A Dynamic Tool to Describe Lamb Growth and Its Use as a Decision Support System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Model Framework
2.2. Diet Composition
2.3. Simulation Procedure
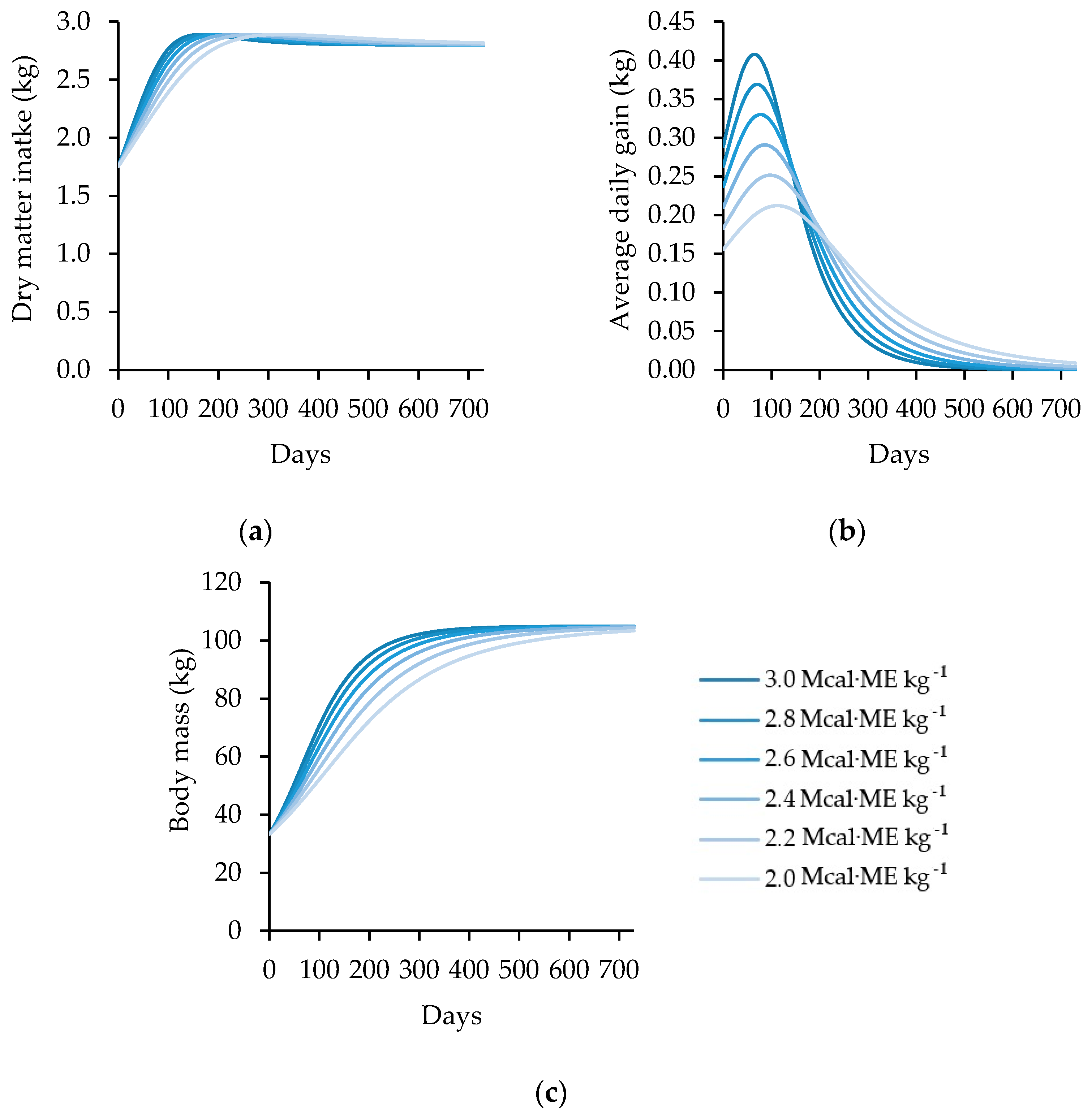
- Input Data: The initial input data consisted of the animal’s initial body mass (kg), standard final mass (kg), age (days), sex (1 or 1.15), and diet metabolizable energy concentration (Mcal.kg−1).
- Nutritional Requirements: The model calculated the nutritional requirements for maintenance (Mcal EM·day−1) and estimated the dry matter intake (kg·day−1). The metabolizable energy intake above maintenance (dimensionless) was then calculated, given the metabolizable energy available for growth (Mcal EM·day−1).
- Energy Content of Gain: Based on the metabolizable energy available for growth and the degree of maturity, the energy content of gain (Mcal·day−1) was estimated as the sum of the retained energy from protein (Mcal·day−1) and the retained energy from fat (Mcal·day−1). The protein in gain (g·day−1) and the fat in gain (g·day−1) were then calculated based on the retained energy from protein and fat, respectively. These values were accumulated in the total body protein (kg) and total body fat (kg).
- Net Energy on Gain: Based on the proportion of energy retained as protein, the kG was calculated and multiplied by the metabolizable energy to gain, giving the net energy on gain.
- Average Daily Gain: The average daily gain was estimated using the net energy available for growth. This calculation considers the initial body mass and its respective maintenance energy requirement. This change was automatically computed to the animal body mass, closing the feedback loop, and dynamically influencing the intake capacity and nutritional requirements over time.
2.4. Model Evaluation as a Support Decision Tool
| Reference | Treatments | Observations | SFM a (kg) | Genotype |
|---|---|---|---|---|
| [23] | 4 | 20 | 55 | Pelibuey |
| [24] | 3 | 18 | 100 | Santa Inês |
| [21] | 2 | 16 | 105 | Mutton Merino |
| [25] | 2 | 14 | 80 | Awassi |
| [26] | 3 | 54 | 81 | Mehbraban |
| [27] | 6 | 36 | 35 | Taleshi |
| [28] | 4 | 24 | 100 | Santa Inês |
| [29] | 4 | 24 | 100 | Santa Inês |
| [30] | 3 | 27 | 100 | Santa Inês |
| [31] | 1 | 10 | 105 | Dorper × Katadhin |
| [32] | 4 | 80 | 80 | Chall |
| [33] | 4 | 24 | 100 | Santa Inês |
| [34] | 5 | 45 | 100 | Santa Inês |
| [35] | 3 | 72 | 119 | Hampshire |
| [36] | 6 | 36 | 80 | Chall |
| [37] | 4 | 24 | 81 | Mehbraban |
| [38] | 4 | 40 | 75 | Pelibuey × Katahdin |
| Item | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Days on feed | 68.85 | 18.99 | 35.00 | 105.00 |
| Dietary ME (Mcal·day−1 of DM) | 2.54 | 0.21 | 2.10 | 2.96 |
| Initial body mass (kg) | 25.85 | 5.90 | 18.05 | 39.10 |
| Final body mass (kg) | 39.94 | 8.87 | 29.15 | 61.70 |
| Dry matter intake (kg·day−1) | 1.30 | 0.30 | 0.87 | 2.33 |
| Average daily gain (kg·day−1) | 0.22 | 0.05 | 0.10 | 0.31 |
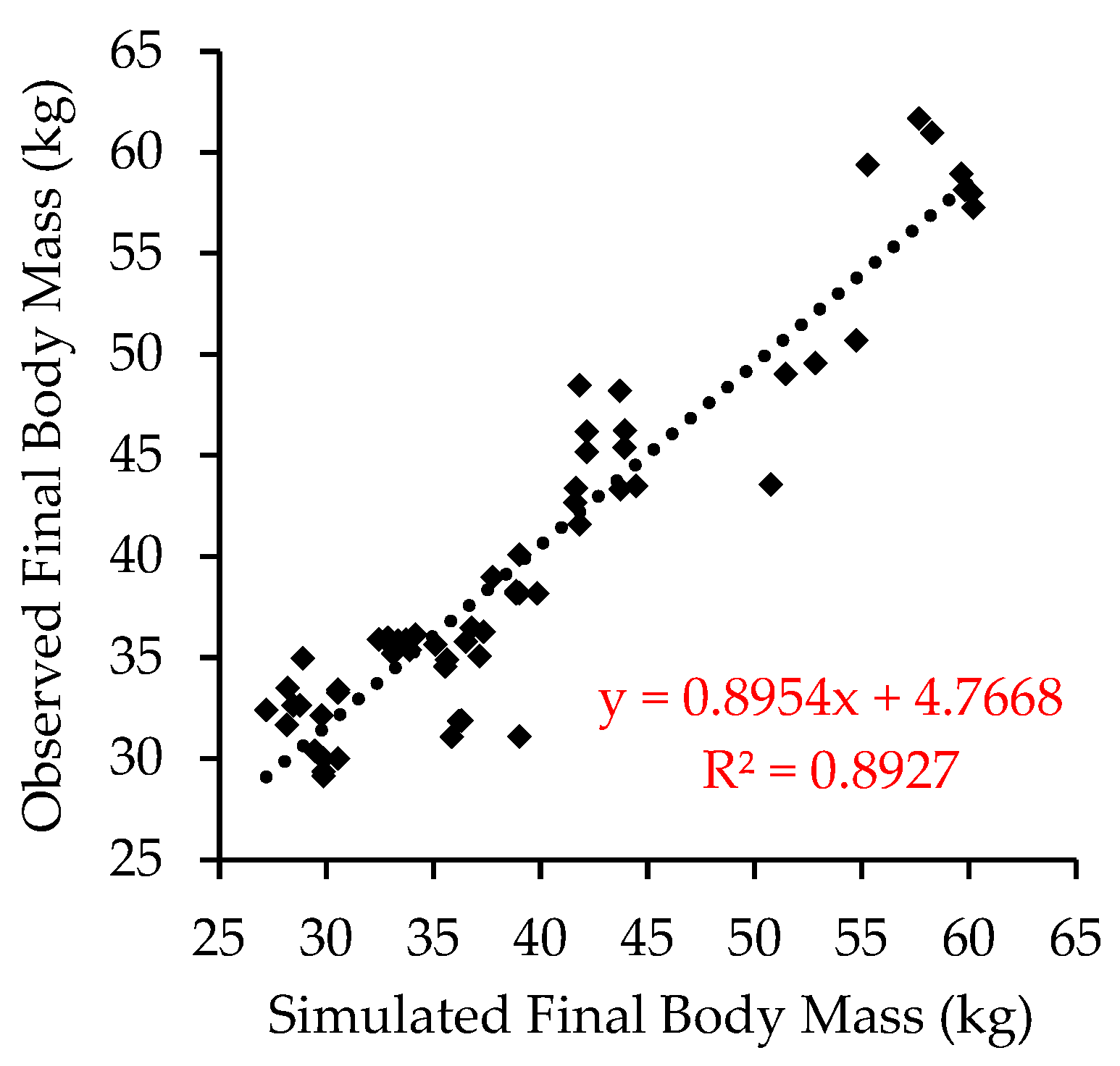
3. Results
Model Evaluation
- The mean bias (MB) was 0.656 kg, corresponding to 1.64% of the average final body mass of 39.94 kg;
- The mean square error of prediction (MSE) was 9.68;
- The root mean square error of prediction (RMSE) was 3.11 kg;
- The mean bias (UM), systematic bias (US), and random error (UC) values were 0.044, 0.024, and 0.93, respectively.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Bezner Kerr, R.; Madsen, S.; Stüber, M.; Liebert, J.; Enloe, S.; Borghino, N.; Parros, P.; Mutyambai, D.M.; Prudhon, M.; Wezel, A. Can Agroecology Improve Food Security and Nutrition? A Review. Glob. Food Secur. 2021, 29, 100540. [Google Scholar] [CrossRef]
- Ajibade, S.; Simon, B.; Gulyas, M.; Balint, C. Sustainable Intensification of Agriculture as a Tool to Promote Food Security: A Bibliometric Analysis. Front. Sustain. Food Syst. 2023, 7, 1101528. [Google Scholar] [CrossRef]
- Guimarães, V.P.; Tedeschi, L.O.; Rodrigues, M.T. Development of a Mathematical Model to Study the Impacts of Production and Management Policies on the Herd Dynamics and Profitability of Dairy Goats. Agric. Syst. 2009, 101, 186–196. [Google Scholar] [CrossRef]
- Gregorini, P.; Villalba, J.J.; Provenza, F.D.; Beukes, P.C.; Forbes, J.M. Modelling Preference and Diet Selection Patterns by Grazing Ruminants: A Development in a Mechanistic Model of a Grazing Dairy Cow, MINDY. Anim. Prod. Sci. 2015, 55, 360–375. [Google Scholar] [CrossRef]
- Herrero, M.; Thornton, P.K.; Kruska, R.; Reid, R.S. Systems Dynamics and the Spatial Distribution of Methane Emissions from African Domestic Ruminants to 2030. Agric. Ecosyst. Environ. 2008, 126, 122–137. [Google Scholar] [CrossRef]
- Russell, J.B.; Muck, R.E.; Weimer, P.J. Quantitative Analysis of Cellulose Degradation and Growth of Cellulolytic Bacteria in the Rumen. FEMS Microbiol. Ecol. 2009, 67, 183–197. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Nicholson, C.F.; Rich, E. Using System Dynamics Modelling Approach to Develop Management Tools for Animal Production with Emphasis on Small Ruminants. Small Rumin. Res. 2011, 98, 102–110. [Google Scholar] [CrossRef]
- Puillet, L.; Martin, O.; Tichit, M.; Sauvant, D. Simple Representation of Physiological Regulations in a Model of Lactating Female: Application to the Dairy Goat. Animal 2008, 2, 235–246. [Google Scholar] [CrossRef]
- Garcia, F.; Sainz, R.D.; Agabriel, J.; Barioni, L.G.; Oltjen, J.W. Comparative Analysis of Two Dynamic Mechanistic Models of Beef Cattle Growth. Anim. Feed Sci. Technol. 2008, 143, 220–241. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Fox, D.G.; Guiroy, P.J. A Decision Support System to Improve Individual Cattle Management. 1. A Mechanistic, Dynamic Model for Animal Growth. Agric. Syst. 2004, 79, 171–204. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Cannas, A.; Fox, D.G. A Nutrition Mathematical Model to Account for Dietary Supply and Requirements of Energy and Nutrients for Domesticated Small Ruminants: The Development and Evaluation of the Small Ruminant Nutrition System. Small Rumin. Res. 2010, 89, 174–184. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Fox, D.G. The Ruminant Nutrition System: An Applied Model for Predicting Nutrient Requirements and Feed Utilization in Ruminants; XanEdu Publishing Inc.: Livonia, MI, USA, 2018; ISBN 1583902368. [Google Scholar]
- NASEM. Nutrient Requirements of Beef Cattle, 8th ed.; National Academies Press: Washington, DC, USA, 2016; ISBN 978-0-309-31702-3. [Google Scholar]
- NASEM. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2021; ISBN 978-0-309-67777-6. [Google Scholar]
- Tedeschi, L.O. ASAS-NANP Symposium: Mathematical Modeling in Animal Nutrition: The Progression of Data Analytics and Artificial Intelligence in Support of Sustainable Development in Animal Science. J. Anim. Sci. 2022, 100, skac111. [Google Scholar] [CrossRef]
- Cannas, A.; Tedeschi, L.O.; Fox, D.G.; Pell, A.N.; Van Soest, P.J. A Mechanistic Model for Predicting the Nutrient Requirements and Feed Biological Values for Sheep. J. Anim. Sci. 2004, 82, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Sterman, J.D. Business Dynamics: Systems Thinking and Modeling for a Complex World; McGraw-Hill Higher Education: New York, NY, USA, 2000; ISBN 0072311355. [Google Scholar]
- Vensim® PLE Software 7.0; Ventana Systems Inc.: Harvard, MA, USA, 2017.
- Thornley, J.H.M.; France, J. Mathematical Models in Agriculture: Quantitative Methods for the Plant, Animal and Ecological Sciences; CABI: Wallingford, UK, 2007; ISBN 1845932404. [Google Scholar]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007; ISBN 978-0-309-47323-1.
- Sheridan, R.; Ferreira, A.V.; Hoffman, L.C. Production Efficiency of South African Mutton Merino Lambs and Boer Goat Kids Receiving Either a Low or a High Energy Feedlot Diet. Small Rumin. Res. 2003, 50, 75–82. [Google Scholar] [CrossRef]
- Kemp, S.; Mamo, Y.; Asrat, B.; Dessie, T. Domestic Animal Genetic Resources Information System. Available online: http://dagris.info/ (accessed on 31 December 2017).
- Fimbres, H.; Hernández-Vidal, G.; Picón-Rubio, J.F.; Kawas, J.R.; Lu, C.D. Productive Performance and Carcass Characteristics of Lambs Fed Finishing Ration Containing Various Forage Levels. Small Rumin. Res. 2002, 43, 283–288. [Google Scholar] [CrossRef]
- Alves, K.S.; Fernando, F.; De Carvalho, R.; Sherlânea, A.; Véras, C.; De Andrade, M.F.; Costa, R.G.; Maria, Â.; Batista, V.; De Medeiros, N.; et al. Níveis de Energia Em Dietas Para Ovinos Santa Inês: Desempenho 1 Dietary Levels of Energy for Santa Inês Sheep: Performance. Rev. Bras. Zootec. 2003, 2003, 1937–1944. [Google Scholar] [CrossRef]
- Haddad, S.G.; Younis, H.M. The Effect of Adding Ruminally Protected Fat in Fattening Diets on Nutrient Intake, Digestibility and Growth Performance of Awassi Lambs. Anim. Feed Sci. Technol. 2004, 113, 61–69. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Ahmadi, H.R.; Zamiri, M.J.; Rowghani, E. Effect of Energy and Protein Levels on Feedlot Performance and Carcass Characteristics of Mehraban Ram Lambs. Pak. J. Biol. Sci. 2007, 10, 1679–1684. [Google Scholar] [CrossRef]
- Kioumarsi, H.; Khorshidi, K.J.; Zahedifar, M.; Seidavi, A.R.; Mirhosseini, S.Z.; Taherzadeh, M.R. The Effect of Dietary Energy and Protein Level on Performance, Efficiency and Carcass Characteristics of Taleshi Lambs. Asian J. Anim. Vet. Adv. 2008, 3, 307–313. [Google Scholar] [CrossRef]
- Cunha, M.G.G.; Carvalho, F.F.R.; Véras, A.S.C.; Batista, A.M.V. Desempenho e Digestibilidade Aparente Em Ovinos Confinados Alimentados Com Dietas Contendo Níveis Crescentes de Caroço de Algodão Integral. Rev. Bras. Zootec.-Braz. J. Anim. Sci. 2008, 37, 1103–1111. [Google Scholar] [CrossRef]
- Pereira, M.S.; Ribeiro, E.L.D.A.; Mizubuti, I.Y.; Da Rocha, M.A.; Kuraoka, J.T.; Nakaghi, E.Y.O. Consumo de Nutrientes e Desempenho de Cordeiros Em Confinamento Alimentados Com Dietas Com Polpa Citrica Moida Prensada Em Substituicao Silagem de Milho. Rev. Bras. Zootec. 2008, 37, 134–139. [Google Scholar] [CrossRef]
- Bensimon, A.M.G.; De Moraes, G.V.; Mataveli, M.; De Macedo, F.A.F.; Carneiro, T.C.; Rossi, R.M. Performance and Carcass Characteristics of Lambs Fed on Diets Supplemented with Glycerin from Biodiesel Production. Rev. Bras. Zootec. 2011, 40, 2211–2219. [Google Scholar] [CrossRef]
- Lopez-Carlos, M.A.; Ramirez, R.G.; Aguilera-Soto, J.I.; Plascencia, A.; Rodriguez, H.; Arechiga, C.F.; Rincon, R.M.; Medina-Flores, C.A.; Gutierrez-Bañuelos, H. Effect of Two Beta Adrenergic Agonists and Feeding Duration on Feedlot Performance and Carcass Characteristics of Finishing Lambs. Livest. Sci. 2011, 138, 251–258. [Google Scholar] [CrossRef]
- Papi, N.; Mostafa-Tehrani, A.; Amanlou, H.; Memarian, M. Effects of Dietary Forage-to-Concentrate Ratios on Performance and Carcass Characteristics of Growing Fat-Tailed Lambs. Anim. Feed Sci. Technol. 2011, 163, 93–98. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Rufino, L.M.d.A.; Dos Santo, A.C.R.; Da Silva, L.P.; Bonfá, H.C.; Duarte, E.R.; Geraseev, L.C. Desempenho de Cordeiros Alimentados Com Inclusão de Torta de Macaúba Na Dieta. Pesqui. Agropecu. Bras. 2012, 47, 1663–1668. [Google Scholar] [CrossRef]
- Costa, R.G.; Treviño, I.H.; De Medeiros, G.R.; Medeiros, A.N.; Pinto, T.F.; De Oliveira, R.L. Effects of Replacing Corn with Cactus Pear (Opuntia Ficus Indica Mill) on the Performance of Santa Inês Lambs. Small Rumin. Res. 2012, 102, 13–17. [Google Scholar] [CrossRef]
- Jaborek, J.R.; Lowe, G.D.; Fluharty, F.L. Effects of Pen Flooring Type and Bedding on Lamb Growth and Carcass Characteristics. Small Rumin. Res. 2016, 144, 28–34. [Google Scholar] [CrossRef]
- Tadayon, Z.; Rouzbehan, Y.; Rezaei, J. Effects of Feeding Different Levels of Dried Orange Pulp and Recycled Poultry Bedding on the Performance of Fattening Lambs1. J. Anim. Sci. 2017, 95, 1751–1765. [Google Scholar] [CrossRef]
- Sadri, K.; Rouzbehan, Y.; Fazaeli, H.; Rezaei, J. Influence of Dietary Feeding Different Levels of Mixed Potato-Wheat Straw Silage on the Diet Digestibility and the Performance of Growing Lambs. Small Rumin. Res. 2017, 159, 84–89. [Google Scholar] [CrossRef]
- Estrada-Angulo, A.; Castro-Pérez, B.I.; Urías-Estrada, J.D.; Ríos-Rincón, F.G.; Arteaga-Wences, Y.J.; Barreras, A.; López-Soto, M.A.; Plascencia, A.; Zinn, R.A. Influence of Protein Level on Growth Performance, Dietary Energetics and Carcass Characteristics of Pelibuey × Katahdin Lambs Finished with Isocaloric Diets. Small Rumin. Res. 2018, 160, 59–64. [Google Scholar] [CrossRef]
- Theil, H. The Measurement of Inequality by Components of Income. Econ. Lett. 1979, 2, 197–199. [Google Scholar] [CrossRef]
- Brand, T.S.S.; Van Der Westhuizen, E.J.J.; Van Der Merwe, D.A.A.; Hoffman, L.C.C. Effect of Days in Feedlot on Growth Performance and Carcass Characteristics of Merino, South African Mutton Merino and Dorper Lambs. S. Afr. J. Anim. Sci. 2017, 47, 26–33. [Google Scholar] [CrossRef]
- CSIRO. Nutrient Requirements of Domesticated Ruminants; CSIRO Publishing: Clayton, Australia, 2007; ISBN 9780643095106.
- SCA. Feeding Standards for Australian Livestock; Standing Committee on Agriculture, Ruminants Subcommittee; CSIRO Publications: Victoria, Australia, 1990; 266p. [Google Scholar]
- Corbett, J.L.; Freer, M. Past and Present Definitions of the Energy and Protein Requirements of Ruminants. Asian-Australas. J. Anim. Sci. 2003, 16, 609–624. [Google Scholar] [CrossRef]
- Xu, G.S.; Ma, T.; Ji, S.K.; Deng, K.D.; Tu, Y.; Jiang, C.G.; Diao, Q.Y. Energy Requirements for Maintenance and Growth of Early-Weaned Dorper Crossbred Male Lambs. Livest. Sci. 2015, 177, 71–78. [Google Scholar] [CrossRef]
- Deng, K.-D.; Diao, Q.-Y.; Jiang, C.-G.; Tu, Y.; Zhang, N.-F.; Liu, J.; Ma, T.; Zhao, Y.-G.; Xu, G.-S. Energy Requirements for Maintenance and Growth of Dorper Crossbred Ram Lambs. Livest. Sci. 2012, 150, 102–110. [Google Scholar] [CrossRef]
- Souza, L.A.; Carneiro, P.L.S.; Malhado, C.H.M.; e Silva, F.F.; da Silveira, F.G. Traditional and Alternative Nonlinear Models for Estimating the Growth of Morada Nova Sheep. Rev. Bras. Zootec. 2013, 42, 651–655. [Google Scholar] [CrossRef]
- Warriss, P.D. Meat Science: An Introductory Text; CABI Publishing: Wallingford, UK, 2000; pp. 234–239. [Google Scholar]
- Hojjati, F.; Hossein-Zadeh, N. Comparison of Non-Linear Growth Models to Describe the Growth Curve of Mehraban Sheep. J. Appl. Anim. Res. 2017, 2119, 499–504. [Google Scholar] [CrossRef]
- Regadas Filho, J.G.L.; Tedeschi, L.O.; Rodrigues, M.T.; Brito, L.F.; Oliveira, T.S. Comparison of Growth Curves of Two Genotypes of Dairy Goats Using Nonlinear Mixed Models. J. Agric. Sci. 2014, 152, 829–842. [Google Scholar] [CrossRef]
- Hossein-Zadeh, N.G. Modeling the Growth Curve of Iranian Shall Sheep Using Non-Linear Growth Models. Small Rumin. Res. 2015, 130, 60–66. [Google Scholar] [CrossRef]
- Rocha, N.S.; Vieira, R.A.M.; Abreu, M.L.C.; Araujo, R.P.; Glória, L.S.; Tamy, W.P.; Camisa Nova, C.H.P.; Fernandes, A.M. Traditional and Biphasic Nonlinear Models to Describe the Growth of Goat Kids of Specialized Dairy Breeds. Small Rumin. Res. 2015, 123, 35–46. [Google Scholar] [CrossRef]
- Brody, S.; Lardy, H.A. Bioenergetics and Growth. J. Phys. Chem. 1946, 50, 168–169. [Google Scholar] [CrossRef]



| Variable | Unit | Description |
|---|---|---|
| a | Average simulated value | |
| a | ith observed or measured value | |
| dimensionless | Standard deviation of the Y variable | |
| a1 | NEM kg 0.75 | Maintenance requirement |
| ADG | g·day−1 | Average daily gain |
| AF | % | Proportion of empty body fat |
| Age | Days | Current age |
| AP | % | Proportion of empty body protein |
| BCS | 1 to 5 | Body condition score |
| BM | kg | Body mass |
| CP | g·day−1 | Crude protein |
| DBF(t) | kg | Dynamic body fat |
| DBM(t) | kg | Dynamic body mass |
| DBP(t) | kg | Dynamic body protein |
| DMI | kg·day−1 | Dry matter intake |
| EBM | kg | Empty body mass |
| EVG | Mcal·day−1 | Energy content of gain |
| kG | dimensionless | Partial efficiency of ME to NE for growth |
| kM | dimensionless | Partial efficiency of ME to NE for maintenance |
| L | dimensionless | Intake above maintenance |
| MB | dimensionless | Mean bias |
| ME | Mcal.kg−1 | Metabolizable energy |
| MEG | Mcal·day−1 | ME available for growth |
| MEI | Mcal·day−1 | ME intake |
| MEM | Mcal·day−1 | ME requirement for maintenance |
| MP | g·day−1 | Metabolizable protein |
| MPM | g·day−1 | MP for maintenance |
| MSE | a | Mean square error of prediction |
| NEG | Mcal·day−1 | Net energy available for growth |
| P | dimensionless | Degree of maturity |
| r | dimensionless | Pearson correlation coefficient. |
| R2 | dimensionless | Coefficient of determination |
| REFat | Mcal·day−1 | Retained energy from fat |
| REP | dimensionless | Proportion of retained energy as protein |
| REprot | Mcal·day−1 | Retained energy from protein |
| RMSE | % | Root mean square error of prediction |
| S | dimensionless | Factor. 1 to female and castrated male and 1.15 to intact males |
| SBM | kg | Shrunk body mass |
| SBM0.75 | kg | Metabolic SBM |
| SFM | kg | Standard final mass |
| t0 | Days | Initial time |
| TE | Mcal | Total energy |
| TF | kg | Total body fat |
| ti | Days | Time in i moment |
| TP | kg | Total body protein |
| UC | dimensionless | MSE decomposition into random error |
| UM | dimensionless | MSE decomposition into mean bias |
| US | dimensionless | MSE decomposition into systematic bias |
| unit | Sample size |
| Variable | Equation | Equation Number |
|---|---|---|
| ADG | (1) | |
| AF | (2) | |
| AP | (3) | |
| DMI a | (4) | |
| DBM(t) b | (5) | |
| DBF(t) b | (6) | |
| DBP(t) b | (7) | |
| EBM | (8) | |
| EVG | (9) | |
| kG | (10) | |
| MEM c | (11) | |
| MPM | (12) | |
| NEG | (13) | |
| REFat | (14) | |
| REP | (15) | |
| REProt | ( | (16) |
| SBM | (17) | |
| TE | (18) | |
| TF | (19) | |
| TP | (20) |
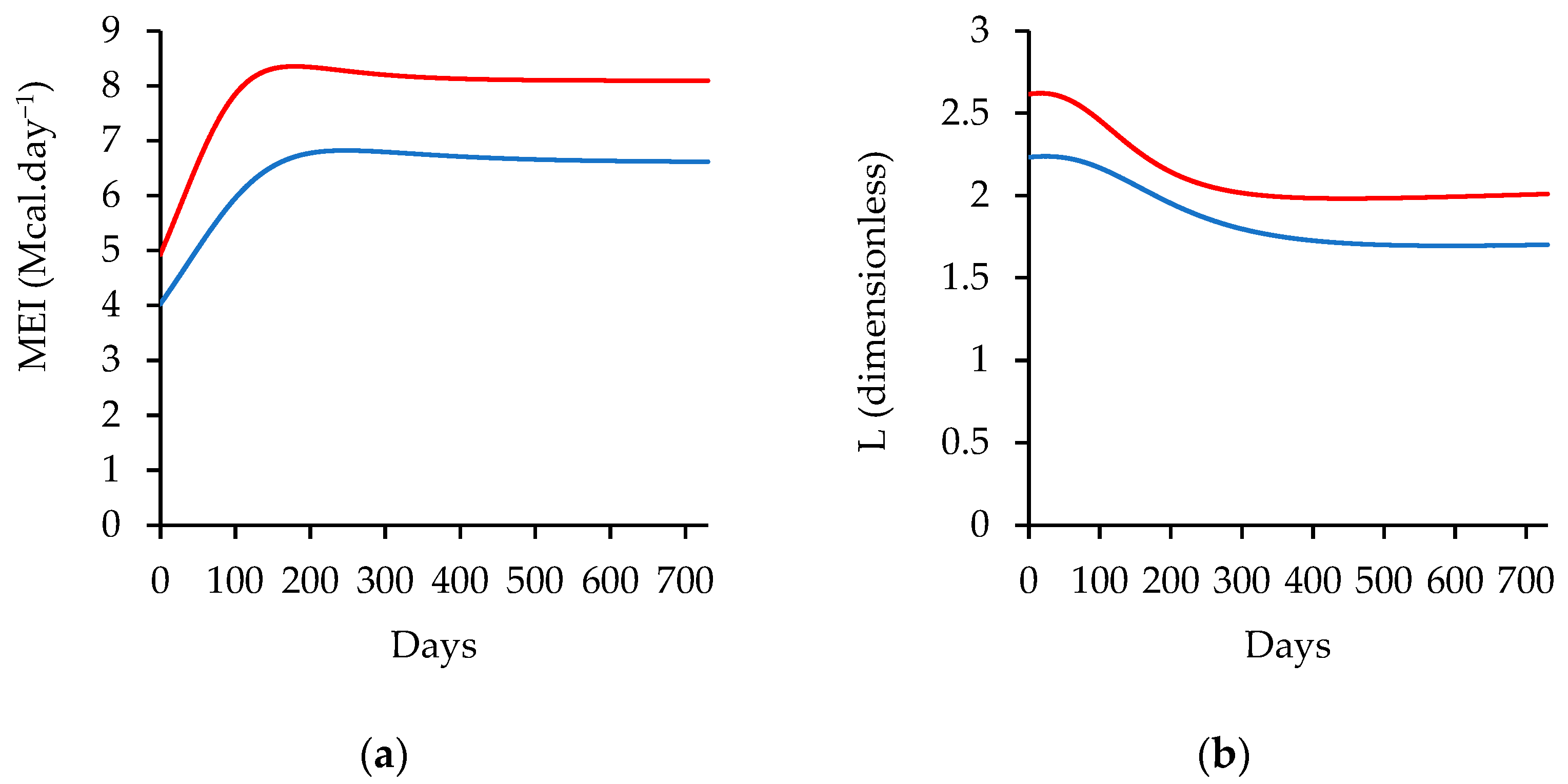
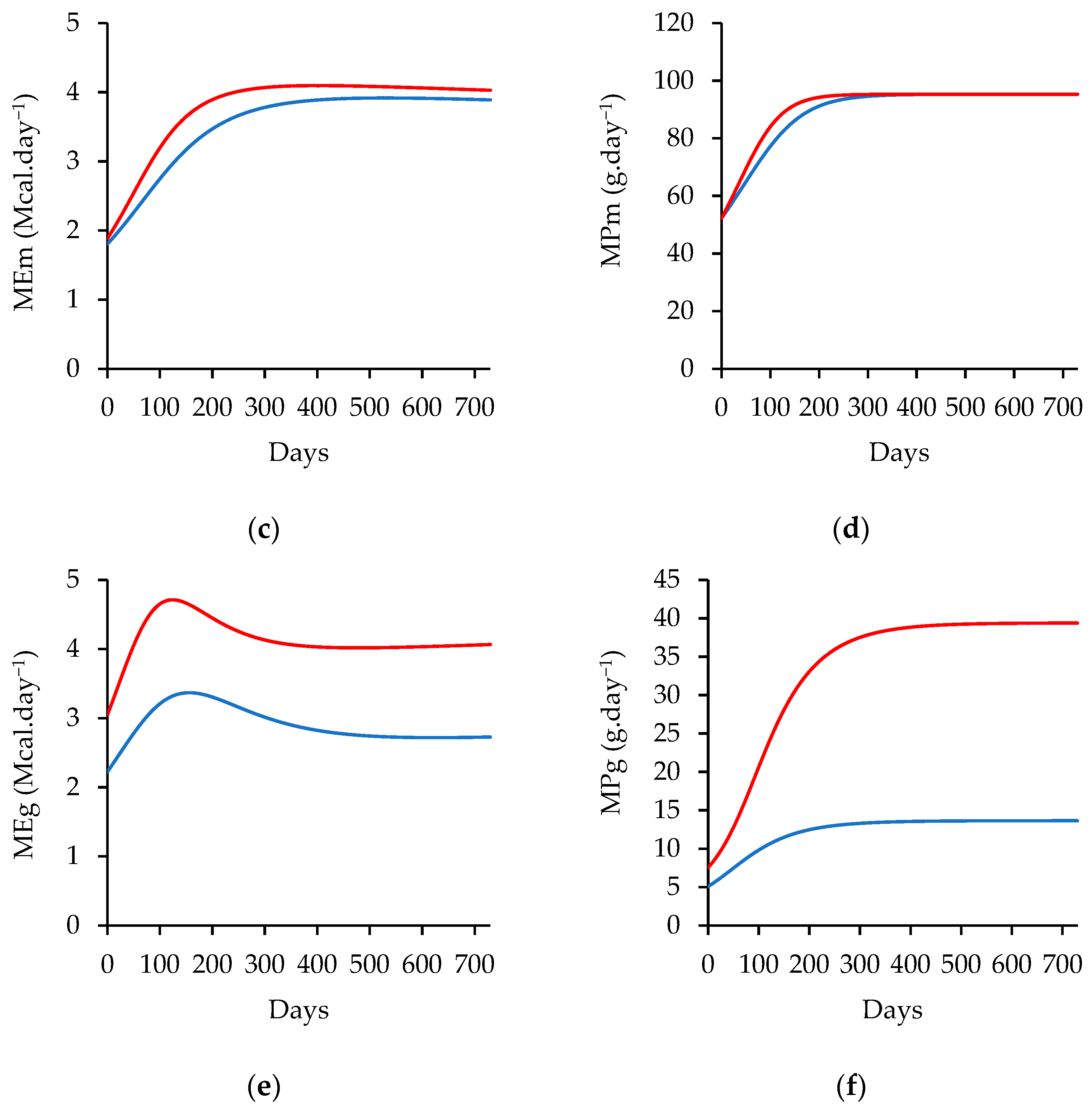
| Item/Ingredient | Low-Energy Diet (%) | High-Energy Diet (%) |
|---|---|---|
| Wheat Bran | 1.50 | 0.00 |
| Maize Meal | 30.00 | 38.00 |
| Sunflower Oilcake | 1.64 | 4.46 |
| Groundnut Oilcake | 3.33 | 0.00 |
| Limestone | 0.07 | 0.58 |
| Urea | 0.70 | 0.59 |
| Maize Germ | 0.00 | 9.31 |
| Supermax Premix | 0.00 | 10.45 |
| Vitamin and Minerals Premix | 0.21 | 0.21 |
| Monocalcium Phosphate | 0.00 | 0.11 |
| Salt | 0.50 | 0.50 |
| Citrus Ruman Flavor | 0.02 | 0.02 |
| Ammonium Chloride | 0.75 | 0.75 |
| Taurotec | 0.03 | 0.03 |
| NaOH Wheat Straw | 21.26 | 15.00 |
| Lucerne Hay | 35.00 | 15.00 |
| Molasses Cuber | 5.00 | 5.00 |
| Chemical Composition | Content | Content |
| Ash (%) | 9.49 | 8.44 |
| Crude Fat (%) | 2.18 | 5.59 |
| Crude Protein (%) | 14.29 | 14.56 |
| Acid Detergent Fiber (%) | 24.74 | 17.78 |
| Neutral Detergent Fiber (%) | 44.17 | 35.92 |
| Gross Energy (Mcal kg−1) | 3.82 | 4.00 |
| Metabolizable Energy (Mcal kg−1) | 2.36 | 2.89 |
| Calcium (%) | 0.44 | 0.74 |
| Phosphorus (%) | 0.27 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, R.M.; Rodrigues, M.T.; Schultz, E.B.; Reis, C.E.R. A Dynamic Tool to Describe Lamb Growth and Its Use as a Decision Support System. Animals 2024, 14, 2246. https://doi.org/10.3390/ani14152246
Amaral RM, Rodrigues MT, Schultz EB, Reis CER. A Dynamic Tool to Describe Lamb Growth and Its Use as a Decision Support System. Animals. 2024; 14(15):2246. https://doi.org/10.3390/ani14152246
Chicago/Turabian StyleAmaral, Rafael Marzall, Marcelo Teixeira Rodrigues, Erica Beatriz Schultz, and Cristiano Eduardo Rodrigues Reis. 2024. "A Dynamic Tool to Describe Lamb Growth and Its Use as a Decision Support System" Animals 14, no. 15: 2246. https://doi.org/10.3390/ani14152246






