradation in response to feed deprivation in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R1332–R1342. [Google Scholar] [CrossRef]
- Pérez-Sánchez, J.; Simó-Mirabet, P.; Naya-Català, F.; Martos-Sitcha, J.A.; Perera, E.; Bermejo-Nogales, A.; Benedito-Palos, L.; Calduch-Giner, J.A. Somatotropic axis regulation unravels the differential effects of nutritional and environmental factors in growth performance of marine farmed fishes. Front. Endocrinol. 2018, 9, 687. [Google Scholar] [CrossRef]
- Naya-Català, F.; Simó-Mirabet, P.; Calduch-Giner, J.; Pérez-Sánchez, J. Transcriptomic profiling of Gh/Igf system reveals a prompted tissue-specific differentiation and novel hypoxia responsive genes in gilthead sea bream. Sci. Rep. 2021, 11, 16466. [Google Scholar] [CrossRef]
- Caballero, M.J.; Izquierdo, M.S.; Kjørsvik, E.; Fernandez, A.J.; Rosenlund, G. Histological alterations in the liver of sea bream, Sparus aurata L., caused by short-or long-term feeding with vegetable oils. Recovery of normal morphology after feeding fish oil as the sole lipid source. J. Fish Dis. 2004, 27, 531–541. [Google Scholar] [CrossRef]
- Ballester-Lozano, G.F.; Benedito-Palos, L.; Estensoro, I.; Sitjà-Bobadilla, A.; Kaushik, S.; Pérez-Sánchez, J. Comprehensive biometric; biochemical and histopathological assessment of nutrient deficiencies in gilthead sea bream fed semi-purified diets. Br. J. Nutr. 2015, 114, 713–726. [Google Scholar] [CrossRef]
- Perera, E.; Turkmen, S.; Simó-Mirabet, P.; Zamorano, M.J.; Xu, H.; Naya-Català, F.; Izquierdo, M.; Pérez-Sánchez, J. Stearoyl-CoA desaturase (scd1a) is epigenetically regulated by broodstock nutrition in gilthead sea bream (Sparus aurata). Epigenetics 2020, 15, 536–553. [Google Scholar] [CrossRef]
- Benedito-Palos, L.; Calduch-Giner, J.A.; Ballester-Lozano, G.F.; Pérez-Sánchez, J. Effect of ration size on fillet fatty acid composition, phospholipid allostasis and mRNA expression patterns of lipid regualtory genes in gilthead sea bream (Sparus aurata). Br. J. Nutr. 2013, 109, 1175–1187. [Google Scholar] [CrossRef][Green Version]
- Bai, L.; Li, H. Innate immune regulatory networks in hepatic lipid metabolism. J. Mol. Med. 2019, 97, 593–604. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, C.; Xu, L.; Yuan, Y.; Zhao, J.; Zhao, W.; Chen, Y.; Qiu, J.; Meng, M.; Zheng, Y.; et al. N (6)-methyladenosine reader protein Ythdc2 suppresses liver steatosis via regulation of mRNA stability of lipogenic genes. Hepatology 2021, 73, 91–103. [Google Scholar] [CrossRef]
- Tripathy, S.; Lytle, K.A.; Stevens, R.D.; Bain, J.R.; Newgard, C.B.; Greenberg, A.S.; Huang, L.S.; Jump, D.B. Fatty acid elongase-5 (Elovl5) regulates hepatic triglyceride catabolism in obese C57BL/6J mice. J. Lipid Res. 2014, 55, 1448–1464. [Google Scholar] [CrossRef]
- Shikama, A.; Shinozaki, H.; Takeuchi, Y.; Matsuzaka, T.; Aita, Y.; Murayama, T.; Sawada, Y.; Piao, X.; Toya, N.; Oya, Y.; et al. Identification of human ELOVL5 enhancer regions controlled by SREBP. Biochem. Biophys. Res. Commun. 2015, 465, 857–863. [Google Scholar] [CrossRef]
- Ruiz, A.; Andree, K.B.; Furones, D.; Holhorea, P.G.; Calduch-Giner, J.A.; Viñas, M.; Pérez-Sánchez, J.; Gisbert, E. Modulation of gut microbiota and intestinal immune response in gilthead seabream (Sparus aurata) by dietary bile salt supplementation. Front. Microbiol. 2023, 14, 1123716. [Google Scholar] [CrossRef]
- Ruiz, A.; Sanahuja, I.; Andree, K.B.; Furones, D.; Holhorea, P.G.; Calduch-Giner, J.A.; Pastor, J.J.; Viñas, M.; Pérez-Sánchez, J.; Morais, S.; et al. The potential of a combination of pungent spices as a novel supplement in gilthead seabream (Sparus aurata) diets to aid in the strategic use of fish oil in aquafeeds: A holistic perspective. Front. Immunol. 2023, 14, 1222173. [Google Scholar] [CrossRef]
- Ruiz, A.; Sanahuja, I.; Andree, K.B.; Furones, D.; Holhorea, P.G.; Calduch-Giner, J.A.; Pastor, J.J.; Viñas, M.; Pérez-Sánchez, J.; Morais, S.; et al. Supplementation of gilthead seabream (Sparus aurata) diets with spices as a functional strategy to control excess adiposity through lipid; cholesterol and bile acid metabolism; and to induce an immunomodulatory intestinal regulation. Aquaculture 2024, 581, 740378. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, W.-D.; Wang, Y.-D. Gut microbiota: An integral moderator in health and disease. Front. Microbiol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Butt, R.L.; Volkoff, H. Gut microbiota and energy homeostasis in fish. Front. Endocrinol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Rekha, K.; Venkidasamy, B.; Samynathan, R.; Nagella, P.; Rebezov, M.; Khayrullin, M.; Ponomarev, E.; Bouyahya, A.; Sarkari, T.; Shariati, M.A.; et al. Short-chain fatty acid: An updated review on signaling, metabolism, and therapeutic effects. Crit. Rev. Food Sci. Nutr. 2024, 64, 2461–2489. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.; Mendes, M.A. Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food Supplements; an overview. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Bonsignore, M.; Messina, C.M.; Bellante, A.; Manuguerra, S.; Arena, R.; Santulli, A.; Maricchiolo, G.; Del Core, M.; Sprovieri, M. Chemical and biochemical responses to sub−lethal doses of mercury and cadmium in gilthead seabream (Sparus aurata). Chemosphere 2022, 307, 135822. [Google Scholar]
- Secombes, C.J.; Zou, J.; Bird, S. Fish cytokines: Discovery, activities and potential applications. In Fish Defenses: Immunology; Zaccone, G., Meseguer, J., Garcia-Ayala, A., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2009; pp. 1–36. [Google Scholar]
- Guo, M.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. The effects of IL-1β, IL-8, G-CSF and TNF-α as molecular adjuvant on the immune response to an E. tarda subunit vaccine in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2018, 77, 374–384. [Google Scholar] [CrossRef]
- Tran, H.B.; Chen, S.C.; Chaung, H.C.; Cheng, T.C. Molecular cloning of IL-6, IL-10, IL-11, IFN-ɤ and modulation of pro-and anti-inflammatory cytokines in cobia (Rachycentron canadum) after Photobacterium damselae subsp. piscicida infection. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 230, 10–18. [Google Scholar] [CrossRef]
- Scapini, P.; Lapinet-Vera, J.A.; Gasperini, S.; Calzetti, F.; Bazzoni, F.; Cassatella, M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000, 177, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Sigh, J.; Lindenstrøm, T.; Buchmann, K. Expression of pro-inflammatory cytokines in rainbow trout (Oncorhynchus mykiss) during an infection with Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2004, 17, 75–86. [Google Scholar] [CrossRef]
- Piazzon, C.; Galindo-Villegas, J.; Pereiro, P.; Estensoro, I.; Calduch-Giner, J.A.; Gómez-Casado, E.; Novoa, B.; Mulero, V.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Differential Modulation of IgT and IgM upon parasitic, bacterial, viral and dietary challenges in a perciform fish. Front. Immunol. 2016, 7, 637. [Google Scholar] [CrossRef]
- Zhang, Y.-A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827. [Google Scholar] [CrossRef]
| Ingredients (%) | CTRL | NOPAP | MIX | PAP |
|---|---|---|---|---|
| Fishmeal LT70 | 20.00 | |||
| Fishmeal 60 (by-products) | 5.00 | |||
| Fish hydrolysate (by-products) | 5.00 | 5.00 | 5.00 | |
| Insect meal | 5.00 | 10.00 | 5.00 | |
| Microbial protein meal | 5.00 | 10.00 | 5.00 | |
| Yeast protein meal | 2.50 | 2.50 | 2.50 | |
| Feather meal hydrolysate | 5.00 | 5.00 | ||
| Porcine blood meal | 3.00 | 3.00 | ||
| Poultry meal 65 | 5.00 | 10.00 | 20.00 | |
| Microalgae meal (Spirulina) | 5.00 | 5.00 | ||
| Microalgae meal (Chlorella) | 0.50 | 0.50 | ||
| Soy protein concentrate | 9.00 | |||
| Pea protein concentrate | 4.10 | |||
| Wheat gluten | 4.00 | 4.00 | ||
| Corn gluten meal | 10.00 | 15.00 | 1.40 | 4.50 |
| Soybean meal 48 | 12.00 | |||
| Rapeseed meal | 4.00 | 11.50 | 7.00 | 5.70 |
| Wheat meal | 8.47 | |||
| Pea starch | 3.00 | 7.90 | 9.00 | 6.00 |
| Yellow peas | 6.20 | 3.00 | 7.03 | 14.58 |
| Fish oil | 6.00 | |||
| Salmon oil | 3.00 | 3.00 | 3.00 | |
| DHA-rich algae (Schizochytrium) | 3.20 | 3.70 | 3.60 | |
| Rapeseed oil | 8.26 | 8.50 | 6.30 | 6.00 |
| Rapeseed lecithin | 0.60 | 1.00 | 1.00 | 1.00 |
| Vitamin and mineral premix * | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin C (35%) | 0.10 | 0.10 | 0.10 | 0.10 |
| Brewer’s yeast | 4.00 | 4.00 | 4.00 | |
| Macroalgae MIX | 2.00 | 2.00 | 2.00 | |
| Antioxidant ** | 0.20 | 0.20 | 0.20 | 0.20 |
| Sodium propionate | 0.10 | 0.10 | 0.10 | 0.10 |
| Monocalcium phosphate | 2.00 | 2.50 | 2.20 | 1.90 |
| L-Tryptophan | 0.05 | 0.18 | 0.15 | 0.15 |
| DL-Methionine | 0.20 | 0.30 | 0.15 | |
| L-Taurine | 0.50 | 0.50 | 0.50 | |
| Yttrium oxide | 0.02 | 0.02 | 0.02 | 0.02 |
| Diet composition (as feed basis) | ||||
| Dry matter (%) | 94.11 | 93.64 | 93.47 | 93.23 |
| Ash (%) | 8.47 | 7.19 | 8.05 | 8.35 |
| Crude Protein (%) | 44.20 | 44.88 | 45.30 | 44.71 |
| Crude Lipid (%) | 17.84 | 17.62 | 16.41 | 16.29 |
| EPA + DHA (%) | 1.70 | 0.79 | 0.93 | 0.96 |
| Gross Energy (KJ/g) | 21.50 | 21.90 | 21.20 | 20.65 |
| NPE (MJ/kg feed) | 3.30 | 3.30 | 3.50 | 3.40 |
| PE ratio (MJ Gross Energy/g Crude Protein) | 21.2 | 21.1 | 21.0 | 21.1 |
| CTRL | NOPAP | MIX | PAP | p-Value | |
|---|---|---|---|---|---|
| IBW 1 (g) | 56.22 ± 0.80 | 55.81 ± 0.33 | 55.91 ± 0.53 | 55.80 ± 0.35 | 0.713 |
| FBW 2 (g) | 136.69 ± 3.27 | 134.63 ± 6.35 | 132.04 ± 0.85 | 128.43 ± 3.88 | 0.070 |
| Weight gain (g) | 80.47 ± 2.88 | 78.82 ± 6.48 | 76.13 ± 0.77 | 72.63 ± 4.20 | 0.090 |
| RGR 3 (% BW/d) | 1.16 ± 0.03 | 1.15 ± 0.07 | 1.12 ± 0.01 | 1.09 ± 0.05 | 0.104 |
| VFI 4 (%/day) | 1.63 ± 0.06 | 1.70 ± 0.11 | 1.76 ± 0.03 | 1.76 ± 0.07 | 0.082 |
| PER 5 | 1.57 ± 0.01 a | 1.50 ± 0.03 a | 1.39 ± 0.04 b | 1.33 ± 0.01 b | <0.0001 |
| FCR 6 | 1.40 ± 0.02 a | 1.48 ± 0.03 b | 1.56 ± 0.02 c | 1.62 ± 0.02 d | <0.0001 |
| Survival (%) | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| CTRL | NOPAP | MIX | PAP | p-Value | |
|---|---|---|---|---|---|
| Protease (%) | 7.27 ± 3.07 | 8.02 ± 3.01 | 7.69 ± 3.52 | 7.73 ± 3.35 | 0.783 |
| Anti-protease (%) | 81.33 ± 2.07 a | 79.86 ± 3.60 ab | 78.96 ± 5.31 b | 81.13 ± 1.40 a | 0.008 |
| IgM (absorbance) | 0.29 ± 0.08 ab | 0.33 ± 0.16 ab | 0.35 ± 0.11 a | 0.27 ± 0.14 b | 0.031 |
| Bactericidal activity (%) | 30.94 ± 7.48 | 35.69 ± 18.91 | 29.47 ± 6.06 | 31.39 ± 6.70 | 0.080 |
| CTRL | NOPAP | MIX | PAP | p-Value | |
|---|---|---|---|---|---|
| Catalase (U/mg protein) | 46.37 ± 9.35 a | 36.42 ± 24.16 b | 28.67 ± 10.71 b | 32.05 ± 15.19 b | <0.0001 |
| TBARS (nmol/g) | 12.81 ± 1.25 | 13.12 ± 1.42 | 13.54 ± 2.15 | 12.68 ± 0.98 | 0.081 |
| CTRL | NOPAP | MIX | PAP | p-Value | |
|---|---|---|---|---|---|
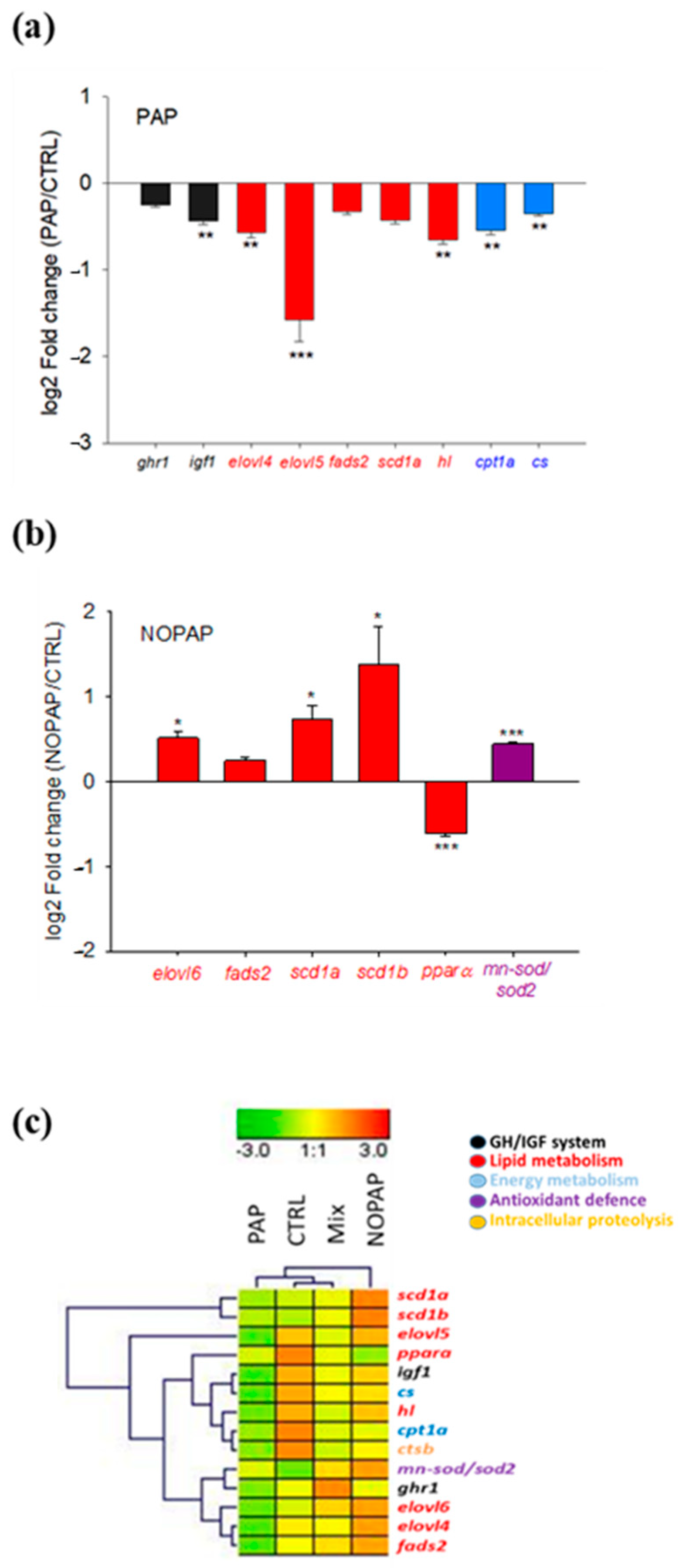
| ghr1 | 2.24 ± 0.25 | 2.23 ± 0.20 | 2.56 ± 0.26 | 1.92 ± 0.18 | 0.320 |
| ghr2 | 0.97 ± 0.07 | 0.98 ± 0.11 | 0.82 ± 0.10 | 0.81 ± 0.11 | 0.470 |
| igf1 | 7.82 ± 0.83 a | 7.47 ± 0.76 a | 6.88 ± 0.61 ab | 5.80 ± 0.53 b | 0.040 |
| igf2 | 2.77 ± 0.43 | 2.64 ± 0.62 | 2.42 ± 0.25 | 2.23 ± 0.40 | 0.836 |
| igfbp1a | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.118 |
| igfbp1b | 6.09 ± 1.59 | 5.02 ± 1.62 | 4.60 ± 0.84 | 5.19 ± 1.11 | 0.881 |
| igfbp2a | 1.52 ± 0.13 | 1.49 ± 0.11 | 1.42 ± 0.15 | 1.36 ± 0.11 | 0.802 |
| igfbp2b | 1.67 ± 0.17 | 2.03 ± 0.14 | 1.68 ± 0.15 | 1.54 ± 0.11 | 0.300 |
| igfbp4 | 0.58 ± 0.05 | 0.64 ± 0.04 | 0.69 ± 0.06 | 0.59 ± 0.04 | 0.417 |
| elovl1 | 7.90 ± 0.49 | 7.82 ± 0.58 | 7.01 ± 0.52 | 6.66 ± 0.34 | 0.252 |
| elovl4 | 0.18 ± 0.02 a | 0.19 ± 0.02 a | 0.16 ± 0.02 ab | 0.12 ± 0.01 b | 0.036 |
| elovl5 | 2.53 ± 0.59 a | 2.49 ± 0.66 a | 1.58 ± 0.39 ab | 0.81 ± 0.14 b | 0.022 |
| elovl6 | 1.71 ± 0.28 ab | 2.44 ± 0.40 a | 2.14 ± 0.52 ab | 1.32 ± 0.19 b | 0.050 |
| fads2 | 2.04 ± 0.34 | 2.42 ± 0.37 | 2.18 ± 0.46 | 1.60 ± 0.17 | 0.417 |
| scd1a | 0.16 ± 0.02 ab | 0.27 ± 0.06 a | 0.20 ± 0.04 ab | 0.12 ± 0.01 b | 0.044 |
| scd1b | 0.32 ± 0.06 a | 0.59 ± 0.13 b | 0.53 ± 0.11 ab | 0.32 ± 0.10 a | 0.049 |
| hl | 7.93 ± 0.62 a | 7.61 ± 0.65 a | 6.24 ± 0.59 ab | 5.25 ± 0.44 b | 0.007 |
| atgl | 0.47 ± 0.11 | 0.42 ± 0.09 | 0.41 ± 0.06 | 0.60 ± 0.20 | 0.688 |
| lpl | 8.09 ± 1.26 | 7.49 ± 0.85 | 8.63 ± 0.91 | 7.58 ± 0.70 | 0.823 |
| pla2g6 | 0.11 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.113 |
| cyp7a1 | 1.12 ± 0.18 | 1.33 ± 0.18 | 1.29 ± 0.16 | 1.45 ± 0.22 | 0.653 |
| pparα | 2.22 ± 0.18 a | 1.46 ± 0.09 b | 1.69 ± 0.14 ab | 1.61 ± 0.17 ab | 0.005 |
| pparβ | 0.69 ± 0.09 | 0.80 ± 0.06 | 0.82 ± 0.08 | 0.76 ± 0.10 | 0.694 |
| pparγ | 0.29 ± 0.02 | 0.32 ± 0.02 | 0.28 ± 0.02 | 0.27 ± 0.02 | 0.156 |
| cpt1a | 0.71 ± 0.05 a | 0.59 ± 0.04 ab | 0.58 ± 0.05 ab | 0.53 ± 0.05 b | 0.050 |
| hfabp | 45.2 ± 4.15 | 51.7 ± 3.54 | 53.4 ± 4.99 | 43.1 ± 1.87 | 0.179 |
| cs | 0.51 ± 0.03 a | 0.48 ± 0.03 ab | 0.46 ± 0.03 ab | 0.41 ± 0.03 b | 0.015 |
| sirt1 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.00 | 0.711 |
| sirt2 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.489 |
| ucp1 | 9.27 ± 0.94 | 11.0 ± 0.92 | 8.59 ± 0.74 | 8.35 ± 0.70 | 0.123 |
| gpx1 | 1.29 ± 0.07 | 1.15 ± 0.06 | 1.26 ± 0.11 | 1.19 ± 0.11 | 0.674 |
| gpx4 | 9.74 ± 1.14 | 11.4 ± 1.00 | 9.77 ± 1.00 | 8.48 ± 0.82 | 0.123 |
| prdx3 | 0.75 ± 0.06 | 0.87 ± 0.06 | 0.88 ± 0.07 | 0.76 ± 0.07 | 0.309 |
| prdx5 | 0.73 ± 0.06 | 0.69 ± 0.04 | 0.73 ± 0.07 | 0.64 ± 0.06 | 0.652 |
| cu-zn-sod/sod1 | 3.53 ± 0.23 | 3.73 ± 0.18 | 3.80 ± 0.30 | 3.36 ± 0.19 | 0.516 |
| mn-sod/sod2 | 0.83 ± 0.06 a | 1.13 ± 0.05 b | 1.04 ± 0.10 ab | 0.95 ± 0.09 ab | 0.050 |
| grp170 | 1.00 ± 0.12 | 1.03 ± 0.09 | 1.03 ± 0.17 | 0.80 ± 0.09 | 0.509 |
| grp94 | 3.51 ± 0.50 | 4.65 ± 0.39 | 4.05 ± 0.71 | 3.72 ± 0.42 | 0.442 |
| grp75 | 0.47 ± 0.04 | 0.45 ± 0.03 | 0.46 ± 0.04 | 0.43 ± 0.04 | 0.893 |
| ctsb | 1.75 ± 0.17 | 1.55 ± 0.09 | 1.46 ± 0.08 | 1.38 ± 0.06 | 0.099 |
| ctsd | 1.07 ± 0.08 | 1.21 ± 0.12 | 0.95 ± 0.11 | 1.03 ± 0.09 | 0.344 |
| ctsl | 5.44 ± 0.56 | 6.14 ± 0.33 | 5.83 ± 0.47 | 5.35 ± 0.49 | 0.618 |
| CTRL | NOPAP | MIX | PAP | p-Value | |
|---|---|---|---|---|---|
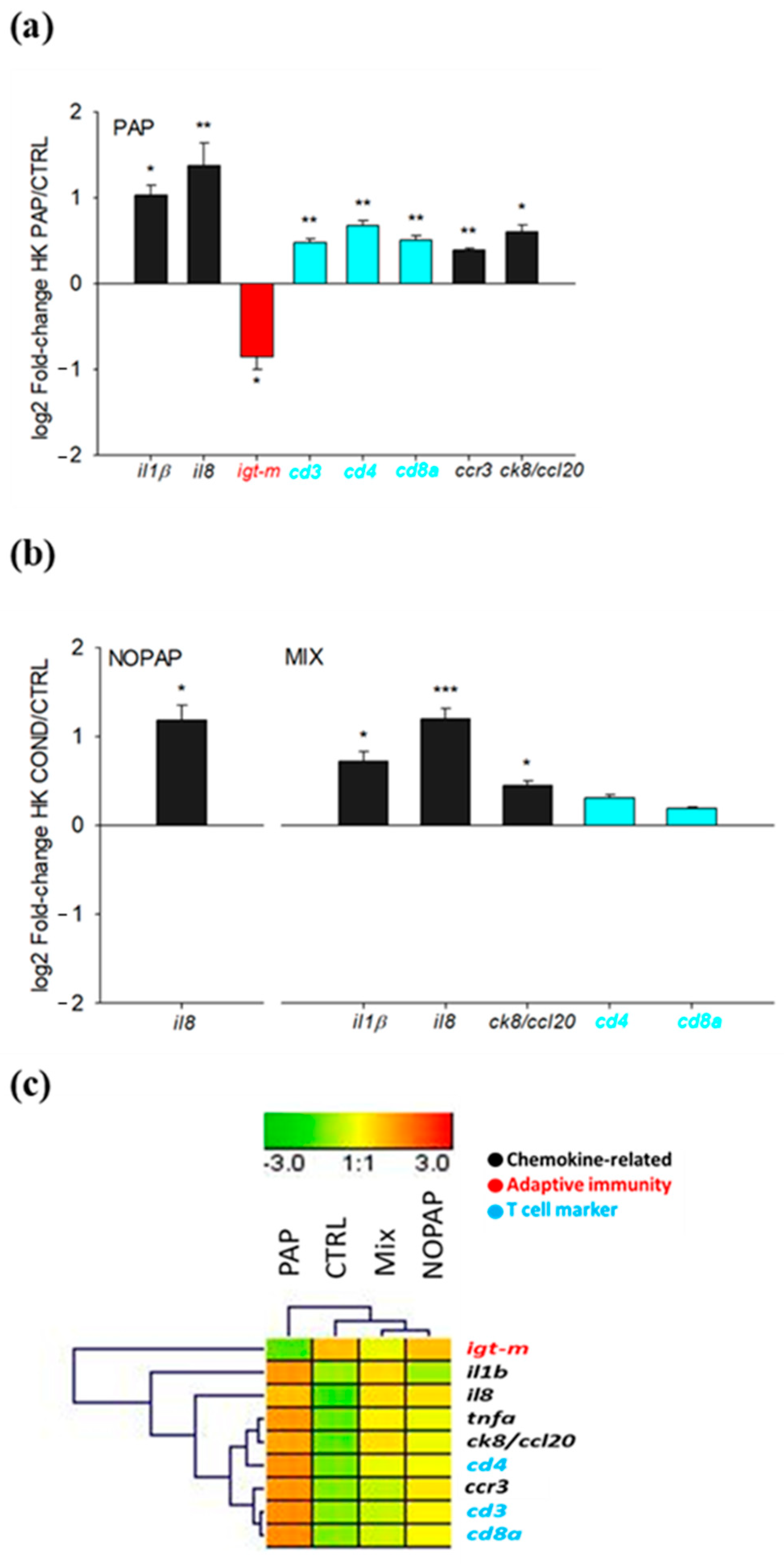
| il1β | 0.38 ± 0.08 a | 0.39 ± 0.04 a | 0.62 ± 0.10 b | 0.77 ± 0.09 c | 0.005 |
| il6 | 0.07 ± 0.01 | 0.08 ± 0.02 | 0.07 ± 0.01 | 0.09 ± 0.02 | 0.170 |
| il7 | 1.01 ± 0.11 | 1.02 ± 0.08 | 0.99 ± 0.08 | 1.18 ± 0.08 | 0.460 |
| il8 | 0.09 ± 0.01 a | 0.22 ± 0.03 b | 0.22 ± 0.02 b | 0.25 ± 0.05 b | <0.001 |
| il10 | 1.01 ± 0.04 | 1.22 ± 0.10 | 1.26 ± 0.11 | 1.09 ± 0.08 | 0.233 |
| il12 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.622 |
| il15 | 0.34 ± 0.04 | 0.34 ± 0.02 | 0.32 ± 0.02 | 0.36 ± 0.04 | 0.867 |
| il34 | 3.49 ± 0.31 | 3.31 ± 0.26 | 3.04 ± 0.09 | 3.57 ± 0.16 | 0.401 |
| tnfα | 0.24 ± 0.02 a | 0.28 ± 0.02 ab | 0.29 ± 0.02 ab | 0.34 ± 0.04 b | 0.030 |
| ccr3 | 7.02 ± 0.62 a | 8.26 ± 0.62 ab | 7.51 ± 0.57 ab | 9.21 ± 0.39 b | 0.044 |
| ck8/ccl20 | 1.57 ± 0.15 a | 1.96 ± 0.19 ab | 2.14 ± 0.26 ab | 2.39 ± 0.33 b | 0.027 |
| igm | 264.3 ± 32.5 | 295.2 ± 28.0 | 230.6 ± 21.4 | 314.2 ± 28 | 0.175 |
| igt-m | 3.47 ± 0.58 a | 3.44 ± 0.39 a | 2.77 ± 0.34 b | 1.92 ± 0.32 c | 0.048 |
| a2m | 0.11 ± 0.01 | 0.13 ± 0.03 | 0.12 ± 0.02 | 0.15 ± 0.03 | 0.300 |
| b2m | 143.8 ± 6.8 | 159.6 ± 11.8 | 133.1 ± 8.97 | 136.6 ± 14.7 | 0.359 |
| c3 | 0.003 ± 0.001 | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.450 |
| casp3 | 0.96 ± 0.05 | 1.18 ± 0.06 | 1.01 ± 0.06 | 1.10 ± 0.08 | 0.242 |
| cd3 | 3.33 ± 0.20 a | 3.92 ± 0.28 ab | 3.59 ± 0.41 ab | 4.63 ± 0.41 b | 0.049 |
| cd4 | 1.08 ± 0.09 a | 1.37 ± 0.10 ab | 1.34 ± 0.16 ab | 1.73 ± 0.15 b | 0.008 |
| cd8α | 1.58 ± 0.09 a | 1.93 ± 0.23 ab | 1.82 ± 0.21 ab | 2.27 ± 0.23 b | 0.024 |
| cd8β | 0.43 ± 0.04 | 0.54 ± 0.06 | 0.47 ± 0.07 | 0.56 ± 0.06 | 0.322 |
| zap70 | 2.33 ± 0.20 | 2.84 ± 0.26 | 2.69 ± 0.31 | 2.85 ± 0.30 | 0.495 |
| csf1r1 | 3.04 ± 0.25 | 3.42 ± 0.28 | 3.78 ± 0.51 | 3.20 ± 0.21 | 0.452 |
| mrc1 | 13.83 ± 1.27 | 16.44 ± 2.19 | 13.71 ± 0.79 | 15.00 ± 1.02 | 0.725 |
| tlr2 | 4.90 ± 0.37 | 6.15 ± 0.42 | 6.08 ± 0.52 | 5.86 ± 0.42 | 0.172 |
| tlr5 | 1.17 ± 0.08 | 1.06 ± 0.06 | 1.02 ± 0.02 | 1.07 ± 0.07 | 0.473 |
| tlr9 | 1.86 ± 0.19 | 2.18 ± 0.23 | 2.36 ± 0.30 | 2.45 ± 0.15 | 0.266 |
| clec10a | 2.48 ± 0.56 | 2.49 ± 0.55 | 2.20 ± 0.52 | 2.93 ± 0.72 | 0.950 |
| fcl | 0.06 ± 0.01 | 0.10 ± 0.02 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.286 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, A.M.; Calduch-Giner, J.À.; Pereira, G.V.; Gonçalves, A.T.; Dias, J.; Johansen, J.; Silva, T.; Naya-Català, F.; Piazzon, C.; Sitjà-Bobadilla, A.; et al. Sustainable Fish Meal-Free Diets for Gilthead Sea Bream (Sparus aurata): Integrated Biomarker Response to Assess the Effects on Growth Performance, Lipid Metabolism, Antioxidant Defense and Immunological Status. Animals 2024, 14, 2166. https://doi.org/10.3390/ani14152166
Fernandes AM, Calduch-Giner JÀ, Pereira GV, Gonçalves AT, Dias J, Johansen J, Silva T, Naya-Català F, Piazzon C, Sitjà-Bobadilla A, et al. Sustainable Fish Meal-Free Diets for Gilthead Sea Bream (Sparus aurata): Integrated Biomarker Response to Assess the Effects on Growth Performance, Lipid Metabolism, Antioxidant Defense and Immunological Status. Animals. 2024; 14(15):2166. https://doi.org/10.3390/ani14152166
Chicago/Turabian StyleFernandes, Ana M., Josep Àlvar Calduch-Giner, Gabriella V. Pereira, Ana Teresa Gonçalves, Jorge Dias, Johan Johansen, Tomé Silva, Fernando Naya-Català, Carla Piazzon, Ariadna Sitjà-Bobadilla, and et al. 2024. "Sustainable Fish Meal-Free Diets for Gilthead Sea Bream (Sparus aurata): Integrated Biomarker Response to Assess the Effects on Growth Performance, Lipid Metabolism, Antioxidant Defense and Immunological Status" Animals 14, no. 15: 2166. https://doi.org/10.3390/ani14152166
APA StyleFernandes, A. M., Calduch-Giner, J. À., Pereira, G. V., Gonçalves, A. T., Dias, J., Johansen, J., Silva, T., Naya-Català, F., Piazzon, C., Sitjà-Bobadilla, A., Costas, B., Conceição, L. E. C., Fernandes, J. M. O., & Pérez-Sánchez, J. (2024). Sustainable Fish Meal-Free Diets for Gilthead Sea Bream (Sparus aurata): Integrated Biomarker Response to Assess the Effects on Growth Performance, Lipid Metabolism, Antioxidant Defense and Immunological Status. Animals, 14(15), 2166. https://doi.org/10.3390/ani14152166








