Investigation of Transmission and Evolution of PEDV Variants and Co-Infections in Northeast China from 2011 to 2022
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Gene Acquisition
2.2. PCR Detection of PEDV and Other Pathogens
2.3. PEDV S Gene Sequencing and Analysis
2.4. Recombination Analysis
2.5. Phylogenetic Analysis
2.6. Molecular Modeling and Analysis of the PEDV S Protein
2.7. Statistical Analysis of Correlation
3. Results
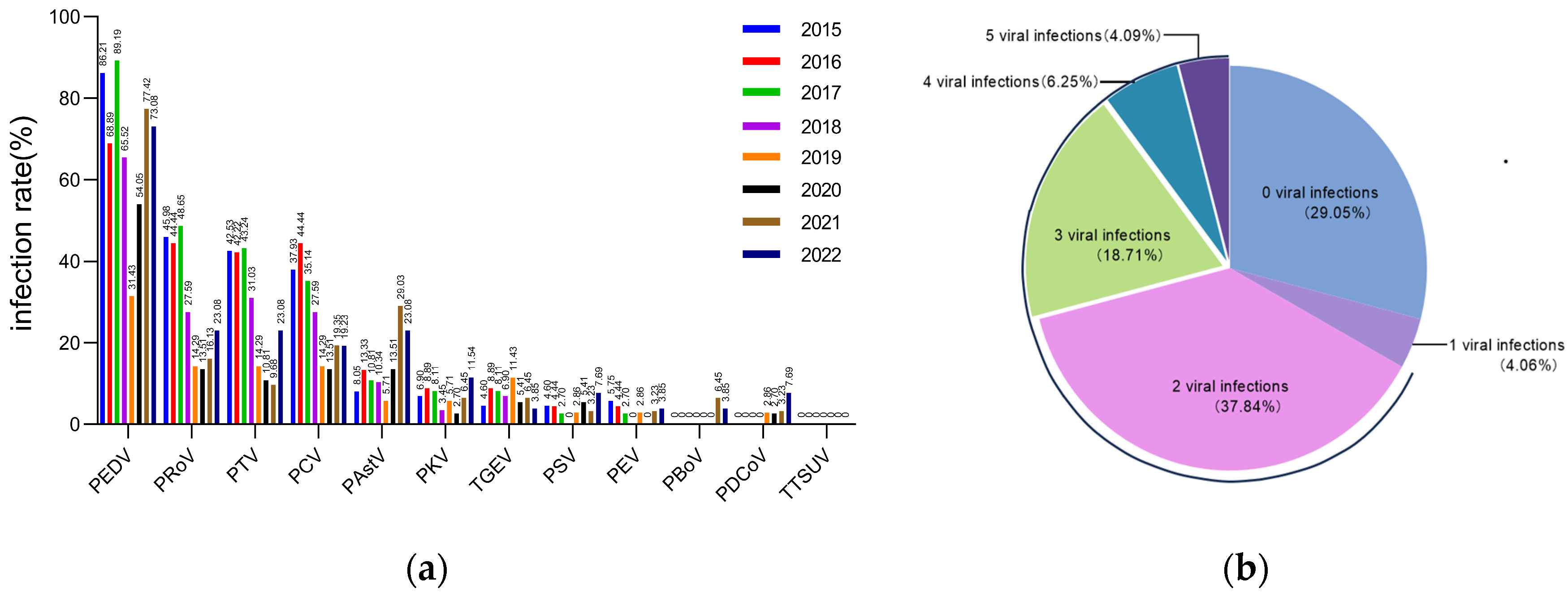
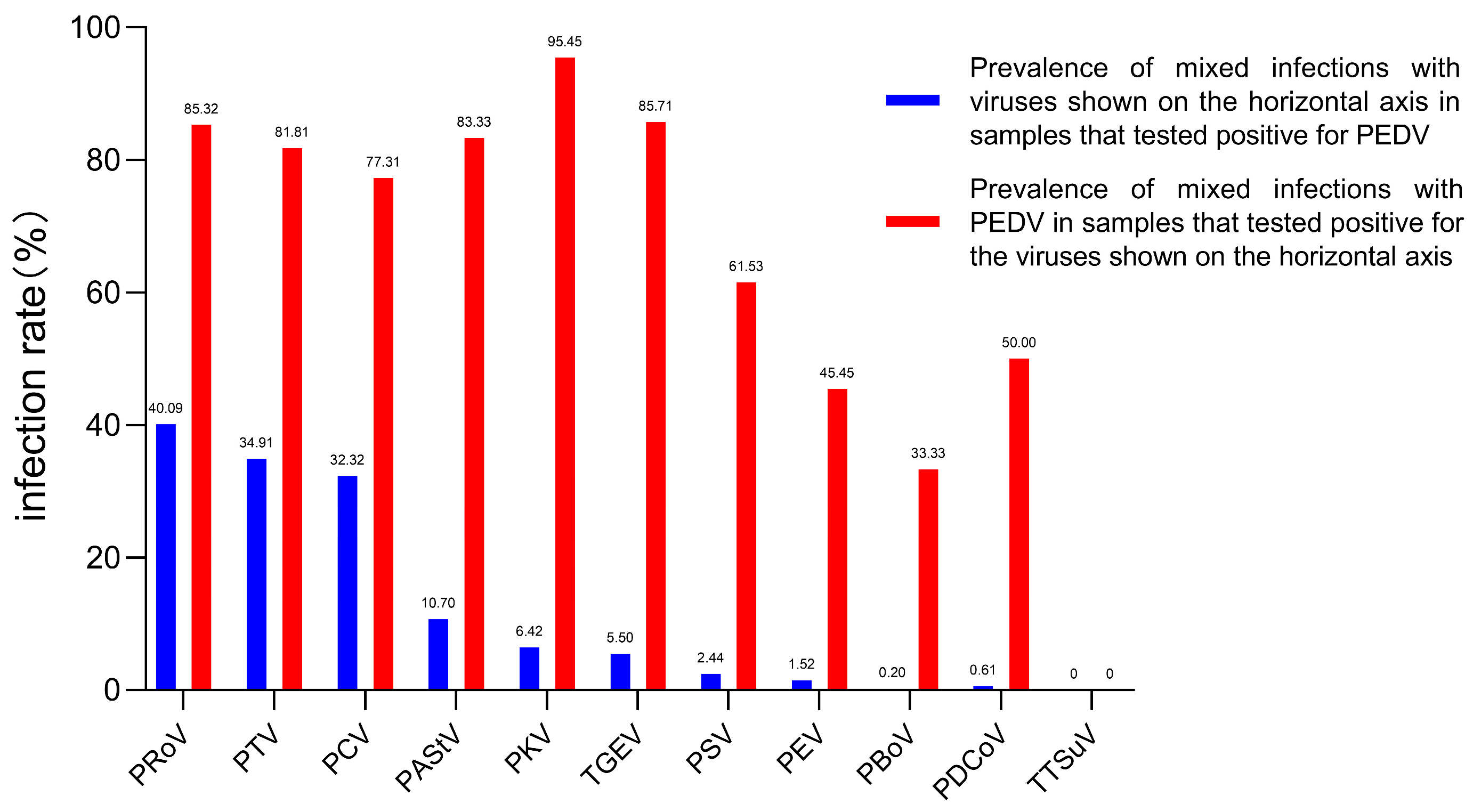
3.1. Co-Infection of PEDV with Multiple Pathogens in Piglets with Diarrhea
3.2. PEDV S Gene Sequencing and Analysis
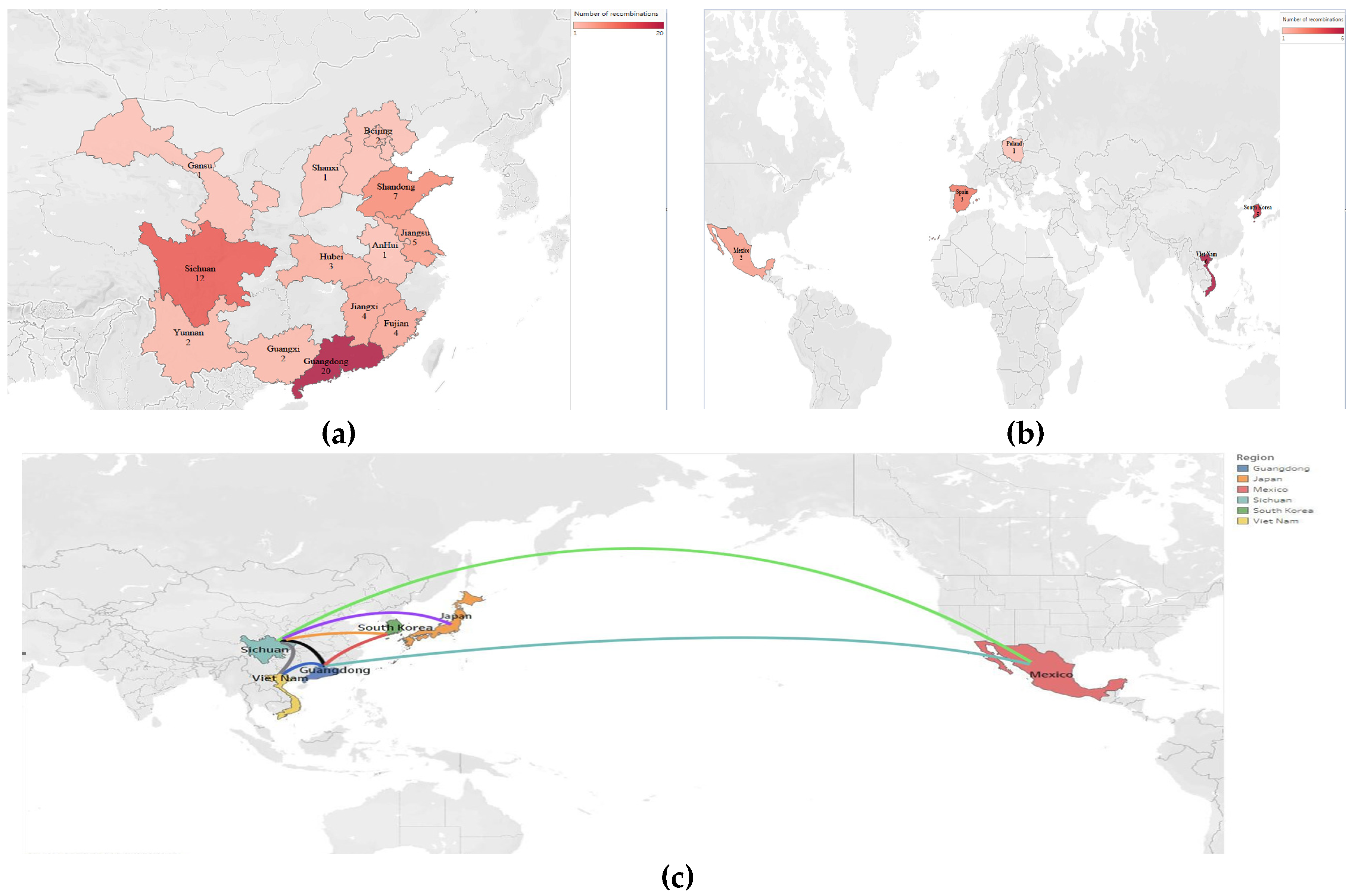
3.3. Recombination Analysis
3.4. Phylogenetic Analysis
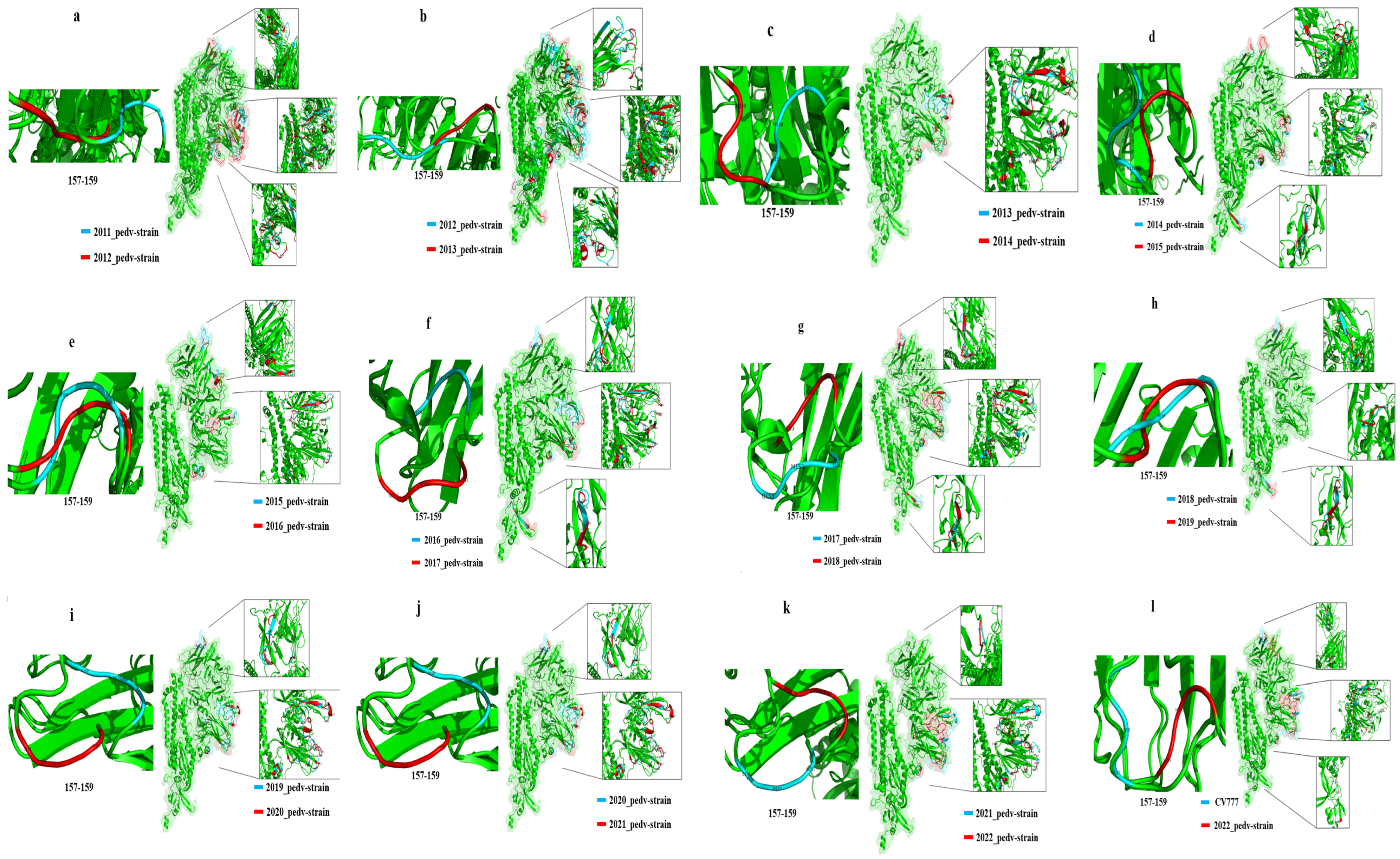
3.5. PEDV S-Protein Modeling Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oldham, J. Letter to the editor. Pig Farming 1972, 10, 72–73. [Google Scholar] [CrossRef]
- Duy, D.T.; Nguyen, T.T.; Puranaveja, S.; Thanawongnuwech, R. Genetic Characterization of Porcine Epidemic Diarrhea Virus (PEDV) Isolates from Southern Vietnam during 2009–2010 Outbreaks. Thai J. Vet. Med. 2011, 41, 55–64. [Google Scholar] [CrossRef]
- Takahashi, K.; Okada, K.; Ohshima, K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Nihon Juigaku Zasshi 1983, 45, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, M.; Tsuda, T.; Yamazaki, K.; Yoshida, K.; Nakazawa, M.; Sato, K.; Minami, T.; Iwashita, K.; Watanabe, M.; Suzuki, Y.; et al. An immunohistochemical investigation of porcine epidemic diarrhoea. J. Comp. Pathol. 1995, 113, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ducatelle, R.; Coussement, W.; Debouck, P. Pathology of experimental CV777 coronavirus enteritis in piglets II. Electron microscopic study. Vet. Pathol. 1982, 19, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, C.; Shi, H.; Qiu, H.; Liu, S.; Chen, X.; Zhang, Z.; Feng, L. Molecular epidemiology of porcine epidemic diarrhea virus in China. Arch. Virol. 2010, 155, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.; Liu, Y.; Pan, Y.; Deng, F.; Song, Y.; Tang, X.; He, Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Infect. Dis. 2012, 18, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Kong, F.; Hou, Y.J.; Wang, Q. Crucial mutation in the exoribonuclease domain of nsp14 of PEDV leads to high genetic instability during viral replication. Cell Biosci. 2021, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.R.; Leibowitz, J.L. Coronavirus Pathogenesis. Adv. Virus Res. 2011, 81, 85–164. [Google Scholar] [PubMed]
- Kocherhans, R.; Bridgen, A.; Ackermann, M.; Tobler, K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes 2001, 23, 137–144. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef] [PubMed]
- ShibataI, T.; Tsuda, T.; Mori, M. Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Vet Microbiol. 2000, 72, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.K.; Whittaker, G.R. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015, 202, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Terada, Y.; Enjuanes, L.; Ohashi, S.; Kamitani, W. S1 Subunit of Spike Protein from a Current Highly Virulent Porcine Epidemic Diarrhea Virus Is an Important Determinant of Virulence in Piglets. Viruses 2018, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lin, C.; Yokoyama, M.; Yount, B.; Marthaler, D.; Douglas, A.; Ghimire, S.; Qin, Y.; Baric, R.; Saif, L.; et al. Deletion of a 197-Amino-Acid Region in the N-Terminal Domain of Spike Protein Attenuates Porcine Epidemic Diarrhea Virus in Piglets. J. Virol. 2017, 91, e00227-17. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Ye, G.; Liu, Q.; Navid, M.T.; Zhong, X.; Li, Y.; Wan, C.; Xiao, S.; He, Q.; Fu, Z.F.; et al. Identification and Comparison of Receptor Binding Characteristics of the Spike Protein of Two Porcine Epidemic Diarrhea Virus Strains. Viruses 2016, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Heald-Sargent, T.; Gallagher, T. Ready, set, fuse! The coronavirus spike protein and acquisition of fusion competence. Viruses 2012, 4, 557–580. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cruz, D.; Shin, H. Clathrin- and serine proteases-dependent uptake of porcine epidemic diarrhea virus into Vero cells. Virus Res. 2014, 191, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Shi, D.; Shi, H.; Zhang, X.; Li, C.; Chi, Y.; Feng, L. Detection and molecular diversity of spike gene of porcine epidemic diarrhea virus in China. Viruses 2013, 5, 2601–2613. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, P.; Guo, L.; Liu, Y.; Du, Y.; Ren, S.; Li, J.; Zhang, Y.; Fan, Y.; Huang, B.; et al. Porcine epidemic diarrhea virus variants with high pathogenicity, China. Emerg. Infect. Dis. 2013, 19, 2048–2049. [Google Scholar] [CrossRef]
- Wang, L.; Byrum, B.; Zhang, Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014, 20, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, X.; Guo, D.; Cao, J.; Cheng, L.; Li, X.; Zou, D.; Zhang, Y.; Xu, J.; Wu, X.; et al. Porcine epidemic diarrhea virus E protein suppresses RIG-I signaling-mediated interferon-β production. Vet. Microbiol. 2021, 254, 108994. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hou, Y.; Prarat, M.; Zhang, Y.; Wang, Q. New variants of porcine epidemic diarrhea virus with large deletions in the spike protein, identified in the United States, 2016–2017. Arch. Virol. 2018, 163, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Receptor recognition mechanisms of coronaviruses: A decade of structural studies. J Virol. 2015, 89, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Murakami, S.; Takahashi, O.; Kodera, A.; Masuda, T.; Itoh, S.; Miyazaki, A.; Ohashi, S.; Tsutsui, T. Molecular characterization of pig epidemic diarrhoea viruses isolated in Japan from 2013 to 2014. Infect. Genet. Evol. 2015, 36, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yim-Im, W.; Chen, Q.; Zheng, Y.; Schumacher, L.; Huang, H.; Gauger, P.; Harmon, K.; Li, G. Identification of porcine epidemic diarrhea virus variant with a large spike gene deletion from a clinical swine sample in the United States. Virus Genes 2018, 54, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Diep, N.V.; Norimine, J.; Sueyoshi, M.; Lan, N.T.; Yamaguchi, R. Novel Porcine Epidemic Diarrhea Virus (PEDV) Variants with Large Deletions in the Spike (S) Gene Coexist with PEDV Strains Possessing an Intact S Gene in Domestic Pigs in Japan: A New Disease Situation. PLoS ONE 2017, 12, e0170126. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; van Kuppeveld, F.J.M.; He, Q.; Rottier, P.J.M.; Bosch, B.-J. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016, 226, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Li, C.; Qi, S.; Yang, D.; Jiang, N.; Yin, B.; Guo, D.; Kong, F.; Yuan, D.; Feng, L.; et al. A molecular epidemiological investigation of PEDV in China: Characterization of co-infection and genetic diversity of S1-based genes. Transbound. Emerg. Dis. 2020, 67, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zhou, X.; Peng, Q.; Chen, Y.; Zhang, F.; Huang, T.; Zhang, T.; Li, A.; Huang, D.; Wu, Q.; et al. Newly Emerged Porcine Deltacoronavirus Associated with Diarrhoea in Swine in China: Identification, Prevalence and Full-Length Genome Sequence Analysis. Transbound. Emerg. Dis. 2015, 62, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Krumbholz, A.; Henke, A.; Birch-Hirschfeld, E.; Stelzner, A.; Doherty, M.; Hoey, E.; Dauber, M.; Prager, D.; Wurm, R. Detection of porcine enteroviruses by nRT-PCR: Differentiation of CPE groups I-III with specific primer sets. J. Virol. Methods 2000, 88, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, R.; Tang, X.; Wu, C.; He, Q.; Zhao, Z.; Chen, H.; Wu, B. Occurrence and investigation of enteric viral infections in pigs with diarrhea in China. Arch. Virol. 2013, 158, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tang, C.; Yue, H.; Ren, Y.; Song, Z. Viral metagenomics analysis demonstrates the diversity of viral flora in piglet diarrhoeic faeces in China. J. Gen. Virol. 2014, 95, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, L.; Zheng, Y.; Zhang, J.; Guo, B.; Yoon, K.; Gauger, P.; Harmon, K.; Main, R.; Li, G. Metagenomic analysis of the RNA fraction of the fecal virome indicates high diversity in pigs infected by porcine endemic diarrhea virus in the United States. Virol. J. 2018, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gauger, P.; Stafne, M.; Thomas, J.; Arruda, P.; Burrough, E.; Madson, D.; Brodie, J.; Magstadt, D.; Derscheid, R.; et al. Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5-day-old neonatal piglets. Virology 2015, 482, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Chen, J.; Feng, L.; Ni, H. Epidemiological survey of porcine epidemic diarrhea virus in 2014. Chin. J. Prev. Vet. Med. 2016, 38, 335–338. [Google Scholar]
- Wang, E.; Guo, D.; Li, C.; Wei, S.; Wang, Z.; Liu, Q.; Zhang, B.; Kong, F.; Feng, L.; Sun, D. Molecular Characterization of the ORF3 and S1 Genes of Porcine Epidemic Diarrhea Virus Non S-INDEL Strains in Seven Regions of China, 2015. PLoS ONE 2016, 11, e0160561. [Google Scholar] [CrossRef]
- Qi, S.; Su, M.; Guo, D.; Li, C.; Wei, S.; Feng, L.; Sun, D. Molecular detection and phylogenetic analysis of porcine circovirus type 3 in 21 Provinces of China during 2015–2017. Transbound. Emerg. Dis. 2019, 66, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Elschner, M.; Prudlo, J.; Hotzel, H.; Otto, P.; Sachse, K. Nested reverse transcriptase-polymerase chain reaction for the detection of group A rotaviruses. J. Vet. Med. B Infect. Dis. Vet. Public Health 2002, 49, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, G.; Opriessnig, T.; Wang, Z.; Yang, Z.; Jiang, Y. Development and validation of a multiplex conventional PCR assay for simultaneous detection and grouping of porcine bocaviruses. J. Virol. Methods 2016, 236, 164–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chu, D.K.; Poon, L.L.; Guan, Y.; Peiris, J.S. Novel astroviruses in insectivorous bats. J. Virol. 2008, 82, 9107–9114. [Google Scholar] [CrossRef] [PubMed]
- Van Dung, N.; Anh, P.H.; Van Cuong, N.; Hoa, N.T.; Carrique-Mas, J.; Hien, V.B.; Sharp, C.; Rabaa, M.; Berto, A.; Campbell, J.; et al. Large-scale screening and characterization of enteroviruses and kobuviruses infecting pigs in Vietnam. J. Gen. Virol. 2016, 97, 378–388. [Google Scholar] [CrossRef] [PubMed]
- den Dunnen, J.; Antonarakis, S. Nomenclature for the description of human sequence variations. Hum. Genet. 2001, 109, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Varsani, A.; Roumagnac, P.; Botha, G.; Maslamoney, S.; Schwab, T.; Kelz, Z.; Kumar, V.; Murrell, B. RDP5: A computer program for analyzing recombination in, and removing signals of recombination from, nucleotide sequence datasets. Virus Evol. 2021, 7, veaa087. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, F.; Yuan, Y.; Zeng, X.; Wei, Z.; Zhu, L.; Sun, B.; Xie, Q.; Cao, Y.; Ma, J.; et al. Sequence and phylogenetic analysis of nucleocapsid genes of porcine epidemic diarrhea virus (PEDV) strains in China. Arch Virol. 2013, 158, 1267–1273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Sungsuwan, S.; Jongkaewwattana, A.; Jaru-Ampornpan, P. Nucleocapsid proteins from other swine enteric coronaviruses differentially modulate PEDV replication. Virology 2020, 540, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Villanova, F.; Cui, S.; Ai, X.; Leal, É. Analysis of full-length genomes of porcine teschovirus (PTV) and the effect of purifying selection on phylogenetic trees. Arch. Virol. 2016, 161, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.; Cui, S.J.; Hu, S.; Zhang, Z.; Guo, Q.; Zell, R. Isolation and characterization of the first Chinese strain of porcine Teschovirus-8. J. Virol. Methods 2010, 167, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ruan, H.; Qiao, S.; Deng, R.; Zhang, G. Co-infection status of porcine circoviruses (PCV2 and PCV3) and porcine epidemic diarrhea virus (PEDV) in pigs with watery diarrhea in Henan province, central China. Microb. Pathog. 2020, 142, 104047. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K. Transcriptional analysis of porcine circovirus type 2. Virology 2003, 305, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, K.; Hou, Y.; Li, H.; Li, X.; Yu, L.; Jiang, Y.; Gao, F.; Tong, W.; Yu, H.; et al. Genetic evolution analysis and pathogenicity assessment of porcine epidemic diarrhea virus strains circulating in part of China during 2011–2017. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2019, 69, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, C.; Peng, O.; Ashraf, U.; Xu, Q.; Gong, L.; Fan, B.; Zhang, Y.; Xu, Z.; Xue, C.; et al. Global Dynamics of Porcine Enteric Coronavirus PEDV Epidemiology, Evolution, and Transmission. Mol. Biol. Evol. 2023, 40, msad052. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Sun, L.; Wang, X.; Xiao, M.; Zeng, L.; Wang, H.; Yang, H.; Lin, F.; Wang, C.; Qin, L.; et al. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus strains circulating in China from 2020 to 2021. BMC Vet. Res. 2022, 18, 392. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yang, Y.; Zhao, J.; Geng, N.; Liu, K.; Zhao, Y.; Wang, F.; Liu, S.; Li, N.; Meng, F.; et al. Molecular epidemiological survey of porcine epidemic diarrhea in some areas of Shandong and genetic evolutionary analysis of S gene. Front. Vet. Sci. 2022, 9, 1015717. [Google Scholar] [CrossRef]
- Chen, J.; Tian, L.; Liu, Y.; Sun, Y.; Li, Z.; Cai, X.; Meng, Q.; Qiao, J. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus in Xinjiang, China, from 2020 to 2022. Arch. Virol. 2024, 169, 96. [Google Scholar] [CrossRef]
- Karte, C.; Platje, N.; Bullermann, J.; Beer, M.; Höper, D.; Blome, S. Re-emergence of porcine epidemic diarrhea virus in a piglet-producing farm in northwestern Germany in 2019. BMC Vet. Res. 2020, 16, 329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Shi, D.; Shi, H.; Zhang, X.; Yuan, J.; Jiang, S.; Feng, L. Immunogenicity and antigenic relationships among spike proteins of porcine epidemic diarrhea virus subtypes G1 and G2. Arch. Virol. 2016, 161, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Gerber, P.F.; Shen, H.; de Castro, A.; Zhang, J.; Chen, Q.; Halbur, P. Evaluation of the efficacy of a commercial inactivated genogroup 2b-based porcine epidemic diarrhea virus (PEDV) vaccine and experimental live genogroup 1b exposure against 2b challenge. Vet. Res. 2017, 48, 69. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Dong, S.; Chen, B.; Liu, Y.; Li, F.; Si, F.; Xie, C.; Li, Z. Antigenicity Alternations of Variant PEDV S Protein Disclosed by Linear B Cell Epitope Mapping. Viruses 2022, 14, 1371. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Weaver, S.; Tegally, H.; San, J.; Shank, S.; Wilkinson, E.; Lucaci, A.; Giandhari, J.; Naidoo, S.; Pillay, Y.; et al. The emergence and ongoing convergent evolution of the SARS-CoV-2 N501Y lineages. Cell 2021, 184, 5189–5200.e7. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Bollen, N.; Xu, Y.; Zhao, J.; Dellicour, S.; Yan, Z.; Gong, W.; Zhang, C.; Zhang, L.; Lu, M.; et al. Phylogeography Reveals Association between Swine Trade and the Spread of Porcine Epidemic Diarrhea Virus in China and across the World. Mol. Biol. Evol. 2022, 39, msab364. [Google Scholar] [CrossRef] [PubMed]
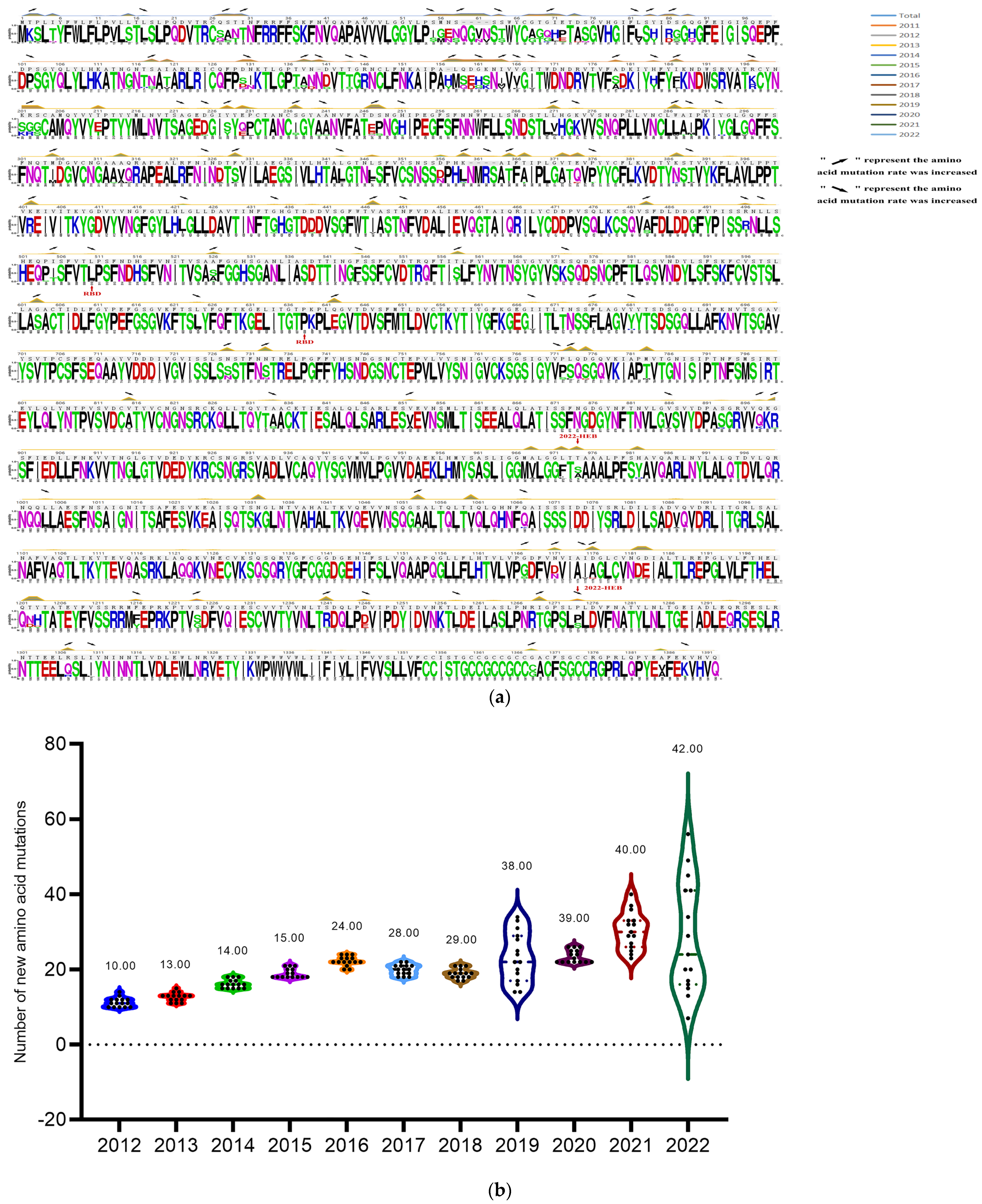


| Year | New Amino Acid Mutations |
|---|---|
| 2012–2013 | Y293H, Q317P, T373I, I505T, S576T, K639I, T833I, V852A, S974A, P1274S |
| 2013–2014 | L510P, Q898K |
| 2014–2015 | V556A, R145L |
| 2015–2016 | L59S, T365I, T496R, P504S, I505T, P772S, V852A, S974A |
| 2016–2017 | I325T, S558T, A834S, V1343A |
| 2017–2018 | P1274S |
| 2018–2019 | L271V, A288T, M289T, N451T, S526T, V886A, S974A, A1095S, A1263S |
| 2019–2020 | G296V |
| 2020–2021 | D327A |
| 2021–2022 | S974A |
| Year of Comparison | NTD (aa) | RBD (aa) |
|---|---|---|
| CV777-HEB (2022) | 55–72, 85–117, 137–140, 156–162, 188, 199–210, 211–231 | 528–536 |
| 2021-HEB (2022) | 55–64, 114–117, 132–141, 151–164, 184, 199–201, 209–213 | 563–565 |
| 2020–2021 | 55–59, 65–68, 111–113, 132–135, 150–160, 184, 195–197, 206–218 | 520–529, 556–557 |
| 2019–2020 | 157–160 | - |
| 2018–2019 | 157–160 | 520–553 |
| 2017–2018 | 57–64, 68–72, 115–117, 138–141, 156–164, 199–201, 210–225 | 524–530 |
| 2016–2017 | 55–60, 65–68, 111–113, 132–135, 151–160, 195–197, 205–219 | 520–527 |
| 2015–2016 | 56–61, 112–114, 134–135, 207–211 | 526 |
| 2014–2015 | 56–61, 112–114, 134–135, 207–211 | 526 |
| 2013–2014 | 52–56, 65–68, 111–113, 126–135, 153–159, 196–198, 206–220 | 520–529, 556–557 |
| 2012–2013 | 38–40, 43–51, 56–76, 84–88, 99–102, 115–117, 133–147, 155–161, 177–179, 184–185, 197–202, 209–217 | 522–532, 560–567 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Ma, X.; Yang, J.; Wei, Z.; Li, J.; Jiang, Y.; Cui, W.; Shan, Z.; Tang, L. Investigation of Transmission and Evolution of PEDV Variants and Co-Infections in Northeast China from 2011 to 2022. Animals 2024, 14, 2168. https://doi.org/10.3390/ani14152168
Zhao F, Ma X, Yang J, Wei Z, Li J, Jiang Y, Cui W, Shan Z, Tang L. Investigation of Transmission and Evolution of PEDV Variants and Co-Infections in Northeast China from 2011 to 2022. Animals. 2024; 14(15):2168. https://doi.org/10.3390/ani14152168
Chicago/Turabian StyleZhao, Feipeng, Xin’ao Ma, Jianfeng Yang, Zhiying Wei, Jiaxuan Li, Yanping Jiang, Wen Cui, Zhifu Shan, and Lijie Tang. 2024. "Investigation of Transmission and Evolution of PEDV Variants and Co-Infections in Northeast China from 2011 to 2022" Animals 14, no. 15: 2168. https://doi.org/10.3390/ani14152168
APA StyleZhao, F., Ma, X., Yang, J., Wei, Z., Li, J., Jiang, Y., Cui, W., Shan, Z., & Tang, L. (2024). Investigation of Transmission and Evolution of PEDV Variants and Co-Infections in Northeast China from 2011 to 2022. Animals, 14(15), 2168. https://doi.org/10.3390/ani14152168







