Establishment of a Steatosis Model in LMH Cells, Chicken Embryo Hepatocytes, and Liver Tissues Based on a Mixture of Sodium Oleate and Palmitic Acid
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Cell Isolation, Culture, and Treatment
2.3. TG Content Detection
2.4. Oil Red O Staining
2.5. RNA Isolation, RNA-Seq Sequencing, and Read Mapping
2.6. Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction
2.7. Bioinformatics and Statistical Analysis
3. Results
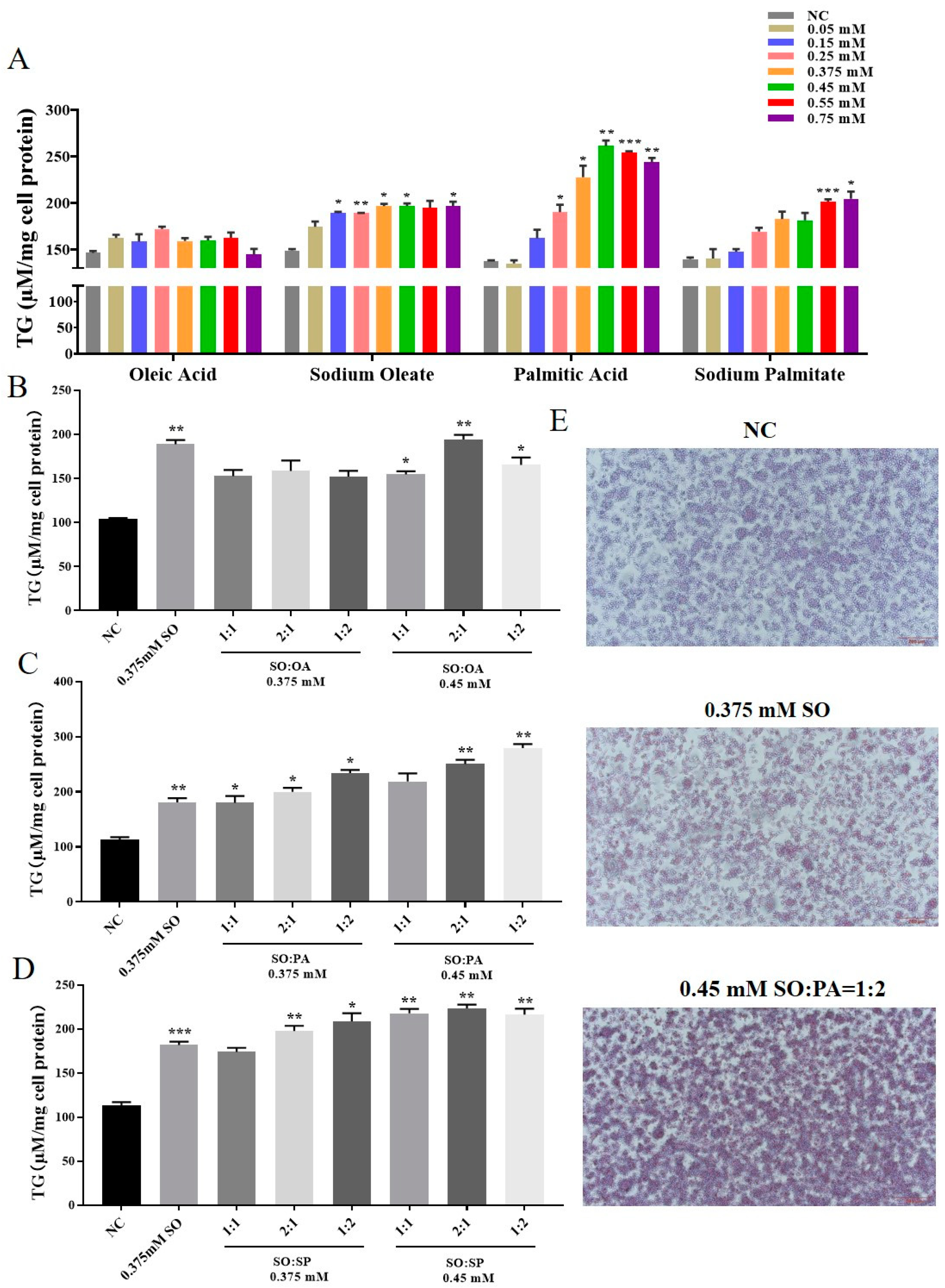
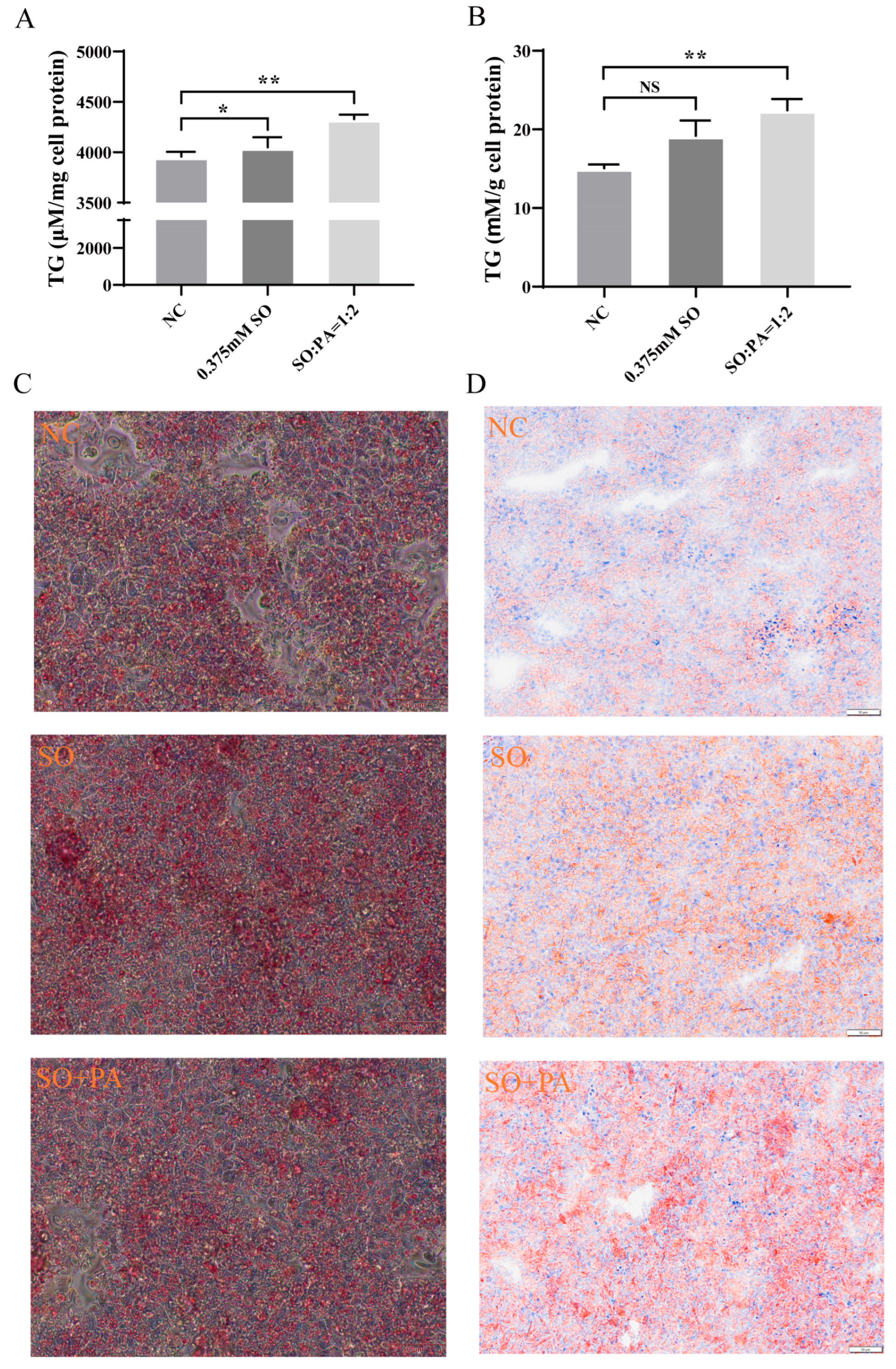
3.1. Identification of the Optimal Conditions for STEATOSIS in LMH Cells
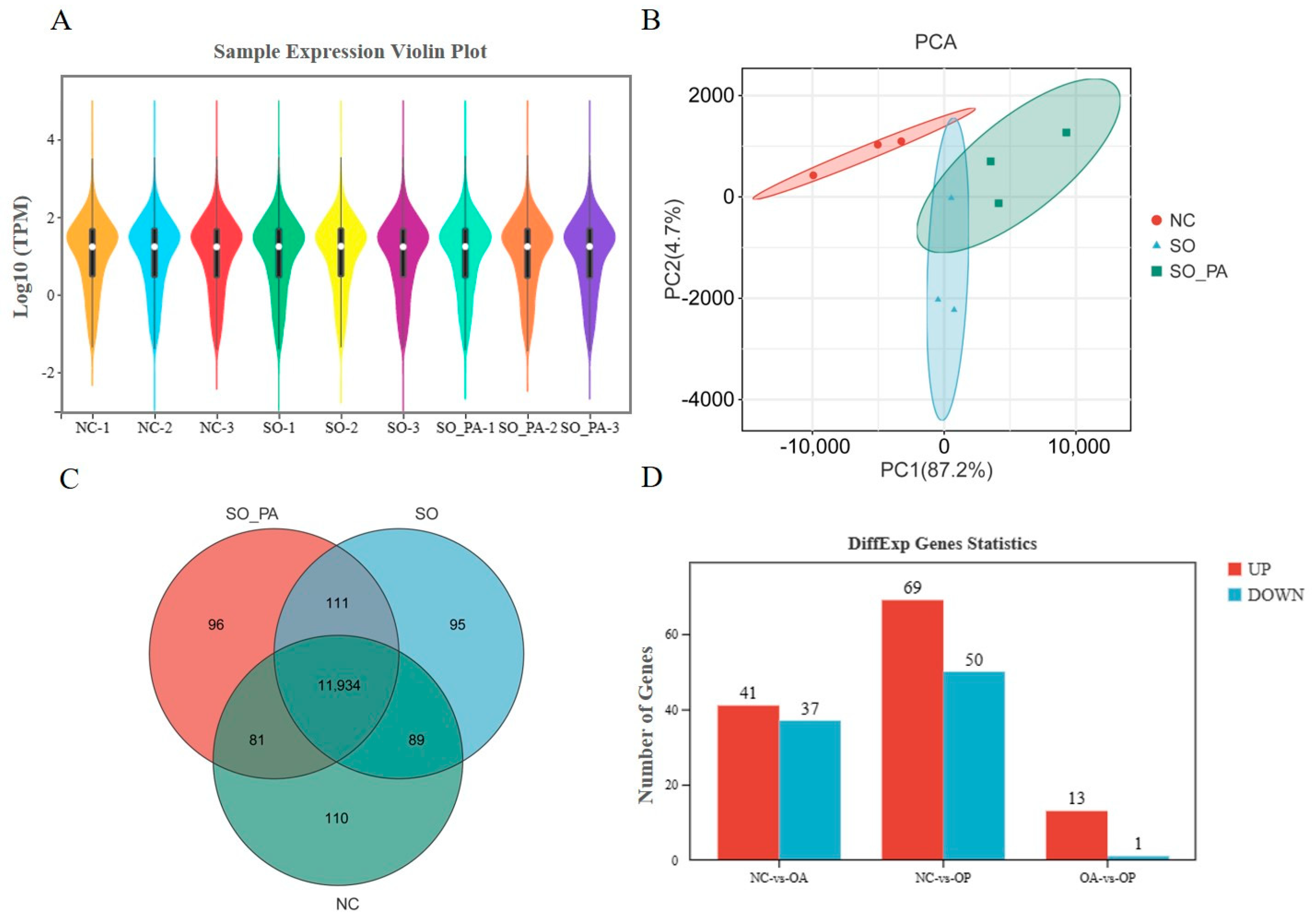
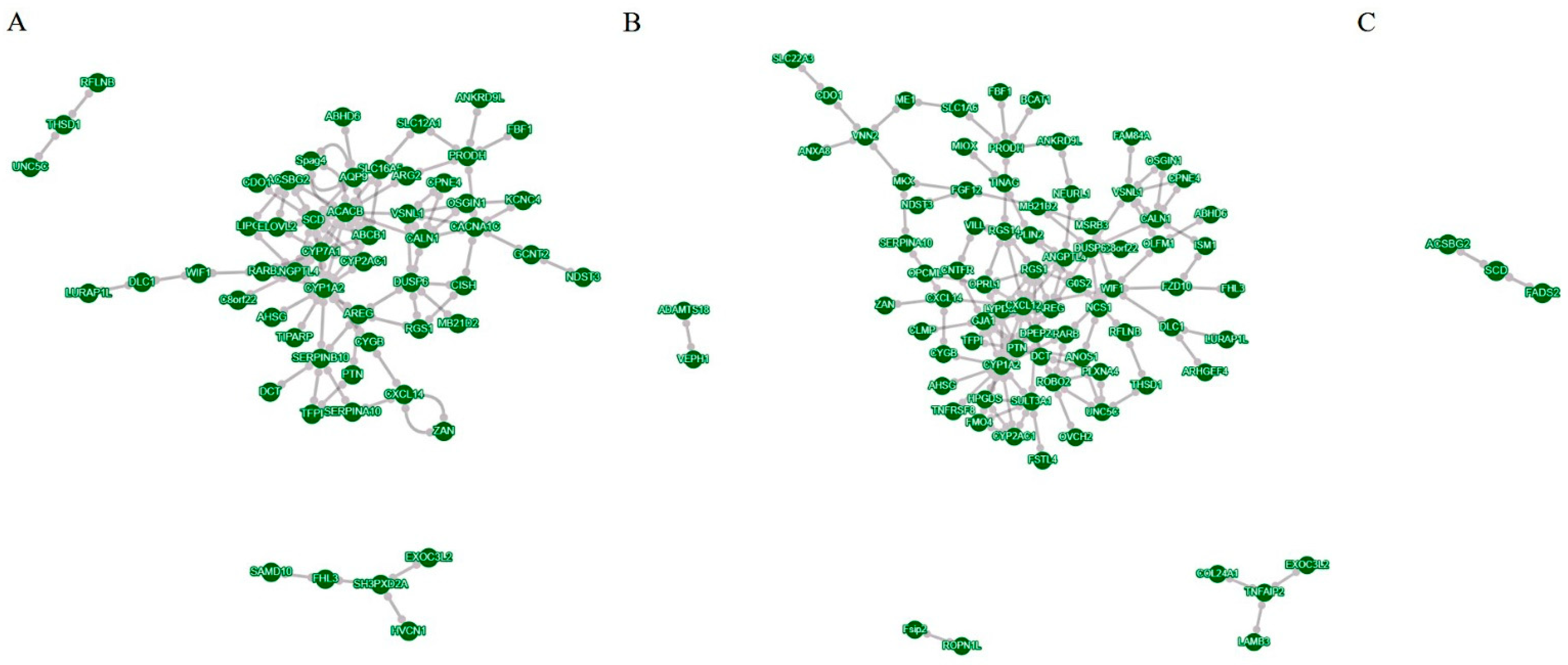
3.2. RNA-Seq Analysis of LMH Cells under Different Steatosis Conditions
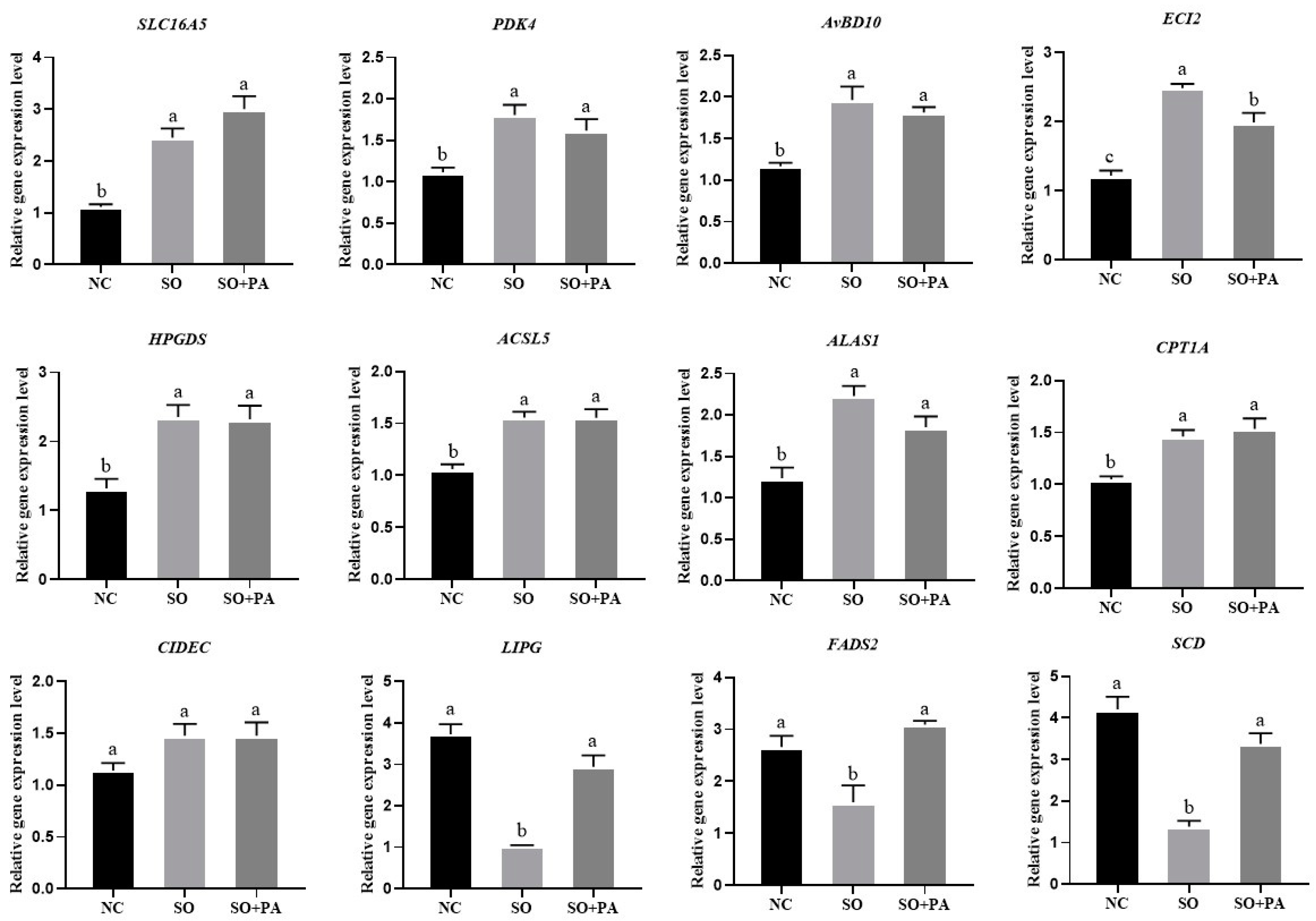
3.3. Quantitative Validation of Differentially Expressed Genes Identified through RNA-Seq
3.4. The Application of Optimal Conditions for Steatosis in Chicken Primary Embryonic Liver Cells and Embryonic Liver Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Bugianesi, E.; Lenzi, M.; Mccullough, A.J.; Natale, S.; Forlani, G.; Melchionda, N. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes 2001, 50, 1844–1850. [Google Scholar] [CrossRef]
- Hurr, C.; Simonyan, H.; Morgan, D.A.; Rahmouni, K.; Young, C.N. Liver sympathetic denervation reverses obesity-induced hepatic steatosis. J. Physiol. 2019, 597, 4565–4580. [Google Scholar] [CrossRef]
- Shini, A.; Shini, S.; Bryden, W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: Impact of production system. Avian Pathol. 2019, 48, 25–34. [Google Scholar] [CrossRef]
- Trott, K.A.; Giannitti, F.; Rimoldi, G.; Hill, A.; Woods, L.; Barr, B.; Anderson, M.; Mete, A. Fatty liver hemorrhagic syndrome in the backyard chicken: A retrospective histopathologic case series. Vet. Pathol. 2014, 51, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Drescher, H.; Weiskirchen, S.; Weiskirchen, R. Current status in testing for nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). Cells 2019, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Zhang, J.Y.; Li, W.X.; Liu, C.; Li, M.L.; Zhao, L.H.; Ji, C.; Ma, Q.G. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult. Sci. 2019, 98, 2509–2521. [Google Scholar] [CrossRef]
- Chen, J.; Hua, G.; Han, D.; Zheng, X.; Dong, X.; Wang, S.; Long, J.; Zheng, Z.; Wang, A.; Wang, J.; et al. An EAV-HP insertion in the promoter region of SLCO1B3 has pleiotropic effects on chicken liver metabolism based on the transcriptome and proteome analysis. Sci. Rep. 2021, 11, 7571. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Song, L.; Niu, X.; Wang, Y.; Liu, W.; Hu, J.; Cai, H.; Song, W.; Liu, L.; Li, H.; et al. Multiscale 3D genome organization underlies duck fatty liver with no adipose inflammation or serious injury. Int. J. Biol. Macromol. 2024, 271, 132452. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, C.; Li, T.; Wang, M.; Serrano, B.R.; Barcenas, A.R.; Qu, L.; Zhao, W.; Shen, M. Quercetin ameliorates lipid deposition in primary hepatocytes of the chicken embryo. Br. Poult. Sci. 2024, 10, 1–8. [Google Scholar] [CrossRef]
- Du, X.; Wang, Y.; Amevor, F.K.; Ning, Z.; Deng, X.; Wu, Y.; Wei, S.; Cao, X.; Xu, D.; Tian, Y.; et al. Effect of high energy low protein diet on lipid metabolism and inflammation in the liver and abdominal adipose tissue of laying hens. Animals 2024, 14, 1199. [Google Scholar] [CrossRef]
- Sun, C.; Lan, F.; Zhou, Q.; Guo, X.; Jin, J.; Wen, C.; Guo, Y.; Hou, Z.; Zheng, J.; Wu, G.; et al. Mechanisms of hepatic steatosis in chickens: Integrated analysis of the host genome, molecular phenomics and gut microbiome. GigaScience 2024, 13, giae023. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and functional characterization of human hepatoma HepG2 cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhu, L.; Lai, S.; Ding, Q.; Xu, T.; Guo, R.; Dou, X.; Chai, H.; Yu, Z.; Li, S. Cimifugin ameliorates lipotoxicity-induced hepatocyte damage and steatosis through TLR4/p38 MAPK- and SIRT1-involved pathways. Oxid. Med. Cell. Longev. 2022, 2022, 4557532. [Google Scholar] [CrossRef] [PubMed]
- Díez-Sainz, E.; Aranaz, P.; Amri, E.; Riezu-Boj, J.I.; Lorente-Cebrián, S.; Milagro, F.I. Plant miR8126-3p and miR8126-5p decrease lipid accumulation through modulation of metabolic genes in a human hepatocyte model that mimics steatosis. Int. J. Mol. Sci. 2024, 25, 1721. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, O.H.; Mroue, K.; Mall, R.; Ullah, E.S.; Al-Akl, N.; Arredouani, A. Investigation of the effect of exendin-4 on oleic acid-induced steatosis in HepG2 cells using fourier transform infrared spectroscopy. Biomedicines 2022, 10, 2652. [Google Scholar] [CrossRef]
- Wang, J.; Du, M.; Du, Y.; Li, J. HA-20 prevents hepatocyte steatosis in metabolic-associated fatty liver disease via regulating Ca2+ relative signalling pathways. Eur. J. Pharmacol. 2022, 921, 174838. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Shao, M.; Lu, Y.; Wang, J.; Wu, T.; Ji, G. Kaempferol alleviates steatosis and inflammation during early non-alcoholic steatohepatitis associated with liver X receptor α-lysophosphatidylcholine acyltransferase 3 signaling pathway. Front. Pharmacol. 2021, 12, 690736. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Jin, J.; Liu, D.; Shi, T.; Fu, X.; Liu, C.; Liu, S.; Wu, R. Oridonin interferes with simple steatosis of liver cells by regulating autophagy. Tissue Cell 2021, 72, 101532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Meng, S.; Li, C.; Yang, Z.; Wang, G.; Wang, X.; Ma, Y. Primary broiler hepatocytes for establishment of a steatosis model. Vet. Sci. 2022, 9, 316. [Google Scholar] [CrossRef]
- Song, H.; Yang, R.; Zhang, J.; Sun, P.; Xing, X.; Wang, L.; Sairijima, T.; Hu, Y.; Liu, Y.; Cheng, H.; et al. Oleic acid-induced steatosis model establishment in LMH cells and its effect on lipid metabolism. Poult. Sci. 2023, 102, 102297. [Google Scholar] [CrossRef]
- Ding, J.; Liu, J.; Chen, J.; Cheng, X.; Cao, H.; Guo, X.; Hu, G.; Zhuang, Y. Sodium butyrate alleviates free fatty acid-induced steatosis in primary chicken hepatocytes via the AMPK/PPARalpha pathway. Poult. Sci. 2024, 103, 103482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, J.; Li, X.; Fang, C.; Wang, L. Identification of functional transcriptional binding sites within chicken Abcg2 gene promoter and screening its regulators. Genes 2020, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Z.; Zhang, J.; Zhuang, W.; Zheng, X. Screening of reliable reference genes for the normalization of RT-qPCR in chicken gastrointestinal tract. Poult. Sci. 2023, 102, 103169. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xu, J.; Zeng, Y.; Chen, L.; Xu, X.L. Polydatin attenuates atherosclerosis in apolipoprotein E-deficient mice: Role of reverse cholesterol transport. Phytomedicine 2019, 62, 152935. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Mu, T.; Li, R.; Li, Y.; Zhao, W.; Li, J.; Dong, X.; Zou, X. Coated sodium butyrate ameliorates high-energy and low-protein diet induced hepatic dysfunction via modulating mitochondrial dynamics, autophagy and apoptosis in laying hens. J. Anim. Sci. Biotechnol. 2024, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Liu, S.; Yu, T.; Su, J.; Chai, K.; Weng, S. Yunpi Heluo decoction reduces ectopic deposition of lipids by regulating the SIRT1-FoxO1 autophagy pathway in diabetic rats. Pharm. Biol. 2022, 60, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, Y.; Zhang, L.; Jia, Y.; Cai, D.; Qian, S.B.; Du, M.; Zhao, R. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m(6)A on lipogenic mRNAs. RNA Biol. 2020, 17, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S.; et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroen. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Pan, X.; Xu, X.; Yu, Y. USP10 alleviates palmitic acid-induced steatosis through autophagy in HepG2 cells. J. Clin. Transl. Hepatol. 2022, 11, 45. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.J.; Ory, D.S.; Schaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef]
- Song, X.; Liu, J.; Kuang, F.; Chen, X.; Zeh, H.J.; Kang, R.; Kroemer, G.; Xie, Y.; Tang, D. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 2021, 34, 108767. [Google Scholar] [CrossRef]
- Thoudam, T.; Chanda, D.; Sinam, I.S.; Kim, B.; Kim, M.; Oh, C.J.; Lee, J.Y.; Kim, M.; Park, S.Y.; Lee, S.Y.; et al. Noncanonical PDK4 action alters mitochondrial dynamics to affect the cellular respiratory status. Proc. Natl. Acad. Sci. USA 2022, 119, e2120157119. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Seol, H.S.; Kamada, T. Tissue distribution of lipase genes related to triglyceride metabolism in laying hens (Gallus gallus). Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2010, 155, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Mao, H.; Luo, T.; Li, Q.; Xu, L.; Xie, Y. HPGDS is a novel prognostic marker associated with lipid metabolism and aggressiveness in lung adenocarcinoma. Front. Oncol. 2022, 12, 894485. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Qiu, D.; Fu, X.; Wu, A.; Yang, P.; Yang, Z.; Wang, Q.; Yan, L.; Xiao, R. Overexpressing HPGDS in adipose-derived mesenchymal stem cells reduces inflammatory state and improves wound healing in type 2 diabetic mice. Stem Cell Res. Ther. 2022, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Jones, R.S.; Morris, M.E. Untargeted metabolomics identifies the potential role of monocarboxylate transporter 6 (MCT6/SLC16A5) in lipid and amino acid metabolism pathways. Pharmacol. Res. Perspect. 2022, 10, e944. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Boekschoten, M.V.; Wopereis, S.; Muller, M.; Kersten, S. Comparative transcriptomic and metabolomic analysis of fenofibrate and fish oil treatments in mice. Physiol. Genom. 2011, 43, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 polymorphisms modulate fatty acid metabolism and dietary impact on health. Annu. Rev. Nutr. 2019, 39, 21–44. [Google Scholar] [CrossRef]
- Hou, T.; Tian, Y.; Cao, Z.; Zhang, J.; Feng, T.; Tao, W.; Sun, H.; Wen, H.; Lu, X.; Zhu, Q.; et al. Cytoplasmic SIRT6-mediated ACSL5 deacetylation impedes nonalcoholic fatty liver disease by facilitating hepatic fatty acid oxidation. Mol. Cell 2022, 82, 4099–4115. [Google Scholar] [CrossRef] [PubMed]
- Sen, U.; Coleman, C.; Sen, T. Stearoyl coenzyme A desaturase-1: Multitasker in cancer, metabolism, and ferroptosis. Trends Cancer 2023, 9, 480–489. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.O.; Jang, H.; Rengaraj, D.; Yang, S.; Han, J.Y.; Lamont, S.J.; Womack, J.E. Tissue expression and antibacterial activity of host defense peptides in chicken. BMC Vet. Res. 2016, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Fulton, J.E.; Wong, E.A. Temporal expression of avian beta defensin 10 and cathelicidins in the yolk sac tissue of broiler and layer embryos. Poult. Sci. 2023, 102, 102334. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, H.; Tian, Y.; Li, J.; Scheben, A.; Zhang, C.; Li, Y.; Wu, J.; Yang, L.; Fan, X.; et al. The chicken pan-genome reveals gene content variation and a promoter region deletion in IGF2BP1 affecting body size. Mol. Biol. Evol. 2021, 38, 5066–5081. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hua, G.; Li, J.; Yang, Y.; Zhang, C.; Yang, L.; Hu, X.; Scheben, A.; Wu, Y.; Gong, P.; et al. Duck pan-genome reveals two transposon insertions caused bodyweight enlarging and white plumage phenotype formation during evolution. iMeta 2024, 3, e154. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elheiga, L.; Matzuk, M.M.; Abo-Hashema, K.A.; Wakil, S.J. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 2001, 291, 2613–2616. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Lei, W.; Wang, H.; Ni, Y.; Liu, Y.; Yan, H.; Tian, Y.; Wang, Z.; Yang, Z.; et al. CXCL12-CXCR4/CXCR7 axis in cancer: From mechanisms to clinical applications. Int. J. Biol. Sci. 2023, 19, 3341–3359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, G.; Ren, L.; Wang, K.; Liu, A. Long non-coding RNA TUG1 recruits miR-29c-3p from its target gene RGS1 to promote proliferation and metastasis of melanoma cells. Int. J. Oncol. 2019, 54, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Welte, M.A.; Gould, A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 1260–1272. [Google Scholar] [CrossRef]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, L.; Li, P. CIDE proteins and lipid metabolism. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wu, L.; Yu, M.; Chen, F.; Arshad, M.; Xia, X.; Ren, H.; Yu, J.; Xu, L.; Xu, D.; et al. Differential roles of cell death-inducing DNA fragmentation factor-α-like effector (CIDE) proteins in promoting lipid droplet fusion and growth in subpopulations of hepatocytes. J. Biol. Chem. 2016, 291, 4282–4293. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Huang, E.; Ruan, J.; Huang, L.; Liang, H.; Wei, Q.; Xie, X.; Zeng, Q.; Huang, J. Effects of a high energy and low protein diet on hepatic and plasma characteristics and Cidea and Cidec mRNA expression in liver and adipose tissue of laying hens with fatty liver hemorrhagic syndrome. Anim. Sci. J. 2019, 90, 247–254. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, W.; Chen, Z.; Shu, X.; Zhang, J.; Zhu, R.; Shen, M.; Chen, J.; Zheng, X. Establishment of a Steatosis Model in LMH Cells, Chicken Embryo Hepatocytes, and Liver Tissues Based on a Mixture of Sodium Oleate and Palmitic Acid. Animals 2024, 14, 2173. https://doi.org/10.3390/ani14152173
Zhuang W, Chen Z, Shu X, Zhang J, Zhu R, Shen M, Chen J, Zheng X. Establishment of a Steatosis Model in LMH Cells, Chicken Embryo Hepatocytes, and Liver Tissues Based on a Mixture of Sodium Oleate and Palmitic Acid. Animals. 2024; 14(15):2173. https://doi.org/10.3390/ani14152173
Chicago/Turabian StyleZhuang, Wuchao, Ziwei Chen, Xin Shu, Jilong Zhang, Runbang Zhu, Manman Shen, Jianfei Chen, and Xiaotong Zheng. 2024. "Establishment of a Steatosis Model in LMH Cells, Chicken Embryo Hepatocytes, and Liver Tissues Based on a Mixture of Sodium Oleate and Palmitic Acid" Animals 14, no. 15: 2173. https://doi.org/10.3390/ani14152173




