Estimation of Genetic Parameters of Early Growth Traits in Dumeng Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Treatment
2.2. Animals and Data Collection
2.3. Statistical Analysis and Genetic Parameter Estimation
3. Results
3.1. Descriptive Statistics
3.2. Environmental Effects of Earth Growth Traits
3.3. Variance Components and Genetic Parameter Estimates
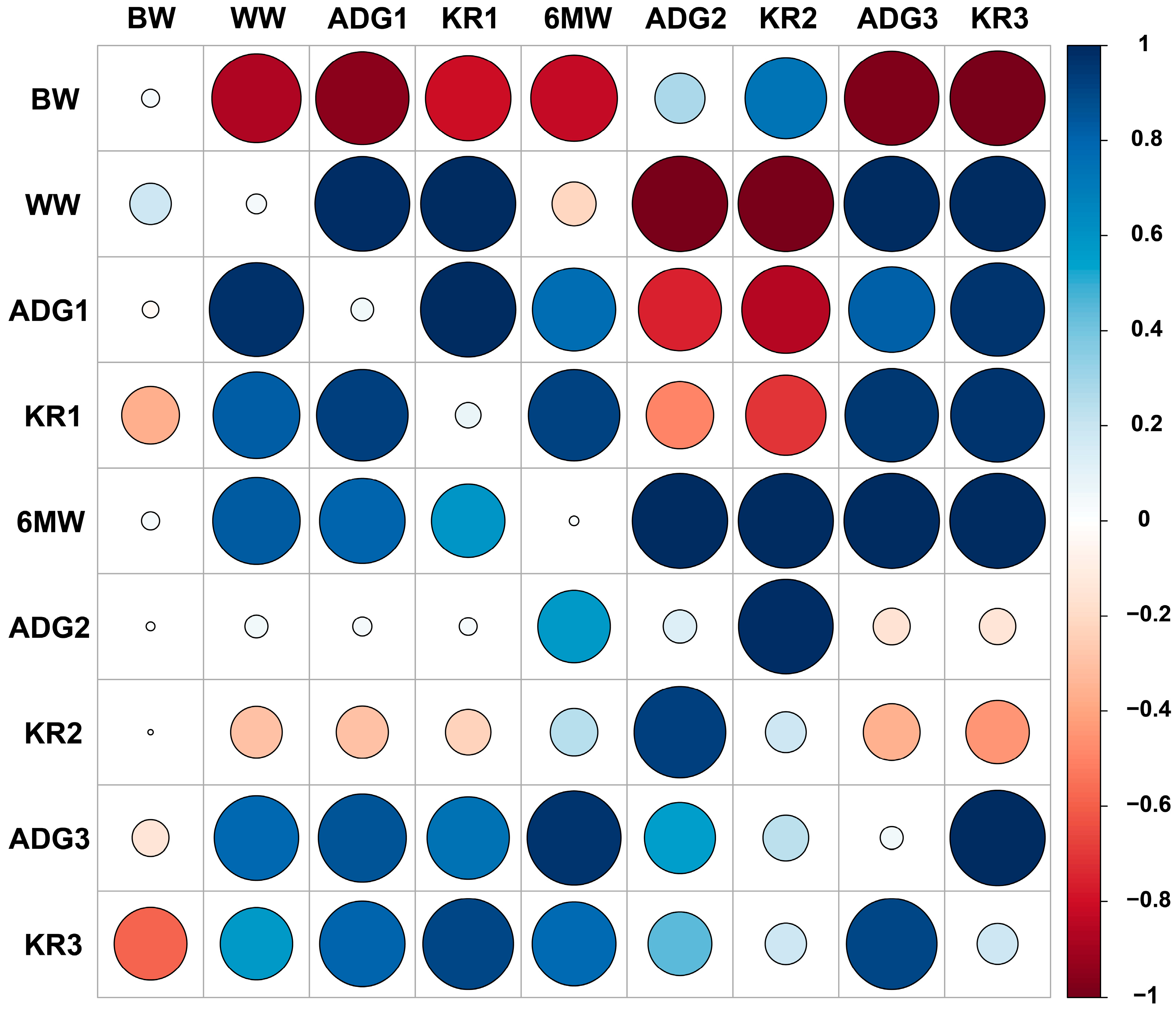
3.4. Heritability, Genetic and Phenotypic Correlation among Traits
4. Discussion
4.1. Environmental Effects
4.2. Model Comparisons
4.3. Genetic Parameters Estimate
4.4. Correlation Estimates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Wang, Q.; Dai, C.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; Hussain, T.; Yang, H. Effect of dietary protein level on growth, carcass characteristics, serum biochemical index, and meat quality of Hu male lambs. Small Rumin. Res. 2021, 194, 106294. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, D.; Su, Y.; Zheng, X.; Li, S.; Chen, L. Research on the authenticity of mutton based on machine vision technology. Foods 2022, 11, 3732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Liu, Y.; Pei, S.; Kong, Y.; Li, F.; Wang, W.; Yue, X. Preliminary genetic parameter estimates of meat quality traits in Hu sheep. Meat Sci. 2024, 212, 109476. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, Q.; Shi, J.; Fu, L.; Cheng, S. Screening of genes related to growth, development and meat quality of Sahan crossbred F1 sheep based on RNA-seq technology. Front. Vet. Sci. 2022, 9, 831519. [Google Scholar] [CrossRef] [PubMed]
- Casey, N.H.; Van Niekerk, W.A. The boer goat. I. Origin, adaptability, performance testing, reproduction and milk production. Small Rumin. Res. 1988, 1, 291–302. [Google Scholar] [CrossRef]
- Cloete, S.W.P.; Snyman, M.A.; Herselman, M.J. Productive performance of Dorper sheep. Small. Rumin. Res. 2000, 36, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Ptáček, M.; Ducháček, J.; Stádník, L.; Beran, J.; Němečková, D. Influence of selected factors on growth performance of Suffolk lambs and their crossbreds. J. Cent. Eur. Agric. 2015, 16, 188–196. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Zhang, Q.; He, Y.; Zhang, X.; Yang, L.; Shi, J. Comparative transcriptome analysis identifying the different molecular genetic markers related to production performance and meat quality in longissimus dorsi tissues of MG × STH and STH sheep. Genes 2020, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Akanno, E.; Chen, L.; Abo-Ismail, M.; Crowley, J.; Wang, Z.; Li, C.; Basarab, J.; Macneil, M.; Plastow, G. Genome-wide association scan for heterotic quantitative trait loci in multi-breed and crossbred beef cattle. Genet. Sel. Evol. 2018, 50, 48. [Google Scholar] [CrossRef]
- Sheet, S.; Jang, S.S.; Kim, J.H.; Park, W.; Kim, D. A transcriptomic analysis of skeletal muscle tissues reveals promising candidate genes and pathways accountable for different daily weight gain in Hanwoo cattle. Sci. Rep. 2024, 14, 315. [Google Scholar] [CrossRef]
- Sharif, N.; Ali, A.; Dawood, M.; Khan, M.I.; Do, D.N. Environmental effects and genetic parameters for growth traits of Lohi sheep. Animals 2022, 12, 3590. [Google Scholar] [CrossRef]
- Washaya, S.; Ngorio, U.; Mudzengi, C.P. Effects of the breeding season, birth type and sex on growth and reproductive performances of sheep breeds. Trop. Anim. Health Prod. 2023, 55, 188. [Google Scholar] [CrossRef] [PubMed]
- Kannan, T.A.; Jaganathan, M.; Ramanujam, R.; Chinnaondi, B.; Illa, S.K.; Kizilkaya, K.; Peters, S.O. Multi-trait Bayesian analysis and genetic parameter estimates in production characters of Mecheri sheep of India. Trop. Anim. Health Prod. 2022, 55, 8. [Google Scholar] [CrossRef] [PubMed]
- Hizli, H.; Takma, C.; Yazgan, E. Comparison of different models for estimation of direct and maternal genetic parameters on body weights in Awassi sheep. Arch. Anim. Breed. 2022, 65, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Jamshid, E. Estimates of (co)variance components and genetic parameters for pre-weaning body weight traits and Kleiber ratio in Sangsari sheep breed. Ital. J. Anim. Sci. 2021, 20, 918–927. [Google Scholar]
- Barazandeh, A.; Moghbeli, S.M.; Vatankhah, M.; Mohammadabadi, M. Estimating non-genetic and genetic parameters of pre-weaning growth traits in Raini cashmere goat. Trop. Anim. Health Prod. 2012, 44, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Areb, E.; Getachew, T.; Kirmani, M.; Abate, Z.; Haile, A. Estimation of (co)variance components, genetic parameters, and genetic trends of growth traits in community-based breeding programs of Bonga sheep. Animal 2021, 15, 100202. [Google Scholar] [CrossRef]
- Habtegiorgis, K.; Haile, A.; Getachew, T.; Kirmani, M.A.; Gemiyo, D. Analysis of genetic parameters and genetic trends for early growth and reproductive traits of Doyogena sheep managed under community-based breeding program. Heliyon 2022, 8, e09749. [Google Scholar] [CrossRef]
- SAS Institute. SAS User’s Guide Version 9.1. Statistics; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Meyer, K. WOMBAT—A tool for mixed model analyses in quantitative genetics by restricted maximum likelihood (REML). J. Zhejiang Univ. Sci. B 2007, 8, 815–821. [Google Scholar] [CrossRef]
- Boujenane, I.; Diallo, I.T. Estimates of genetic parameters and genetic trends for pre-weaning growth traits in Sardi sheep. Small Rumin. Res. 2017, 146, 61–68. [Google Scholar] [CrossRef]
- Balasundaram, B.; Thiruvenkadan, A.K.; Murali, N.; Muralidharan, J.; Cauveri, D.; Peters, S.O. Genetic parameters of growth traits and quantitative genetic metrics for selection and conservation of Mecheri sheep of Tamil Nadu. Animals 2023, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Maghsoudi, A.; Torshizi, R.V.; Jahanshahi, A.S. Estimates of (co)variance components for productive and composite reproductive traits in Iranian cashmere goats. Livest. Sci. 2009, 126, 162–167. [Google Scholar] [CrossRef]
- Alam, M.; Cho, C.I.; Choi, T.J.; Park, B.; Choi, J.G.; Choy, Y.H.; Lee, S.S.; Cho, K.H. Estimation of genetic parameters for somatic cell scores of Holsteins using multi-trait lactation models in Korea. Asian-Australas. J. Anim. Sci. 2015, 28, 303–310. [Google Scholar] [CrossRef]
- Yiting, Y.; Mailin, G.; Xidi, Y.; Ping, Z.; Yun, L.; Bin, L.; Kangping, Z.; Wenqiang, C.; Lei, C.; Ye, Z.; et al. Estimation of genetic parameters of pig reproductive traits. Front. Vet. Sci. 2023, 10, 1172287. [Google Scholar]
- Ou, J.H.; Rönneburg, T.; Carlborg, Ö.; Honaker, C.F.; Siegel, P.B.; Rubin, C.J. Complex genetic architecture of the chicken Growth1 QTL region. PLoS ONE 2024, 19, e0295109. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Latifi, M. Autosomal and sex-linked (co)variance components and genetic parameters for growth traits of Iranian Zandi sheep. Trop. Anim. Health Prod. 2020, 52, 1023–1032. [Google Scholar] [CrossRef]
- Hanoglu Oral, H.; Ozis Altincekic, S.; Duru, S. Effects of non-genetic factors on growth traits and survival rate in Karacabey Merino lambs. Pol. J. Vet. Sci. 2023, 26, 473–481. [Google Scholar] [CrossRef]
- Gul, S.; Arzik, Y.; Kizilaslan, M.; Behrem, S.; Keskin, M. Heritability and environmental influence on pre-weaning traits in Kilis goats. Trop. Anim. Health Prod. 2023, 55, 85. [Google Scholar] [CrossRef]
- Dhakad, G.; Saini, S.; Mallick, P.; Jatav, P.; Gowane, G.; Sharma, P. Non-genetic factors affecting lifetime body weights in ram lamb of Malpura sheep. Indian. J. Small Rumin. 2023, 29, 11–16. [Google Scholar] [CrossRef]
- Bruna Cristina, H.; Saulo Henrique, W.; Dhéri, M.; Cristina Santos, S. Productive performance of lambs born in different seasons of the year. Open. Vet. J. 2023, 13, 932–941. [Google Scholar]
- Aguirre, E.L.; Mattos, E.C.; Eler, J.P.; Barreto Neto, A.D.; Ferraz, J.B. Estimation of genetic parameters and genetic changes for growth characteristics of Santa Ines sheep. Genet. Mol. Res. 2016, 15, 4283. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi-Zefrehei, M.; Safari, A.; Moridi, M.; Khanzadeh, H.; Dehsaraei, A.R. Bayesian estimate of genetic parameters for growth traits in Lori Bakhtiari sheep. Trop. Anim. Health Prod. 2021, 53, 457. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, B.P.; Ajoy, M.; Arora, A.L.; Ramesh, K.; Satish, K.; Notter, D.R. Direct and maternal (co)variance components and heritability estimates for body weights in Chokla sheep. J. Anim. Breed. Genet. 2009, 126, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Khan, N.N.; Ganai, N.A.; Shanaz, S.; Rather, M.A.; Alam, S. Multivariate quantitative genetic analysis of body weight traits in Corriedale sheep. Trop. Anim. Health Prod. 2021, 53, 197. [Google Scholar] [CrossRef] [PubMed]
- Magotra, A.; Bangar, Y.C.; Chauhan, A.; Malik, B.S.; Malik, Z.S. Influence of maternal and additive genetic effects on offspring growth traits in Beetal goat. Reprod. Domest. Anim. 2021, 56, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Tesema, Z.; Alemayehu, K.; Getachew, T.; Kebede, D.; Deribe, B.; Taye, M.; Tilahun, M.; Lakew, M.; Kefale, A.; Belayneh, N.; et al. Estimation of genetic parameters for growth traits and Kleiber ratios in Boer × Central Highland goat. Trop. Anim. Health Prod. 2020, 52, 3195–3205. [Google Scholar] [CrossRef]
- Singh, M.K.; Dige, M.S.; Pourouchottamane, R.; Kumar, A.; Gowane, G.R. Influences of maternal factors on the estimate of genetic parameters for goat feed efficiency traits. Trop. Anim. Health Prod. 2022, 54, 376. [Google Scholar] [CrossRef] [PubMed]
- Besufkad, S.; Goshme, S.; Abebe, A.; Bisrat, A.; Abebe, A.; Zewdie, T.; Areaya, A.; Alemayehu, L.; Kebede, A.; Getachew, T. Genetic evaluation of growth rate and efficiency-related traits in Dorper × local crossbred sheep population. Trop. Anim. Health Prod. 2023, 55, 249. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Zhang, Y.; Xu, D.-Q.; Li, X.; Su, J.; Yang, L.-G. Genetic and phenotypic parameter estimates for growth traits in Boer goat. Livest. Sci. 2009, 124, 66–71. [Google Scholar] [CrossRef]
- Menendez-Buxadera, A.; Alexandre, G.; Mandonnet, N.; Naves, M.; Aumont, G. Direct genetic and maternal effects affecting litter size, birth weight and pre-weaning losses in Creole goats of Guadeloupe. Anim. Sci. 2003, 77, 363–369. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; He, J.; Zhang, M.; Liu, G.; Wei, C.; Zhang, G.; Zhang, W.; Nie, F.; Wang, M.; et al. Estimation of Genetic Parameters for Early Growth Traits in Luzhong Mutton Sheep. Animals 2024, 14, 1754. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, B.; Baneh, H. (Co)variance components and genetic parameters for growth traits in Arabi sheep using different animal models. Genet. Mol. Res. 2012, 11, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Ofori, S.A.; Hagan, J.K. Genetic and non-genetic factors influencing the performance of the West African Dwarf (WAD) goat kept at the Kintampo Goat Breeding Station of Ghana. Trop. Anim. Health Prod. 2020, 52, 2577–2584. [Google Scholar] [CrossRef]
- Dige, M.S.; Rout, P.K.; Singh, M.K.; Dass, G.; Kaushik, R.; Gowane, G.R. Estimation of co (variance) components and genetic parameters for growth and feed efficiency traits in Jamunapari goat. Small Rumin. Res. 2021, 196, 106317. [Google Scholar] [CrossRef]

| Item | BW, kg | WW, kg | ADG1, g | KR1 | 6MW, kg | ADG2, g | ADG3, g | KR2 | KR3 |
|---|---|---|---|---|---|---|---|---|---|
| No. of records | 4474 | 4474 | 4474 | 4474 | 1003 | 1003 | 1003 | 1003 | 1003 |
| No. of sires | 9 | 9 | 9 | 9 | 8 | 8 | 8 | 8 | 8 |
| No. of dams | 620 | 620 | 620 | 620 | 297 | 297 | 297 | 297 | 297 |
| Average litter size of sire | 497 | 497 | 497 | 497 | 125 | 125 | 125 | 125 | 125 |
| Average litter size of dam | 7.2 | 7.2 | 7.2 | 7.2 | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 |
| Mean | 4.20 | 31.39 | 302.08 | 22.63 | 53.93 | 232.20 | 274.55 | 11.58 | 13.74 |
| SD | 1.12 | 5.57 | 63.16 | 1.90 | 8.73 | 78.31 | 48.92 | 3.22 | 0.87 |
| CV (%) | 27 | 18 | 21 | 8 | 16 | 34 | 18 | 28 | 6 |
| Factors | n | BW, kg | WW, kg | ADG1, g | KR1 |
|---|---|---|---|---|---|
| Overall | 4474 | 4.20 ± 0.02 | 31.39 ± 0.08 | 302.08 ± 0.94 | 22.63 ± 0.03 |
| Dam age | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | |
| 2 | 1032 | 4.37 ± 0.03 a | 28.94 ± 0.13 d | 272.64 ± 1.38 d | 21.78 ± 0.05 d |
| 3 | 977 | 4.17 ± 0.02 b | 30.85 ± 0.16 c | 296.50 ± 1.72 c | 22.54 ± 0.05 c |
| 4 | 2277 | 4.19 ± 0.03 b | 32.39 ± 0.12 b | 313.26 ± 1.40 b | 22.91 ± 0.04 b |
| 5 | 188 | 3.41 ± 0.07 c | 35.57 ± 0.48 a | 357.36 ± 5.45 a | 24.37 ± 0.14 a |
| Sex | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | |
| Male | 2310 | 4.27 ± 0.02 a | 33.12 ± 0.12 a | 320.57 ± 1.31 a | 23.09 ± 0.04 a |
| Female | 2164 | 4.12 ± 0.02 b | 29.55 ± 0.11 b | 282.36 ± 1.23 b | 22.15 ± 0.04 b |
| Birth year | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | |
| 2019 | 203 | 4.72 ± 0.07 a | 29.21 ± 0.26 c | 272.08 ± 2.84 d | 21.59 ± 0.09 d |
| 2020 | 1451 | 4.23 ± 0.02 c | 29.55 ± 0.12 c | 281.12 ± 1.29 c | 22.09 ± 0.04 c |
| 2021 | 2201 | 4.33 ± 0.03 b | 31.46 ± 0.11 b | 301.52 ± 1.27 b | 22.56 ± 0.04 b |
| 2022 | 619 | 3.48 ± 0.03 d | 36.16 ± 0.25 a | 363.08 ± 2.83 a | 24.48 ± 0.07 a |
| Birth season | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | |
| Spring | 918 | 4.37 ± 0.03 a | 30.93 ± 0.17 b | 295.14 ± 1.84 b | 22.38 ± 0.05 b |
| Summer | 686 | 4.34 ± 0.03 a | 29.62 ± 0.16 d | 280.91 ± 1.75 d | 22.03 ± 0.06 c |
| Autumn | 847 | 4.05 ± 0.02 c | 30.13 ± 0.17 c | 289.83 ± 1.88 c | 22.42 ± 0.05 b |
| Winter | 2023 | 4.13 ± 0.03 b | 32.73 ± 0.13 a | 317.54 ± 1.57 a | 23.04 ± 0.05 a |
| Herd | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | |
| 1 | 108 | 3.99 ± 0.10 gh | 36.68 ± 0.44 b | 363.24 ± 5.06 b | 24.29 ± 0.13 b |
| 2 | 108 | 4.23 ± 0.09 ef | 35.59 ± 0.39 c | 348.42 ± 4.36 c | 23.86 ± 0.12 c |
| 3 | 804 | 4.36 ± 0.03 de | 29.98 ± 0.12 gh | 284.64 ± 1.29 f | 22.17 ± 0.04 f |
| 4 | 188 | 7.35 ± 0.06 a | 31.09 ± 0.31 ef | 263.87 ± 3.28 gh | 19.94 ± 0.11 i |
| 5 | 272 | 4.15 ± 0.06 fg | 29.64 ± 0.25 gh | 283.29 ± 2.82 f | 22.21 ± 0.09 f |
| 6 | 82 | 5.13 ± 0.15 b | 29.32 ± 0.49 h | 268.74 ± 4.94 g | 21.26 ± 0.16 h |
| 7 | 253 | 4.02 ± 0.06 gh | 33.87 ± 0.33 c | 331.68 ± 3.67 d | 23.52 ± 0.10 d |
| 8 | 176 | 4.30 ± 0.08 def | 31.51 ± 0.33 e | 302.42 ± 3.89 e | 22.63 ± 0.13 e |
| 9 | 408 | 3.65 ± 0.03 i | 26.70 ± 0.19 i | 255.12 ± 2.04 h | 21.67 ± 0.08 g |
| 10 | 250 | 4.44 ± 0.05 d | 30.23 ± 0.24 gh | 286.49 ± 2.66 f | 22.16 ± 0.08 f |
| 11 | 193 | 4.67 ± 0.06 c | 29.91 ± 0.33 gh | 280.39 ± 3.68 f | 21.81 ± 0.12 g |
| 12 | 322 | 3.90 ± 0.07 h | 36.91 ± 0.35 b | 366.83 ± 3.85 b | 24.36 ± 0.10 b |
| 13 | 1040 | 3.90 ± 0.68 h | 30.50 ± 0.16 fg | 295.54 ± 1.83 e | 22.62 ± 0.06 e |
| 14 | 270 | 3.18 ± 0.35 j | 38.07 ± 0.34 a | 387.66 ± 3.78 a | 25.21 ± 0.08 a |
| By*Bs*Herd | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| Factors | n | 6MW, kg | ADG2, g | KR2 | ADG3, g | KR3 |
|---|---|---|---|---|---|---|
| Overall | 1003 | 53.93 ± 0.28 | 232.20 ± 2.47 | 11.58 ± 0.10 | 274.55 ± 1.54 | 13.74 ± 0.03 |
| Dam age | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | |
| 2 | 284 | 48.79 ± 0.42 b | 203.17 ± 3.85 b | 10.91 ± 0.17 b | 246.09 ± 2.32 b | 13.30 ± 0.05 b |
| 3 | 80 | 56.44 ± 0.79 a | 214.66 ± 8.17 b | 10.40 ± 0.37 b | 288.92 ± 4.43 a | 13.99 ± 0.07 a |
| 4 | 623 | 55.90 ± 0.35 a | 246.80 ± 3.24 a | 12.00 ± 0.13 a | 285.34 ± 1.97 a | 13.90 ± 0.03 a |
| 5 | 16 | 56.00 ± 1.52 a | 266.28 ± 13.65 a | 12.99 ± 0.58 a | 287.58 ± 9.71 a | 14.01 ± 0.21 a |
| Sex | <0.001 *** | <0.001 *** | 0.0427 * | <0.001*** | <0.001 *** | |
| Male | 584 | 57.95 ± 0.32 a | 249.20 ± 3.51 a | 11.78 ± 0.15 a | 296.46 ± 1.81 a | 14.07 ± 0.03 a |
| Female | 419 | 48.33 ± 0.33 b | 208.50 ± 2.96 b | 11.29 ± 0.13 b | 244.01 ± 1.88 b | 13.28 ± 0.04 b |
| Birth year | <0.001 *** | <0.001 *** | <0.001 *** | <0.001*** | <0.001 *** | |
| 2019 | 66 | 50.52 ± 0.83 b | 226.19 ± 9.37 b | 11.80 ± 0.37 a | 254.22 ± 37.21 b | 13.38 ± 0.08 b |
| 2020 | 280 | 50.27 ± 0.48 b | 204.96 ± 3.73 c | 10.79 ± 0.16 b | 254.86 ± 44.28 b | 13.46 ± 0.05 b |
| 2021 | 657 | 55.84 ± 0.34 a | 244.41 ± 3.18 a | 11.89 ± 0.13 a | 284.98 ± 48.66 a | 13.90 ± 0.03 a |
| Birth season | <0.001 *** | <0.001 *** | <0.001 *** | <0.001*** | <0.001 *** | |
| Spring | 232 | 54.61 ± 0.49 b | 169.62 ± 5.31 c | 8.43 ± 0.25 c | 276.56 ± 39.29 b | 13.74 ± 0.04 b |
| Summer | 141 | 54.18 ± 0.73 b | 231.30 ± 6.85 b | 11.51 ± 0.28 b | 275.96 ± 34.70 b | 13.77 ± 0.07 a |
| Autumn | 66 | 56.38 ± 0.66 a | 271.12 ± 4.96 a | 13.03 ± 0.17 a | 290.65 ± 37.21 a | 14.05 ± 0.06 b |
| Winter | 564 | 52.25 ± 0.37 c | 232.16 ± 3.10 b | 11.86 ± 0.13 b | 264.38 ± 50.75 c | 13.56 ± 0.04 a |
| Traits | Models | AIC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | model1 | 0.0412 | 0.8022 | 0.8433 | 0.0488 ± 0.027 | 3798.326 | 3802.326 | ||||||
| model2 | 0.0525 | 0.4492 | 0.3722 | 0.8741 | 0.0601 ± 0.027 | 0.514 ± 0.027 | 3681.85 | 3687.854 | |||||
| model3 | 0.0067 | 0.0562 | 0.7825 | 0.8455 | 0.008 ± 0.010 | 0.0665 ± 0.014 | 3782.862 | 3788.862 | |||||
| model4 | 0.0323 | 0.1375 | −0.0656 | 0.7575 | 0.8617 | 0.0375 ± 0.049 | 0.1596 ± 0.048 | −0.984 ± 0.475 | 3777.286 | 3785.286 | |||
| model5 | 0.0049 | 0.0539 | 0.4401 | 0.3742 | 0.8722 | 0.0056 ± 0.009 | 0.0608 ± 0.013 | 0.5046 ± 0.027 | 3664.378 | 3672.378 | |||
| model6 | 0.0311 | 0.1217 | −0.0606 | 0.4342 | 0.3584 | 0.5294 | 0.0352 ± 0.049 | 0.1375 ± 0.046 | 0.4907 ± 0.027 | −0.9838 ± 0.511 | 3659.218 | 3669.218 | |
| WW | model1 | 0.9704 | 16.85 | 17.821 | 0.0545 ± 0.024 | 17,333.78 | 17,337.78 | ||||||
| model2 | 0.7913 | 3.8197 | 13.129 | 17.74 | 0.0446 ± 0.023 | 0.2153 ± 0.031 | 17,282.722 | 17,288.722 | |||||
| model3 | 0.9745 | 0.001 | 16.849 | 17.824 | 0.0547 ± 0.043 | 0.0001 ± 0.013 | 17,333.784 | 17,339.784 | |||||
| model4 | 1.0311 | 0.0014 | −0.0195 | 16.823 | 17.836 | 0.0578 ± 0.057 | 0.0001 ± 0.058 | −0.520 ± failed | 17,333.776 | 17,341.776 | |||
| model5 | 0.8021 | 0.001 | 3.8203 | 13.125 | 17.748 | 0.0452 ± 0.037 | 0.0001 ± 0.012 | 0.2153 ± 0.031 | 17,282.73 | 17,290.73 | |||
| model6 | 0.948 | 0.0051 | −0.0697 | 3.8296 | 13.086 | 17.799 | 0.0533 ± 0.051 | 0.0003 ± 0.104 | 0.2152 ± 0.031 | −0.999 ± failed | 17,282.534 | 17,292.534 | |
| ADG1 | model1 | 140.02 | 1984.1 | 2124.1 | 0.0659 ± 0.026 | 38,541.64 | 38,545.64 | ||||||
| model2 | 119.27 | 405.16 | 1591.5 | 2116 | 0.0564 ± 0.025 | 0.1915 ± 0.032 | 38,501.8 | 38,507.79 | |||||
| model3 | 139.99 | 0.0035 | 1984.1 | 2124.1 | 0.066 ± 0.045 | 0.000 ± 0.013 | 38,541.64 | 38,547.64 | |||||
| model4 | 152.79 | 0.2921 | −5.6014 | 1978.5 | 2126 | 0.0719 ± 0.062 | 0.0001 ± 0.047 | −0.838 ± failed | 38,541.61 | 38,549.61 | |||
| model5 | 119.19 | 0.0013 | 405.06 | 1591.7 | 2115.9 | 0.0563 ± 0.040 | 0.000 ± 0.012 | 0.1914 ± 0.032 | 38,501.79 | 38,509.79 | |||
| model6 | 120.23 | 0.001 | 0.3467 | 405.55 | 1593.2 | 2119.3 | 0.0567 ± 0.049 | 0.000 ± 0.048 | 0.1914 ± 0.032 | −1.000 ± failed | 38,501.81 | 38,511.81 | |
| KR1 | model1 | 0.1504 | 1.7 | 1.8505 | 0.0813 ± 0.028 | 7246.636 | 7250.636 | ||||||
| model2 | 0.1336 | 0.2721 | 1.4404 | 1.8461 | 0.0724 ± 0.027 | 0.1474 ± 0.033 | 7225.854 | 7231.854 | |||||
| model3 | 0.1327 | 0.0074 | 1.7066 | 1.8467 | 0.0719 ± 0.045 | 0.004 ± 0.014 | 7246.53 | 7252.53 | |||||
| model4 | 0.1252 | 0.0031 | 0.0063 | 1.7103 | 1.845 | 0.0679 ± 0.055 | 0.0017 ± 0.035 | 0.321 ± failed | 7246.52 | 7254.52 | |||
| model5 | 0.1313 | 0.0010 | 0.2718 | 1.4415 | 1.8457 | 0.0712 ± 0.045 | 0.0005 ± 0.014 | 0.1473 ± 0.033 | 7225.856 | 7233.856 | |||
| model6 | 0.1304 | 0.001 | 0.0005 | 0.2717 | 1.442 | 1.8456 | 0.0707 ± 0.057 | 0.001 ± 0.037 | 0.1473 ± 0.034 | 0.041 ± failed | 7225.856 | 7235.856 | |
| 6MW | model1 | 0.4701 | 48.319 | 48.79 | 0.010 ± 0.033 | 4894.378 | 4898.378 | ||||||
| model2 | 0.3762 | 6.1416 | 42.243 | 48.761 | 0.008 ± 0.031 | 0.126 ± 0.118 | 4893.094 | 4899.094 | |||||
| model3 | 0.4741 | 0.001 | 48.317 | 48.792 | 0.010 ± 0.035 | 0.000 ± 0.038 | 4894.378 | 4900.378 | |||||
| model4 | 0.5019 | 0.001 | 0.0223 | 48.385 | 48.91 | 0.0103 ± 0.036 | 0.000 ± 0.181 | 0.9978 ± failed | 4894.39 | 4902.39 | |||
| model5 | 0.3763 | 0.001 | 6.1428 | 42.243 | 48.763 | 0.008 ± 0.032 | 0.000 ± 0.040 | 0.126 ± 0.123 | 4893.096 | 4901.096 | |||
| model6 | 0.4103 | 0.001 | 0.019 | 6.2784 | 42.24 | 48.949 | 0.0084 ± 0.033 | 0.000 ± 0.178 | 0.1283 ± 0.124 | 0.940 ± failed | 4893.12 | 4903.12 | |
| ADG2 | model1 | 478.39 | 3415.7 | 3894.1 | 0.123 ± 0.092 | 9222.832 | 9226.832 | ||||||
| model2 | 496.09 | 11.855 | 3417.3 | 3925.2 | 0.1264 ± 0.095 | 0.003 ± 0.140 | 9222.898 | 9228.898 | |||||
| model3 | 270.79 | 127.68 | 3453.2 | 3851.7 | 0.0703 ± 0.078 | 0.0331 ± 0.042 | 9222.266 | 9228.266 | |||||
| model4 | 304.62 | 523.96 | −399.34 | 3428.7 | 3857.9 | 0.079 ± 0.089 | 0.1358 ± 0.207 | −1.000 ± failed | 9221.762 | 9229.762 | |||
| model5 | 270.87 | 128.82 | 24.683 | 3472.6 | 3897 | 0.0695 ± 0.078 | 0.0331 ± 0.043 | 0.0063 ± 0.142 | 9222.428 | 9230.428 | |||
| model6 | 304.73 | 523.58 | −399.4 | 0.0179 | 3428.6 | 3857.5 | 0.079 ± 0.089 | 0.1357 ± 0.207 | 0.000 ± 0.139 | −1.000 ± failed | 9221.762 | 9231.762 | |
| ADG3 | model1 | 91.285 | 1483.4 | 1574.7 | 0.058 ± 0.067 | 8338.078 | 8342.078 | ||||||
| model2 | 77.436 | 174.94 | 1319.4 | 1571.8 | 0.0493 ± 0.062 | 0.1113 ± 0.122 | 8337.174 | 8343.174 | |||||
| model3 | 69.365 | 20.03 | 1481.1 | 1570.5 | 0.0442 ± 0.065 | 0.0128 ± 0.039 | 8337.974 | 8343.974 | |||||
| model4 | 61.819 | 8.2343 | 20.938 | 1480 | 1571 | 0.0394 ± 0.064 | 0.0052 ± 0.200 | 0.928 ± failed | 8337.838 | 8345.838 | |||
| model5 | 75.273 | 2.2468 | 172.57 | 1321.3 | 1571.4 | 0.048 ± 0.069 | 0.0014 ± 0.040 | 0.110 ± 0.126 | 8337.174 | 8345.174 | |||
| model6 | 63.827 | 2.2126 | 11.862 | 162.08 | 1329.2 | 1569.2 | 0.041 ± 0.065 | 0.0014 ± 0.192 | 0.1033 ± 0.126 | 0.998 ± failed | 8337.108 | 8347.108 | |
| KR2 | model1 | 1.2795 | 5.7266 | 7.0062 | 0.1826 ± 0.103 | 2925.038 | 2929.038 | ||||||
| model2 | 1.4163 | 0.0026 | 5.9146 | 7.3355 | 0.1931 ± 0.108 | 0.0006 ± 0.142 | 2925.964 | 2931.964 | |||||
| model3 | 0.6241 | 0.2951 | 5.9449 | 6.8641 | 0.0909 ± 0.096 | 0.043 ± 0.044 | 2924.254 | 2930.254 | |||||
| model4 | 0.6061 | 0.9196 | −0.5659 | 5.9033 | 6.8631 | 0.0883 ± 0.100 | 0.134 ± 0.178 | −0.758 ± failed | 2923.618 | 2931.618 | |||
| model5 | 0.624 | 0.2953 | 0.001 | 5.9462 | 6.8665 | 0.0909 ± 0.096 | 0.043 ± 0.045 | 0.0001 ± 0.142 | 2924.256 | 2932.256 | |||
| model6 | 0.6052 | 0.9215 | −0.5678 | 0.001 | 5.9047 | 6.8647 | 0.0882 ± 0.100 | 0.1342 ± 0.178 | 0.0001 ± 0.141 | −0.760 ± failed | 2923.62 | 2933.62 | |
| KR3 | model1 | 0.1064 | 0.4731 | 0.5796 | 0.1837 ± 0.096 | 447.652 | 451.652 | ||||||
| model2 | 0.0936 | 0.1303 | 0.3533 | 0.5773 | 0.1621 ± 0.095 | 0.2258 ± 0.121 | 444.804 | 450.804 | |||||
| model3 | 0.041 | 0.0452 | 0.4801 | 0.5662 | 0.0724 ± 0.075 | 0.0798 ± 0.043 | 444.032 | 450.032 | |||||
| model4 | 0.0443 | 0.0671 | −0.0213 | 0.4783 | 0.5684 | 0.0779 ± 0.090 | 0.118 ± 0.144 | −0.390 ± failed | 443.406 | 451.406 | |||
| model5 | 0.043 | 0.0387 | 0.1021 | 0.3831 | 0.5669 | 0.0758 ± 0.077 | 0.0682 ± 0.044 | 0.1801 ± 0.125 | 442.308 | 450.308 | |||
| model6 | 0.0409 | 0.0568 | −0.0166 | 0.0998 | 0.3855 | 0.5664 | 0.0722 ± 0.085 | 0.1003 ± 0.143 | 0.1762 ± 0.126 | −0.346 ± failed | 441.768 | 451.768 |
| Model | DF | BW | WW | ADG1 | KR1 | 6MW | ADG2 | ADG3 | KR2 | KR3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1:2 | 1 | 116.472 *** | 51.058 *** | 39.846 *** | 20.782 *** | 1.284 ns | −0.066 ns | −0.002 ns | −0.926 ns | 2.848 ns |
| 1:3 | 1 | 15.464 *** | −0.004 ns | 0 ns | 0.106 ns | 0 ns | 0.566 ns | 1.028 ns | 0.784 ns | 3.62 ns |
| 1:4 | 2 | 21.040 *** | 0.004 ns | 0.022 ns | 0.116 ns | −0.012 ns | 1.07 ns | 1.264 ns | 1.42 ns | 4.246 ns |
| 1:5 | 2 | 133.948 *** | 51.050 *** | 39.846 *** | 20.78 *** | 1.282 ns | 0.404 ns | 1.026 ns | 0.782 ns | 5.344 ns |
| 1:6 | 3 | 139.108 *** | 51.246 *** | 39.826 *** | 20.78 *** | 1.258 ns | 1.07 ns | 1.264 ns | 1.418 ns | 5.884 ns |
| 2:5 | 1 | 17.476 *** | −0.008 ns | 0 ns | −0.002 ns | −0.002 ns | 0.47 ns | 1.028 ns | 1.708 ns | 2.496 ns |
| 2:6 | 2 | 22.636 *** | 0.188 ns | −0.02 ns | −0.002 ns | −0.026 ns | 1.136 ns | 1.266 ns | 2.344 ns | 3.036 ns |
| 3:4 | 1 | 5.576 * | 0.008 ns | 0.022 ns | 0.01 ns | −0.012 ns | 0.504 ns | 0.236 ns | 0.636 ns | 0.626 ns |
| 3:5 | 1 | 118.484 *** | 51.054 *** | 39.846 *** | 20.674 *** | 1.282 ns | −0.162 ns | −0.002 ns | −0.002 ns | 1.724 ns |
| 3:6 | 2 | 123.644 *** | 51.250 *** | 39.826 *** | 20.674 *** | 1.258 ns | 0.504 ns | 0.236 ns | 0.634 ns | 2.264 ns |
| 4:6 | 1 | 118.068 *** | 51.242 *** | 39.804 *** | 20.664 *** | 1.270 ns | 0 ns | 0 ns | −0.002 ns | 1.638 ns |
| 5:6 | 1 | 5.160 * | 0.196 ns | −0.02 ns | 0 ns | −0.024 ns | 0.666 ns | 0.238 ns | 0.636 ns | 0.540 ns |
| Trait | BW | WW | ADG1 | KR1 | 6MW | ADG2 | KR2 | ADG3 | KR3 |
|---|---|---|---|---|---|---|---|---|---|
| BW | 0.0352 | −0.8748 | −0.9548 | −0.8029 | −0.8244 | 0.2756 | 0.7300 | −0.9787 | −0.9909 |
| WW | 0.1890 | 0.0446 | 0.9859 | 0.9911 | −0.2131 | −0.9980 | −0.9989 | 0.9953 | 0.9951 |
| ADG1 | −0.0302 | 0.9752 | 0.0564 | 0.9964 | 0.7623 | −0.7568 | −0.8522 | 0.8119 | 0.9645 |
| KR1 | −0.3665 | 0.8262 | 0.9213 | 0.0724 | 0.9185 | −0.5000 | −0.7088 | 0.9574 | 0.9646 |
| 6MW | 0.0359 | 0.8397 | 0.8039 | 0.5936 | 0.0100 | 0.9978 | 0.9929 | 0.9998 | 0.9998 |
| ADG2 | 0.0085 | 0.0582 | 0.0404 | 0.0351 | 0.5786 | 0.1230 | 0.9840 | −0.1504 | −0.1460 |
| KR2 | 0.0031 | −0.2927 | −0.2988 | −0.227 | 0.2482 | 0.9244 | 0.1826 | −0.3544 | −0.4427 |
| ADG3 | −0.1490 | 0.7836 | 0.8512 | 0.7439 | 0.9691 | 0.5544 | 0.2300 | 0.0580 | 0.9950 |
| KR3 | −0.5829 | 0.5767 | 0.8012 | 0.9033 | 0.7721 | 0.4477 | 0.1878 | 0.9023 | 0.1837 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, X.; Liu, B.; Zhang, L.; Li, J.; Chen, D.; Ma, Y.; He, H.; Liu, J.; Liu, Y.; et al. Estimation of Genetic Parameters of Early Growth Traits in Dumeng Sheep. Animals 2024, 14, 2298. https://doi.org/10.3390/ani14162298
Wang R, Wang X, Liu B, Zhang L, Li J, Chen D, Ma Y, He H, Liu J, Liu Y, et al. Estimation of Genetic Parameters of Early Growth Traits in Dumeng Sheep. Animals. 2024; 14(16):2298. https://doi.org/10.3390/ani14162298
Chicago/Turabian StyleWang, Ruijun, Xinle Wang, Baodong Liu, Lifei Zhang, Jing Li, Dayong Chen, Yunhui Ma, Huijie He, Jie Liu, Yongbin Liu, and et al. 2024. "Estimation of Genetic Parameters of Early Growth Traits in Dumeng Sheep" Animals 14, no. 16: 2298. https://doi.org/10.3390/ani14162298
APA StyleWang, R., Wang, X., Liu, B., Zhang, L., Li, J., Chen, D., Ma, Y., He, H., Liu, J., Liu, Y., & Zhang, Y. (2024). Estimation of Genetic Parameters of Early Growth Traits in Dumeng Sheep. Animals, 14(16), 2298. https://doi.org/10.3390/ani14162298





