Phagocytic Function and Flow Cytometric Phenotype of Asian Elephant Monocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Population, Sample Information, and Institutional Approval
2.2. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
2.3. Monocyte Isolation and Dissociation
2.4. Particle Phagocytosis for Spectrophotometry
2.5. Fluorescence Microscopy
2.6. Flow Cytometry
2.7. Statistical Analysis
3. Results
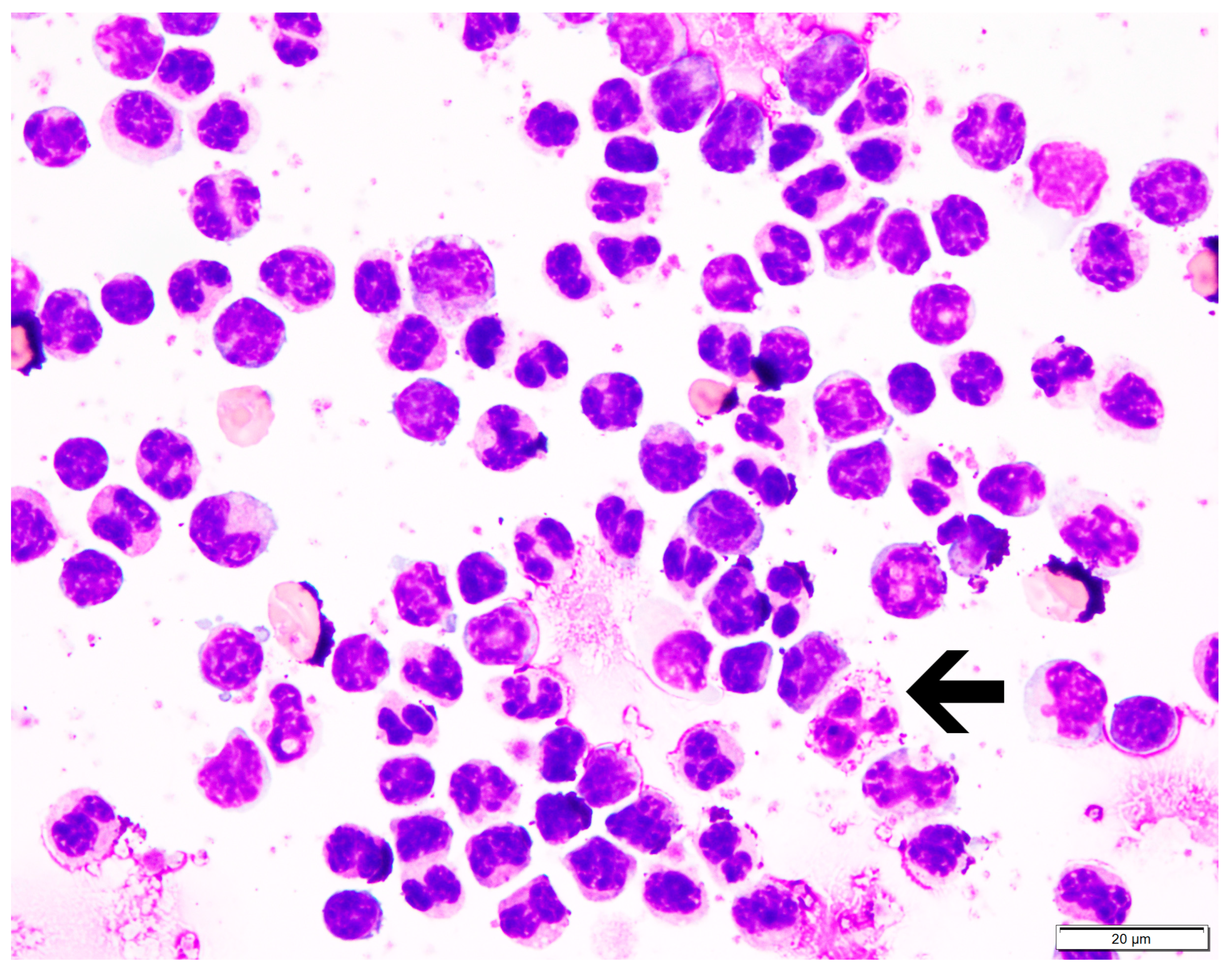
3.1. PBMC Purification via Density Centrifugation
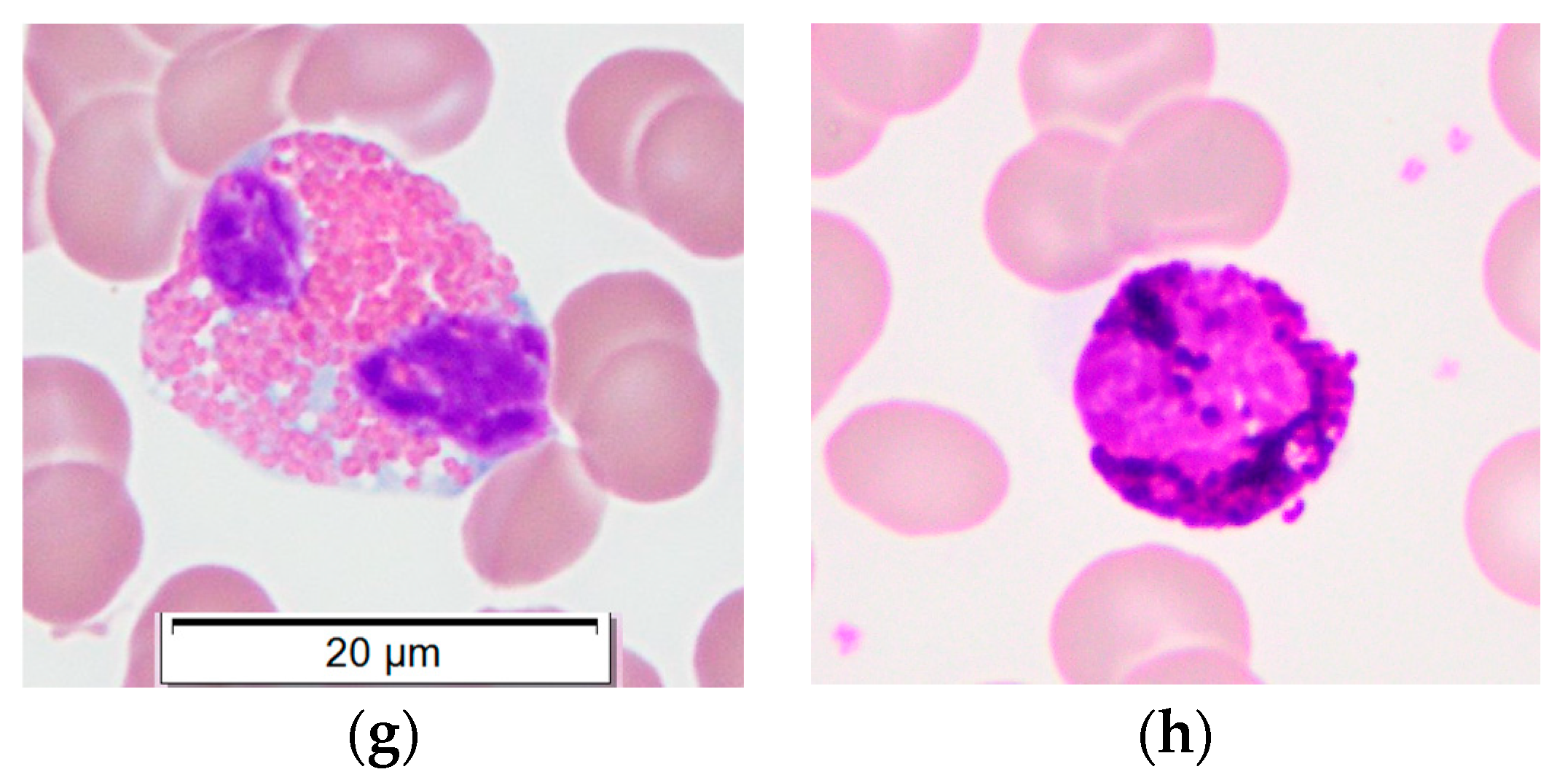
3.2. Monocyte Isolation and Dissociation
3.3. Phagocytosis of Fluorescent Zymosan Particles, Measured Via
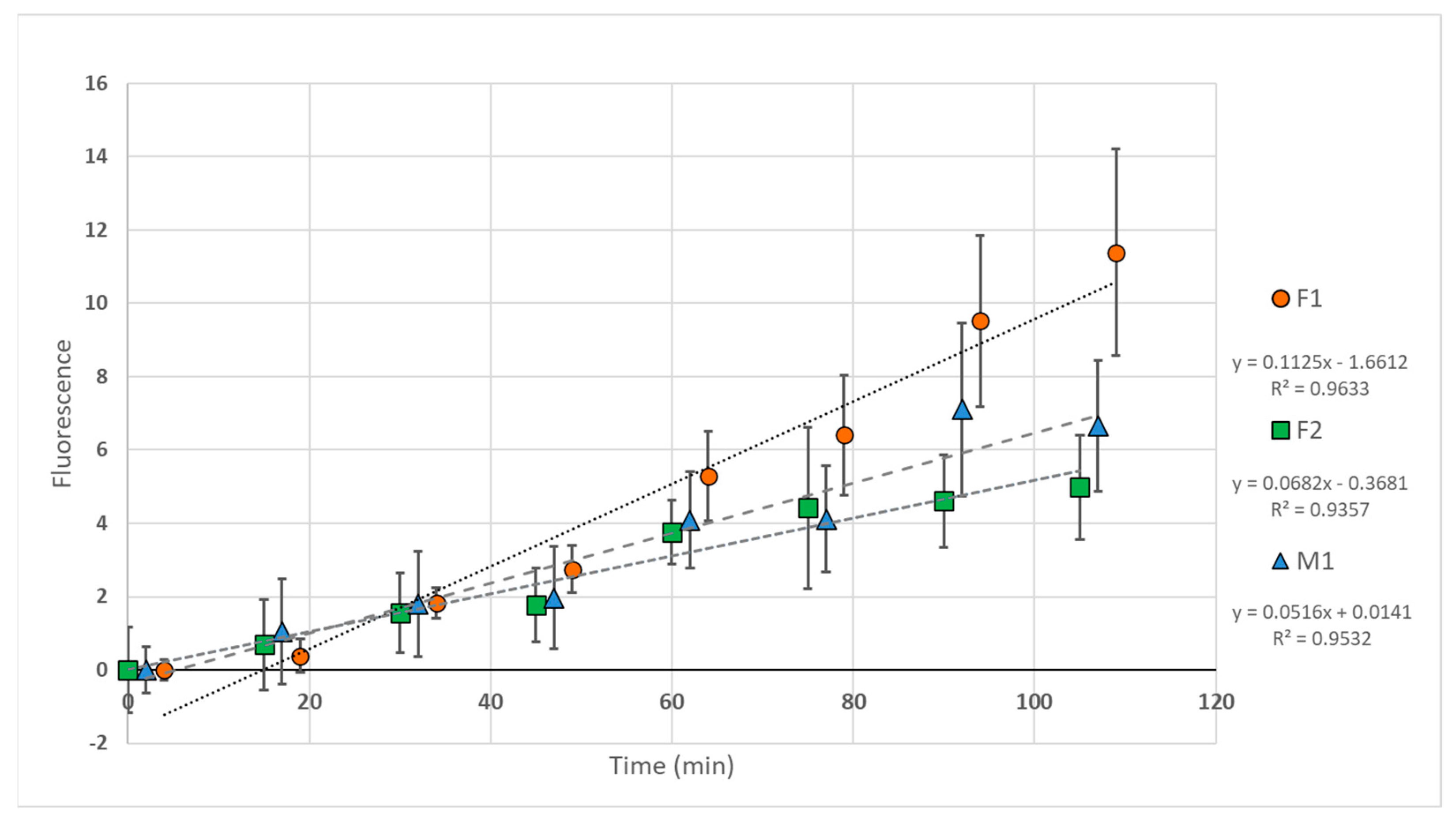
3.3.1. Spectrophotometry
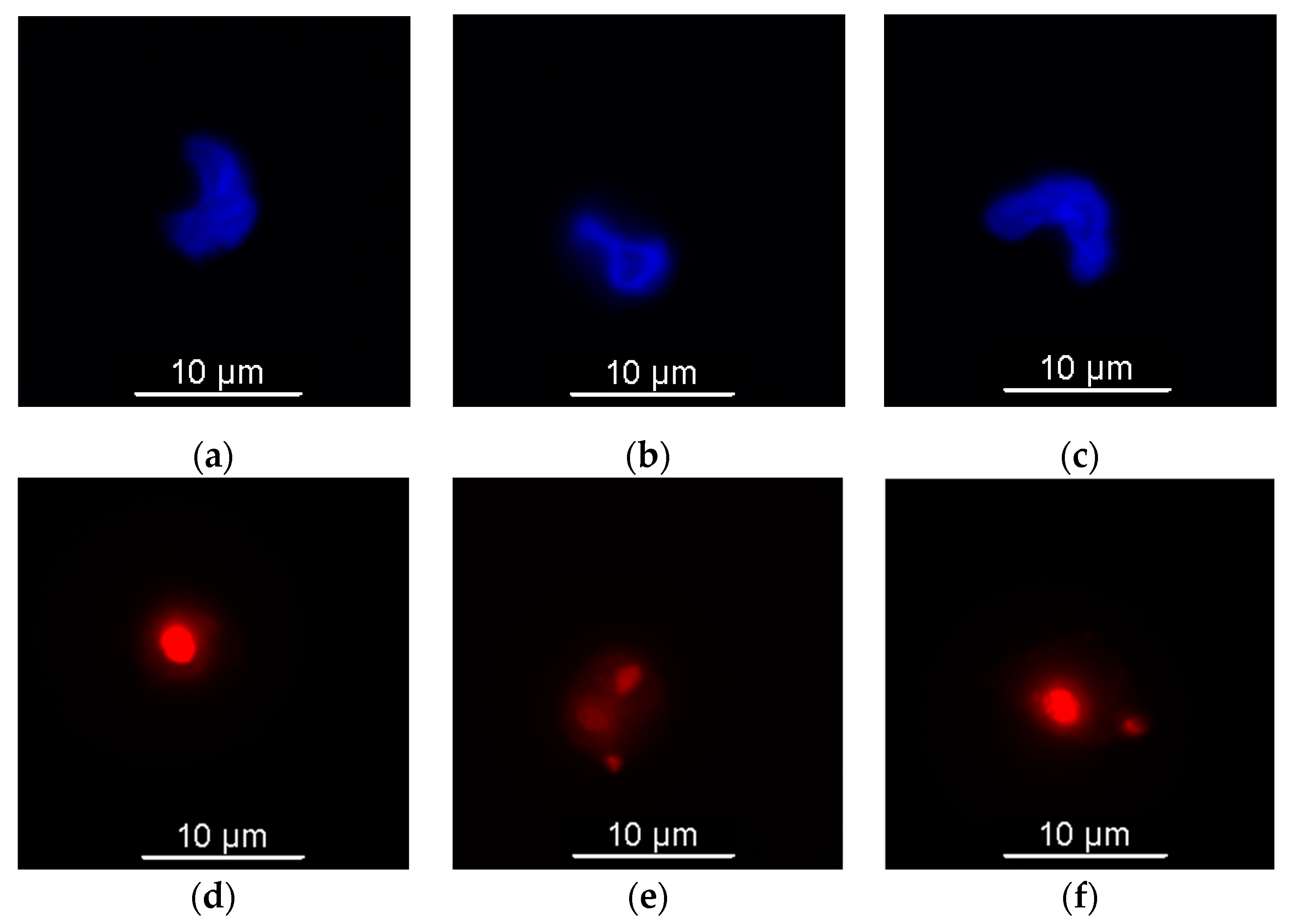
3.3.2. Fluorescence Microscopy
3.4. Flow Cytometry
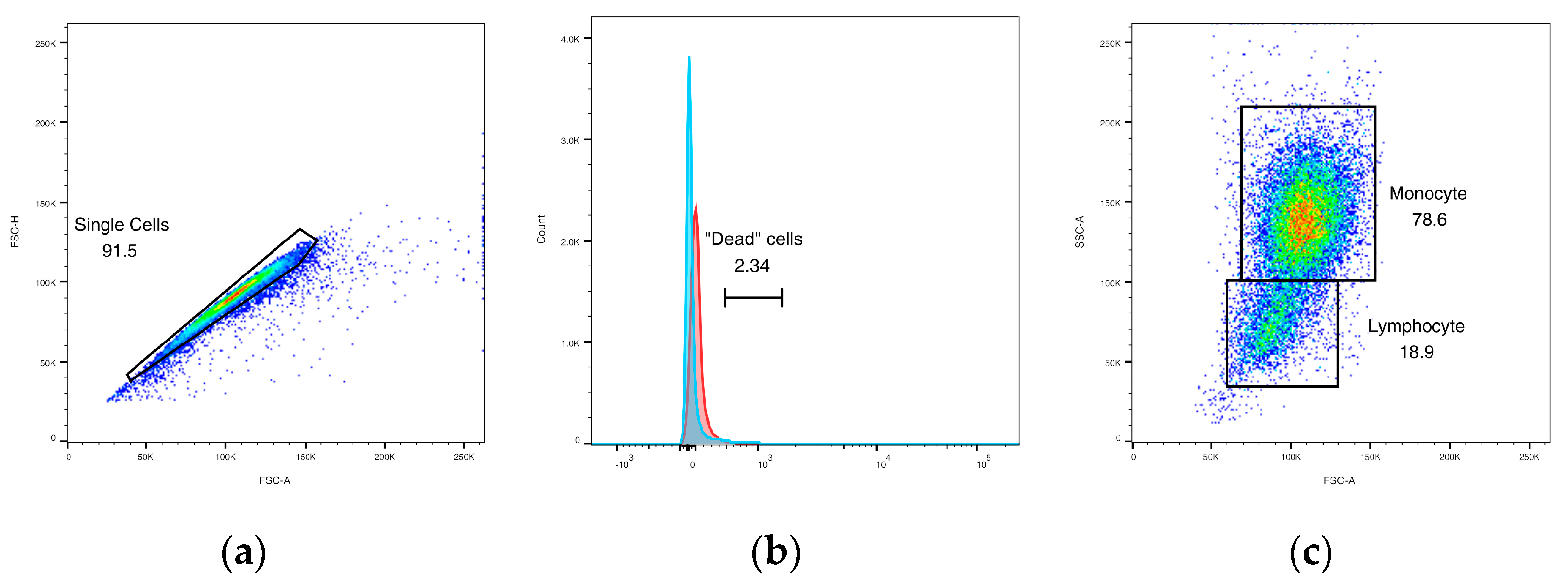
3.4.1. Initial Gating
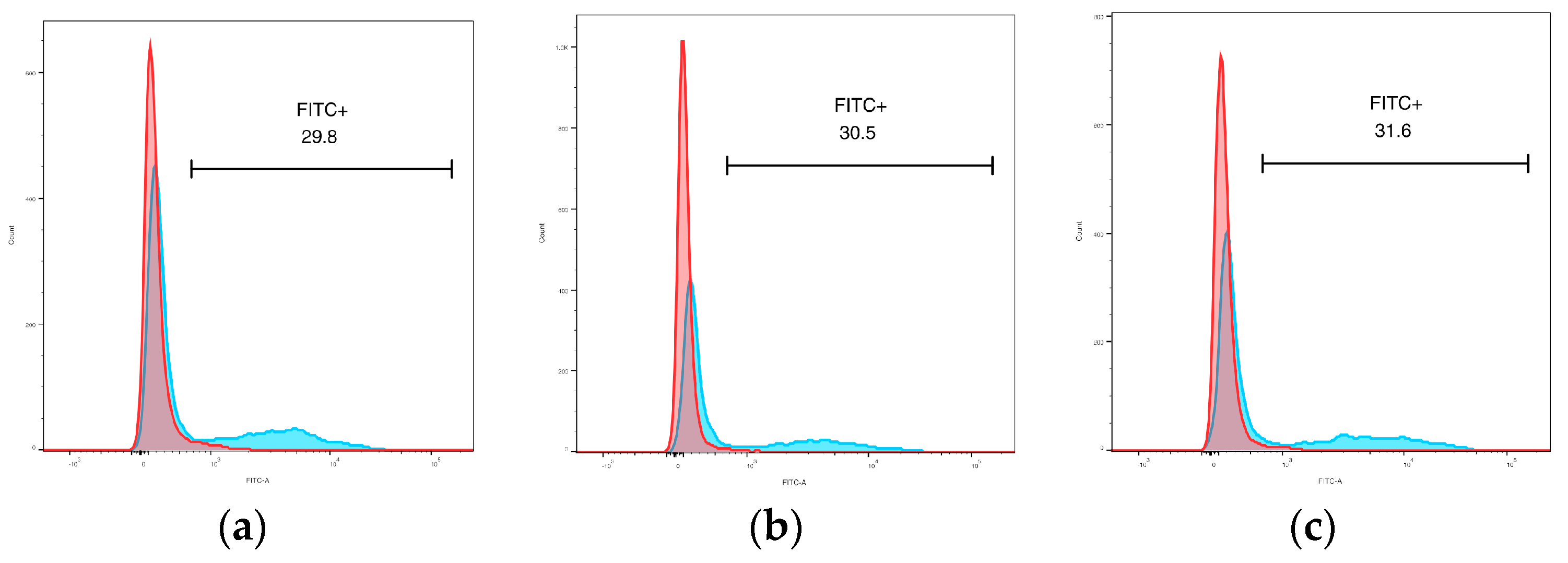
3.4.2. Phagocytic Activity
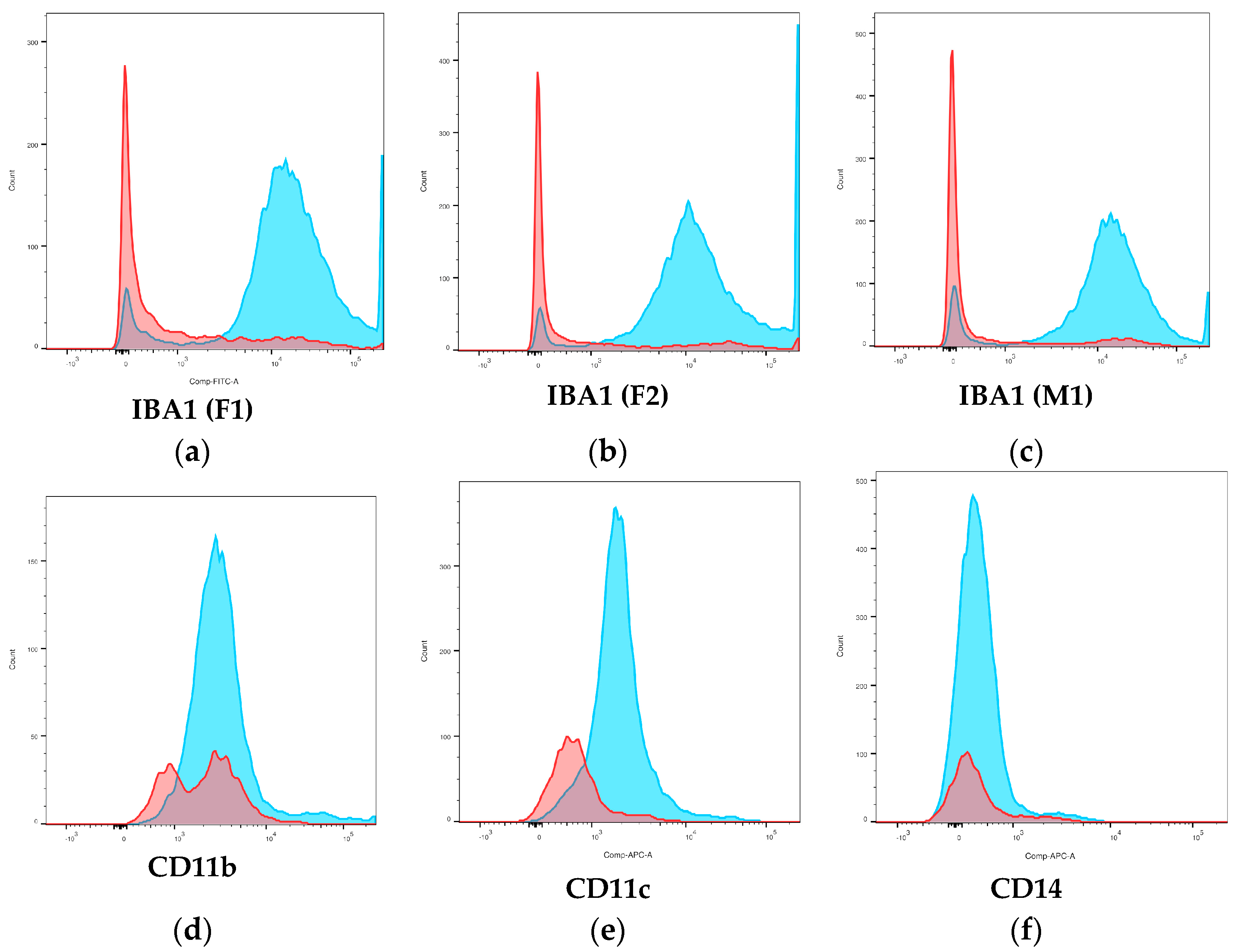
3.4.3. Surface Phenotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gobush, K.S.; Edwards, C.T.T.; Balfour, D.; Wittemyer, G.; Maisels, F.; Taylor, R.D. Loxodonta africana (Amended Version of 2021 Assessment). The IUCN Red List of Threatened Species 2022. Available online: https://www.iucnredlist.org/species/181008073/223031019 (accessed on 12 June 2024).
- Richman, L.K.; Montali, R.J.; Cambre, R.C.; Schmitt, D.; Hardy, D.; Hildbrandt, T.; Bengis, R.G.; Hamzeh, F.M.; Shahkolahi, A.; Hayward, G.S. Clinical and Pathological Findings of a Newly Recognized Disease of Elephants Caused by Endotheliotropic Herpesviruses. J. Wildl. Dis. 2000, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Long, S.Y.; Latimer, E.M.; Hayward, G.S. Review of Elephant Endotheliotropic Herpesviruses and Acute Hemorrhagic Disease. ILAR J. 2016, 56, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Mikota, S.K.; Maslow, J.N. Tuberculosis at the Human–Animal Interface: An Emerging Disease of Elephants. Tuberculosis 2011, 91, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Boonprasert, K.; Yun, Y.; Kosaruk, W.; Towiboon, P.; Tankaew, P.; Punyapornwithaya, V.; Janyamathakul, T.; Muanghong, P.; Brown, J.L.; Thitaram, C.; et al. A Longitudinal Study of Hematology and Stress Biomarker Profiles in Young Asian Elephants (Elephas maximus) in Relation to Elephant Endotheliotropic Herpesvirus (EEHV) in Thailand. Animals 2021, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Stacy, N.I.; Isaza, R.; Wiedner, E. First Report of Changes in Leukocyte Morphology in Response to Inflammatory Conditions in Asian and African Elephants (Elephas maximus and Loxodonta africana). PLoS ONE 2017, 12, e0185277. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, T.C.; Isaza, R.; Cray, C.; Adler, L.; Stacy, N.I. The Importance of Manual White Blood Cell Differential Counts and Platelet Estimates in Elephant Hematology: Blood Film Review Is Essential. Vet. Q. 2021, 41, 30–35. [Google Scholar] [CrossRef]
- Steyrer, C.; Miller, M.; Hewlett, J.; Buss, P.; Hooijberg, E.H. Reference Intervals for Hematology and Clinical Chemistry for the African Elephant (Loxodonta africana). Front. Vet. Sci. 2021, 8, 599387. [Google Scholar] [CrossRef]
- Silva, I.D.; Kuruwita, V.Y. Hematology, Plasma, and Serum Biochemistry Values in Domesticated Elephants (Elephas maximus ceylonicus) in Sri Lanka. J. Zoo Wildl. Med. 1993, 24, 434–439. [Google Scholar]
- Harr, K.; Isaza, R.; Blue, J. Hematology of Elephants. In Schalm’s Veterinary Hematology; Weiss, D., Wardrop, K., Eds.; Blackwell Publishing: Ames, IA, USA, 2010; pp. 942–949. [Google Scholar]
- Allen, J.; Jacobson, E.; Harvey, J.; Boyce, W. Hematologic and Serum Chemical Values for Young African Elephants (Loxodonta african) [Sic] with Variations for Sex and Age. J. Zoo Wildl. Med. 1985, 16, 98–101. [Google Scholar] [CrossRef]
- Harvey, J.W.; Harr, K.E.; Murphy, D.; Walsh, M.T.; Nolan, E.C.; Bonde, R.K.; Pate, M.G.; Deutsch, C.J.; Edwards, H.H.; Clapp, W.L. Hematology of Healthy Florida Manatees (Trichechus manatus). Vet. Clin. Pathol. 2009, 38, 183–193. [Google Scholar] [CrossRef]
- Aroch, I.; King, R.; Baneth, G. Hematology and Serum Biochemistry Values of Trapped, Healthy, Free-Ranging Rock Hyraxes (Procavia capensis) and Their Association with Age, Sex, and Gestational Status. Vet. Clin. Pathol. 2007, 36, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Medway, W.; Black, D.J.; Rathbun, G.B. Hematology of the West Indian Manatee (Trichechus manatus). Vet. Clin. Pathol. 1982, 11, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Cagle, L.A.; Stacy, N.I.; Harvey, J.W.; de Wit, M.; Adler, L.; Walsh, M.; Bonde, R.; Stokol, T. Cytochemical Staining of Leukocytes and Platelets in the Florida Manatee (Trichechus manatus latirostris): Identification of a Bilobed Monocyte Similar to Other Members of the Paenungulata. Front. Vet. Sci. 2023, 10, 1149000. [Google Scholar] [CrossRef] [PubMed]
- Srivorakul, S.; Guntawang, T.; Kochagul, V.; Photichai, K.; Sittisak, T.; Janyamethakul, T.; Boonprasert, K.; Khammesri, S.; Langkaphin, W.; Punyapornwithaya, V.; et al. Possible Roles of Monocytes/Macrophages in Response to Elephant Endotheliotropic Herpesvirus (EEHV) Infections in Asian Elephants (Elephas maximus). PLoS ONE 2019, 14, e0222158. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, A.; Seilern-Moy, K.; Darpel, K.; Steinbach, F.; Molenaar, F. Surviving and Fatal Elephant Endotheliotropic Herpesvirus-1A Infections in Juvenile Asian Elephants—Lessons Learned and Recommendations on Anti-Herpesviral Therapy. BMC Vet. Res. 2016, 12, 178. [Google Scholar] [CrossRef]
- Perrin, K.L.; Kristensen, A.T.; Gray, C.; Nielsen, S.S.; Bertelsen, M.F.; Kjelgaard-Hansen, M. Biological Variation of Hematology and Biochemistry Parameters for the Asian Elephant (Elephas maximus), and Applicability of Population-Derived Reference Intervals. J. Zoo Wildl. Med. 2020, 51, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Fuery, A.; Leen, A.M.; Peng, R.; Wong, M.C.; Liu, H.; Ling, P.D. Asian Elephant T Cell Responses to Elephant Endotheliotropic Herpesvirus. J. Virol. 2018, 92, e01951-17. [Google Scholar] [CrossRef] [PubMed]
- Landolfi, J.A.; Terio, K.A.; Miller, M.; Junecko, B.F.; Reinhart, T. Pulmonary Tuberculosis in Asian Elephants (Elephas maximus): Histologic Lesions with Correlation to Local Immune Responses. Vet. Pathol. 2015, 52, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Miksa, M.; Komura, H.; Wu, R.; Shah, K.G.; Wang, P. A Novel Method to Determine the Engulfment of Apoptotic Cells by Macrophages Using PHrodo Succinimidyl Ester. J. Immunol. Methods 2009, 342, 71–77. [Google Scholar] [CrossRef]
- Pierezan, F.; Mansell, J.; Ambrus, A.; Hoffmann, A.R. Immunohistochemical Expression of Ionized Calcium Binding Adapter Molecule 1 in Cutaneous Histiocytic Proliferative, Neoplastic and Inflammatory Disorders of Dogs and Cats. J. Comp. Pathol. 2014, 151, 347–351. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.-P.; Ziober, A.; Zhang, P.J.; Bagg, A. Ionized Calcium Binding Adaptor Molecule 1 (IBA1). Am. J. Clin. Pathol. 2021, 156, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Perrin, K.L.; Kristensen, A.T.; Bertelsen, M.F.; Denk, D. Retrospective Review of 27 European Cases of Fatal Elephant Endotheliotropic Herpesvirus-Haemorrhagic Disease Reveals Evidence of Disseminated Intravascular Coagulation. Sci. Rep. 2021, 11, 14173. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N. Role of Tissue Factor in Hemostasis and Thrombosis. Blood Cells Mol. Dis. 2006, 36, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Mikota, S.K.; Peddie, L.; Peddie, J.; Isaza, R.; Dunker, F.; West, G.; Lindsay, W.; Larsen, R.S. Epidemiology and Diagnosis of Mycobacterium tuberculosis in Captive Asian Elephants (Elephas maximus). J. Zoo Wildl. Med. 2001, 32, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Finnegan, M.; Storms, T.; Garner, M.; Lyashchenko, K.P. Outbreak of Mycobacterium tuberculosis in a Herd of Captive Asian Elephants (Elephas maximus): Antemortem Diagnosis, Treatment, and Lessons Learned. J. Zoo Wildl. Med. 2018, 49, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Lyashchenko, K.P.; Greenwald, R.; Esfandiari, J.; Olsen, J.H.; Ball, R.; Dumonceaux, G.; Dunker, F.; Buckley, C.; Richard, M.; Murray, S.; et al. Tuberculosis in Elephants: Antibody Responses to Defined Antigens of Mycobacterium tuberculosis, Potential for Early Diagnosis, and Monitoring of Treatment. Clin. Vaccine Immunol. 2006, 13, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Obanda, V.; Poghon, J.; Yongo, M.; Mulei, I.; Ngotho, M.; Waititu, K.; Makumi, J.; Gakuya, F.; Omondi, P.; Soriguer, R.C.; et al. First Reported Case of Fatal Tuberculosis in a Wild African Elephant with Past Human–Wildlife Contact. Epidemiol. Infect. 2013, 141, 1476. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, R.M.; Napit, R.; Manandhar, P.; Raut, R.; Gurung, A.; Poudel, A.; Shrestha, N.; Sadaula, A.; Karmacharya, D.; Gortázar, C.; et al. Phylogenomic Analysis Supports Mycobacterium tuberculosis Transmission between Humans and Elephants. Front. Vet. Sci. 2023, 10, 1133823. [Google Scholar] [CrossRef]
- Zachariah, A.; Pandiyan, J.; Madhavilatha, G.K.; Mundayoor, S.; Chandramohan, B.; Sajesh, P.K.; Santhosh, S.; Mikota, S.K. Mycobacterium tuberculosis in Wild Asian Elephants, Southern India. Emerg. Infect. Dis. 2017, 23, 504. [Google Scholar] [CrossRef]
- Miller, M.A.; Buss, P.; Roos, E.O.; Hausler, G.; Dippenaar, A.; Mitchell, E.; van Schalkwyk, L.; Robbe-Austerman, S.; Waters, W.R.; Sikar-Gang, A.; et al. Fatal Tuberculosis in a Free-Ranging African Elephant and One Health Implications of Human Pathogens in Wildlife. Front. Vet. Sci. 2019, 6, 18. [Google Scholar] [CrossRef]
- Weiss, G.; Schaible, U.E. Macrophage Defense Mechanisms against Intracellular Bacteria. Immunol. Rev. 2015, 264, 182. [Google Scholar] [CrossRef]
- Palkopoulou, E.; Lipson, M.; Mallick, S.; Nielsen, S.; Rohland, N.; Baleka, S.; Karpinski, E.; Ivancevic, A.M.; To, T.-H.; Kortschak, R.D.; et al. A Comprehensive Genomic History of Extinct and Living Elephants. Proc. Natl. Acad. Sci. USA 2018, 115, E2566–E2574. [Google Scholar] [CrossRef]
- Fernando, P.; Vidya, T.N.C.; Payne, J.; Stuewe, M.; Davison, G.; Alfred, R.J.; Andau, P.; Bosi, E.; Kilbourn, A.; Melnick, D.J. DNA Analysis Indicates That Asian Elephants Are Native to Borneo and Are Therefore a High Priority for Conservation. PLoS Biol. 2003, 1, e6. [Google Scholar] [CrossRef]
- Farias-Jofre, M.; Romero, R.; Galaz, J.; Xu, Y.; Tao, L.; Demery-Poulos, C.; Arenas-Hernandez, M.; Bhatti, G.; Liu, Z.; Kawahara, N.; et al. Pregnancy Tailors Endotoxin-Induced Monocyte and Neutrophil Responses in the Maternal Circulation. Inflamm. Res. 2022, 71, 653. [Google Scholar] [CrossRef]
- Faas, M.M.; Spaans, F.; De Vos, P. Monocytes and Macrophages in Pregnancy and Pre-Eclampsia. Front. Immunol. 2014, 5, 298. [Google Scholar] [CrossRef]
- Vogelnest, L.; Hulst, F.; Thompson, P.; Lyashchenko, K.P.; Vinette Herrin, K.A. Diagnosis and Management of Tuberculosis (Mycobacterium tuberculosis) in an Asian Elephant (Elephas maximus) with a Newborn Calf. J. Zoo Wildl. Med. 2015, 46, 77–85. [Google Scholar] [CrossRef]


| Antigen Target | Peripheral WBC Targeted 1 | Clone | Sequence Similarity 2 |
|---|---|---|---|
| CD11b | Granulocytes, monocytes | 5C6 3 | 73.5% |
| CD11c | Monocytes, lymphocytes | 3.9 4 | 72.7% |
| CD115 | Monocytes | 12-3A3-1B10 4 | 79.1% |
| CD14 | Monocytes | Tuk4 3 | 71.7% |
| IBA1 | Monocytes | 1C6A10 4 | 90.6% |
| Live/dead | Viability dye (aqua) | N/A 4 | N/A |
| Elephant | Monocytes (%) via Microscopy | Monocytes (%) via Flow Cytometry | Lymphocytes (%) via Microscopy | Lymphocytes (%) via Flow Cytometry |
|---|---|---|---|---|
| F1 | 83.5 ± 2.7 | 75.3 ± 4.0 | 16.1 ± 1.9 | 18.9 ± 2.1 |
| F2 | 84.0 ± 1.9 | 76.1 ± 3.3 | 16.0 ± 1.7 | 21.2 ± 3.0 |
| M1 | 82.4 ± 2.3 | 71.2 ± 2.5 | 17.5 ± 1.7 | 22.8 ± 2.5 |
| Positive Events (%) | ||
|---|---|---|
| Monocytes | Lymphocytes | |
| CD11b | 22.6 ± 3.6 | 16.7 ± 2.4 |
| CD11c * | 47.3 ± 12.8 | 12.1 ± 3.4 |
| CD115 | 13.7 ± 1.3 | 9.4 ± 2.2 |
| CD14 | 29.5 ± 4.4 | 21.7 ± 1.5 |
| IBA1 * | 86.8 ± 7.5 | 21.4 ± 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johns, J.L.; Baumgartner, T.R.; Sanchez, C.R.; Dolan, B.P. Phagocytic Function and Flow Cytometric Phenotype of Asian Elephant Monocytes. Animals 2024, 14, 2297. https://doi.org/10.3390/ani14162297
Johns JL, Baumgartner TR, Sanchez CR, Dolan BP. Phagocytic Function and Flow Cytometric Phenotype of Asian Elephant Monocytes. Animals. 2024; 14(16):2297. https://doi.org/10.3390/ani14162297
Chicago/Turabian StyleJohns, Jennifer L., Trinity R. Baumgartner, Carlos R. Sanchez, and Brian P. Dolan. 2024. "Phagocytic Function and Flow Cytometric Phenotype of Asian Elephant Monocytes" Animals 14, no. 16: 2297. https://doi.org/10.3390/ani14162297
APA StyleJohns, J. L., Baumgartner, T. R., Sanchez, C. R., & Dolan, B. P. (2024). Phagocytic Function and Flow Cytometric Phenotype of Asian Elephant Monocytes. Animals, 14(16), 2297. https://doi.org/10.3390/ani14162297





