Histological Characteristics of Follicles, Reproductive Hormones and Transcriptomic Analysis of White King Pigeon Illuminated with Red Light
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Reproductive Hormone Concentrations Analysis
2.3. Histological Analysis of Follicles
2.4. Transcriptome of Pigeon Ovary
2.5. RT-qPCR Validation
2.6. Statistical Analysis
3. Results
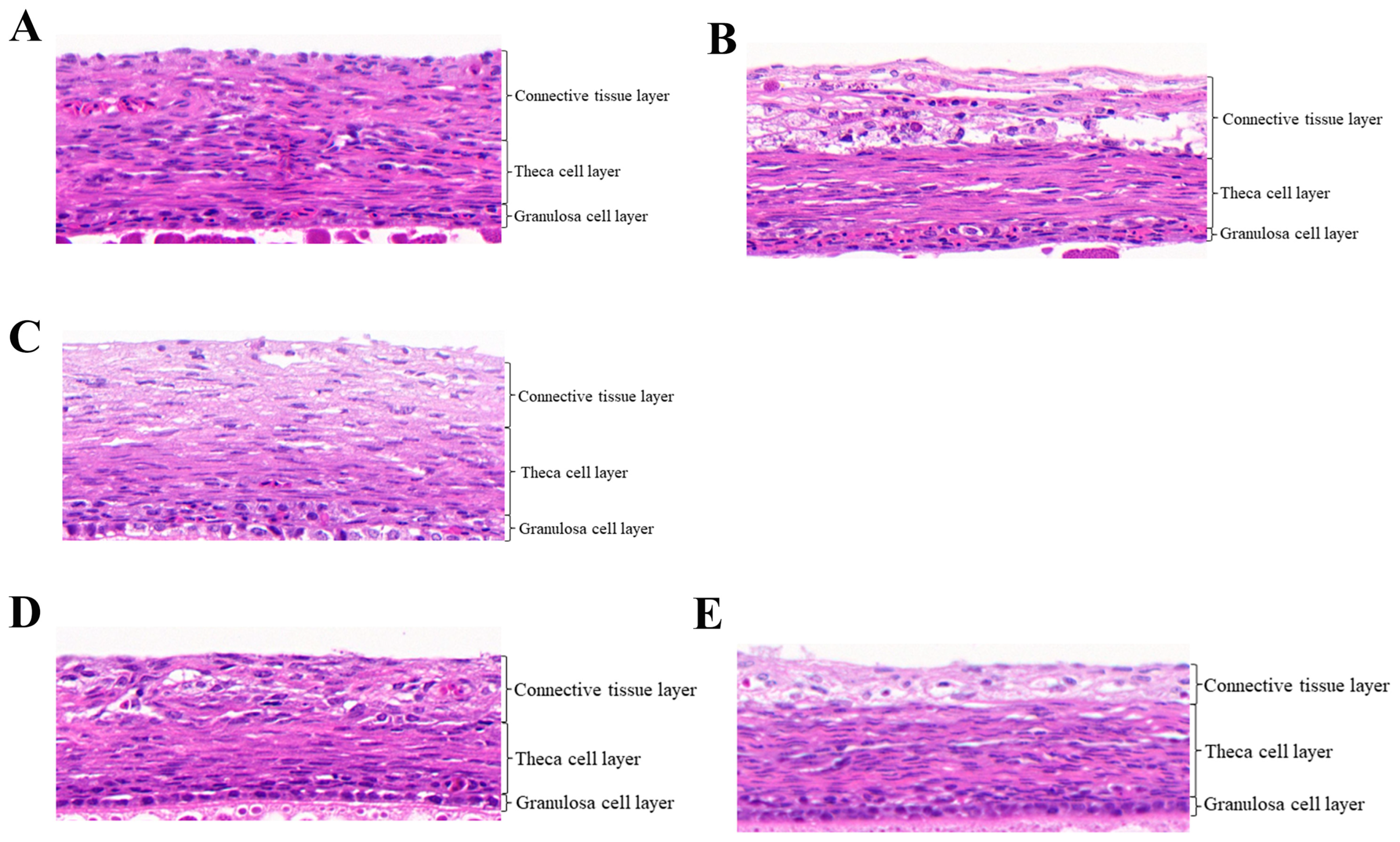
3.1. Histological Observation of Follicles in Pigeon under RL
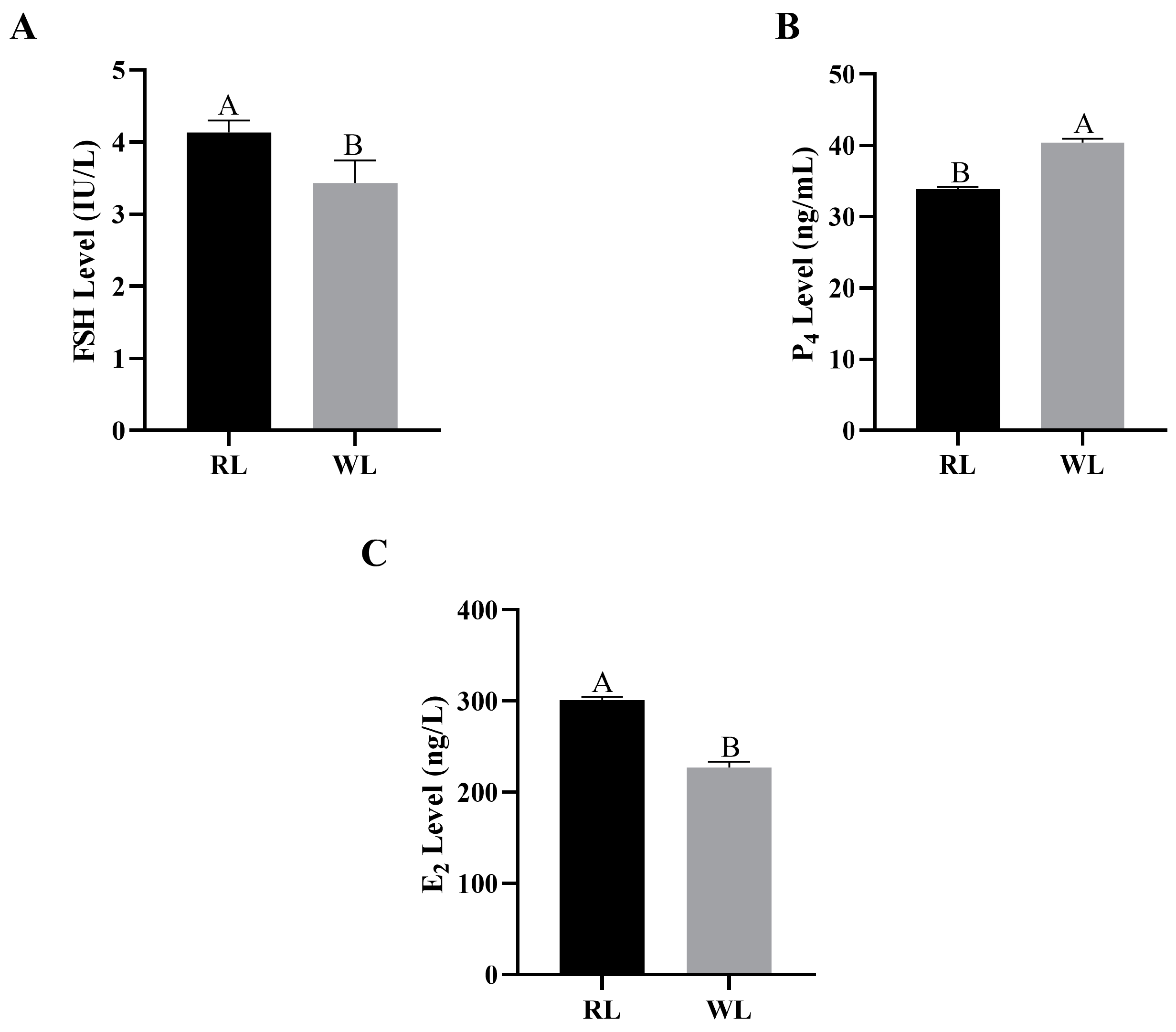
3.2. Concentrations of Reproductive Hormones in Females under RL in LI3
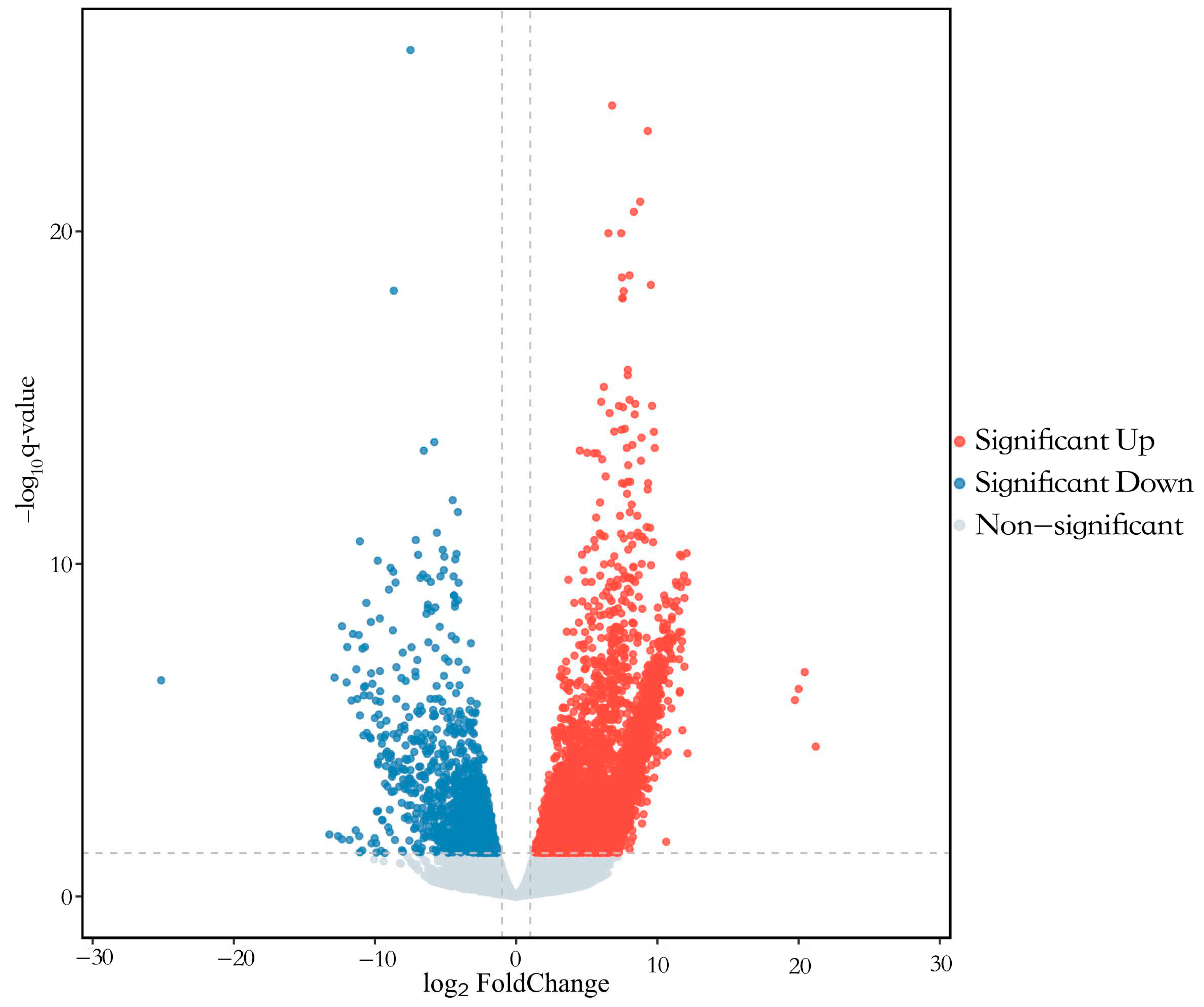
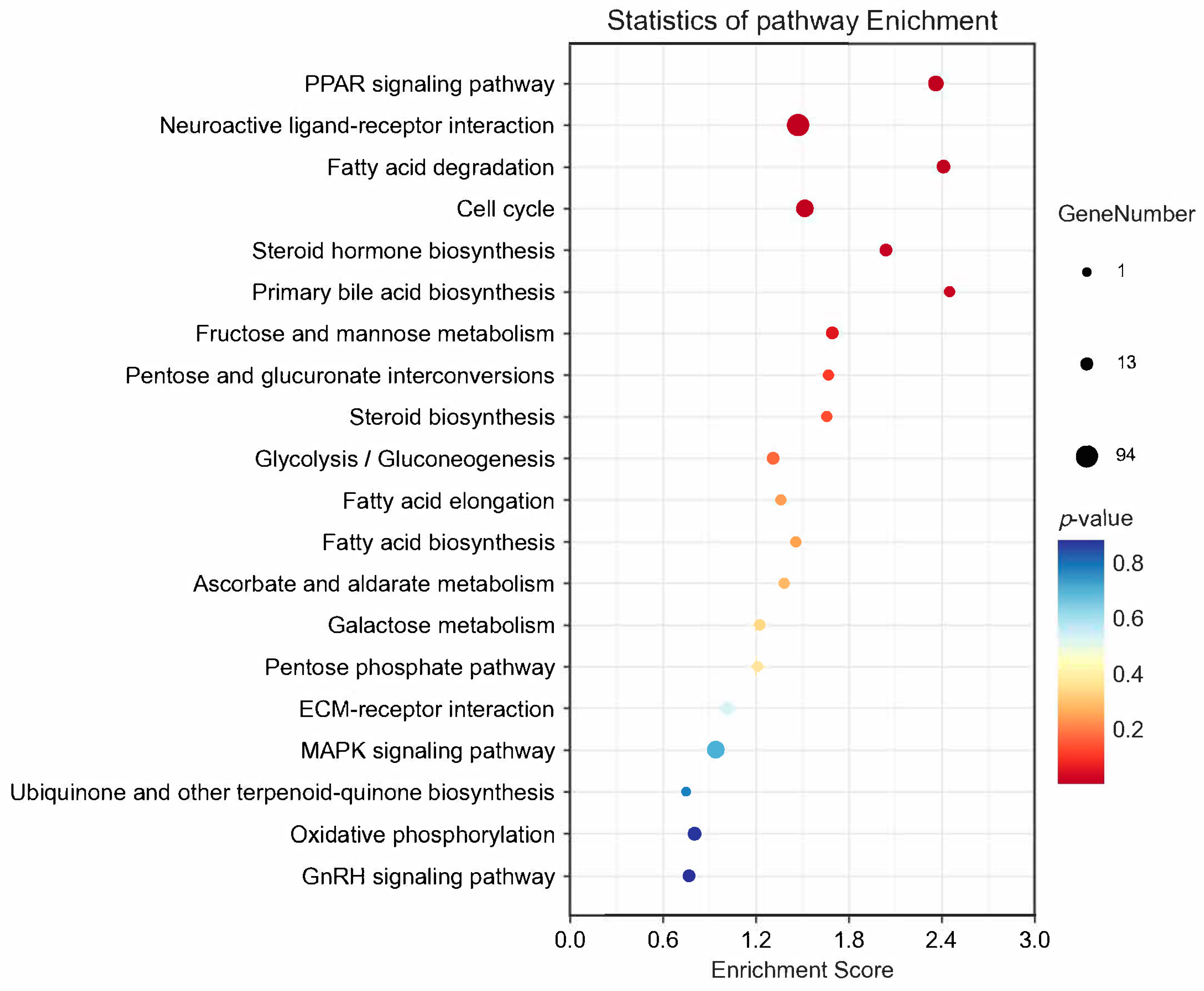
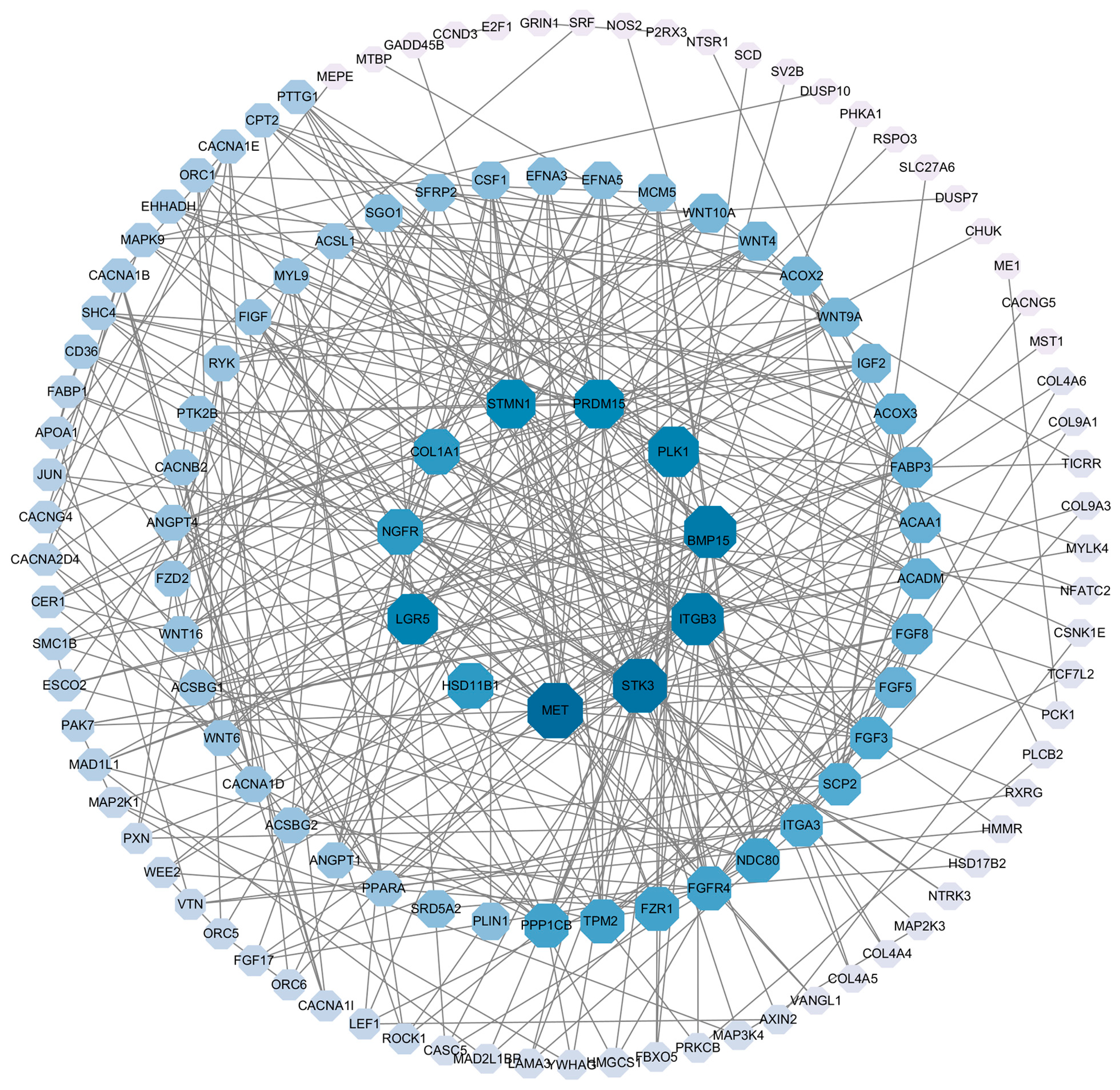
3.3. Transcriptome Analysis of Pigeon Ovary under RL in LI3
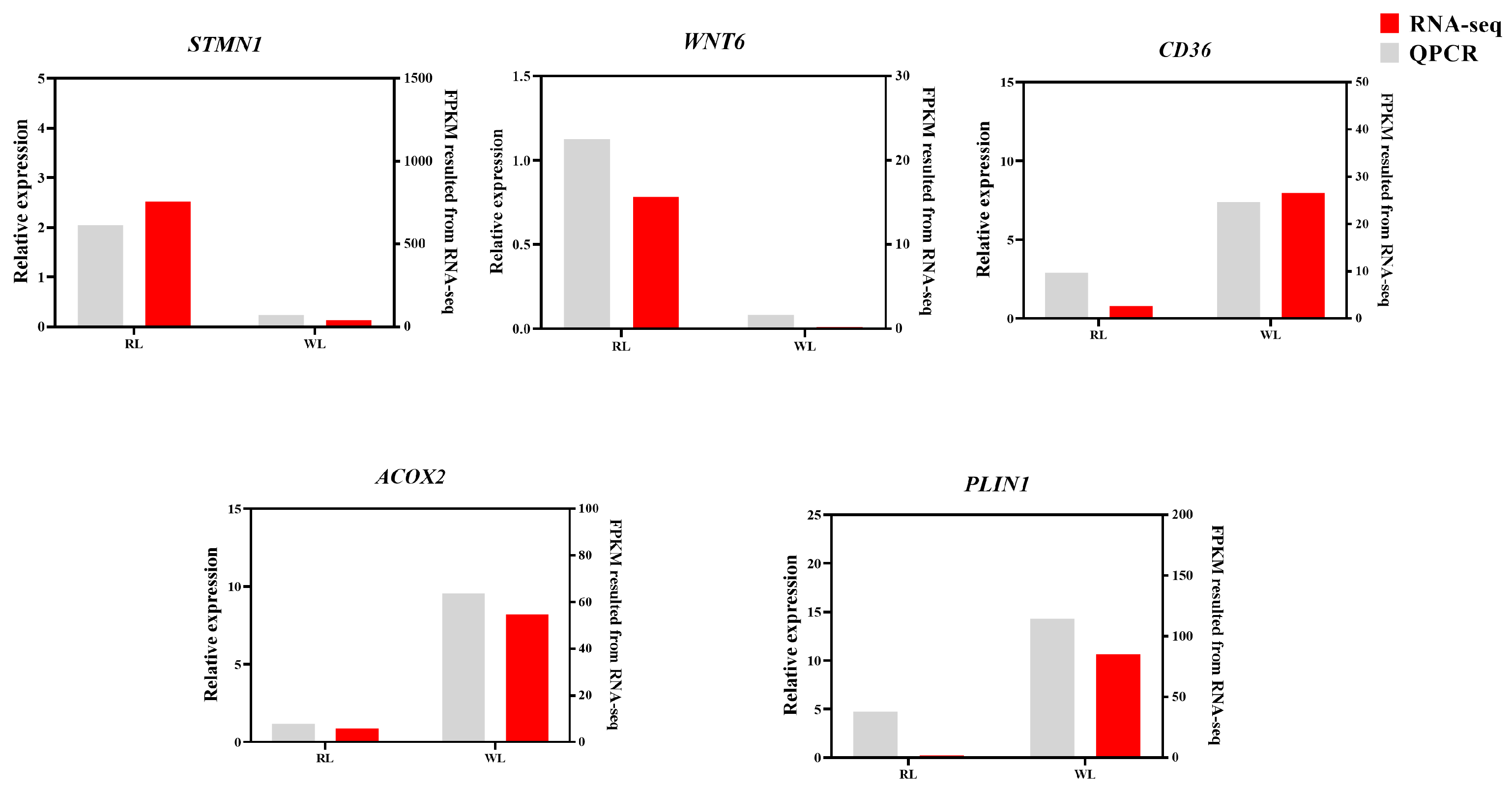
3.4. Validation of Pigeon Ovary Transcriptome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kokoszyński, D.; Stęczny, K.; Żochowska-Kujawska, J.; Sobczak, M.; Kotowicz, M.; Saleh, M.; Fik, M.; Arpášová, H.; Hrnčár, C.; Włodarczyk, K. Carcass Characteristics, Physicochemical Properties, and Texture and Microstructure of the Meat and Internal Organs of Carrier and King Pigeons. Animals 2020, 10, 1315. [Google Scholar] [CrossRef]
- Jin, C.; He, Y.; Jiang, S.; Wang, X.; Yan, H.; Tan, H.; Gao, C. Chemical composition of pigeon crop milk and factors affecting its production: A review. Poult. Sci. 2023, 102, 102681. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, D.Z.; Zhang, C.; Chen, J.; Yang, H.M.; Wang, Z.Y. CircRNAs involved in the red light of effect on follicle selection in pigeons. Poult. Sci. 2024, 103, 104010. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.D.; Morris, T.R. Poultry and coloured light. World Poult. Sci J. 2000, 56, 189–207. [Google Scholar] [CrossRef]
- Tsutsui, K.; Bentley, G.E.; Bédécarrats, G.; Osugi, T.; Ubuka, T.; Kriegsfeld, L.J. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front. Neuroendocrinol. 2010, 31, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Ottinger, M.A.; Abdelnabi, M.; Li, Q.; Chen, K.; Thompson, N.; Harada, N.; Viglietti-Panzica, C.; Panzica, G.C. The Japanese quail: A model for studying reproductive aging of hypothalamic systems. Exp. Gerontol. 2004, 39, 1679–1693. [Google Scholar] [CrossRef]
- Stevenson, T.J.; Bernard, D.J.; McCarthy, M.M.; Ball, G.F. Photoperiod-dependent regulation of gonadotropin-releasing hormone 1 messenger ribonucleic acid levels in the songbird brain. Gen. Comp. Endocrinol. 2013, 190, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Needham, K.B.; Burns, C.B.; Graham, J.L.; Bauer, C.M.; Kittilson, J.D.; Ketterson, E.D.; Hahn, T.; Greives, T.J. Changes in processes downstream of the hypothalamus are associated with seasonal follicle development in a songbird, the dark-eyed junco (Junco hyemalis). Gen. Comp. Endocrinol. 2019, 270, 103–112. [Google Scholar] [CrossRef]
- Baxter, M.; Joseph, N.; Osborne, V.R.; Bédécarrats, G.Y. Red light is necessary to activate the reproductive axis in chickens independently of the retina of the eye. Poult. Sci. 2014, 93, 1289–1297. [Google Scholar] [CrossRef]
- Rozenboim, I.; El Halawani, M.E.; Kashash, Y.; Piestun, Y.; Halevy, O. The effect of monochromatic photostimulation on growth and development of broiler birds. Gen. Comp. Endocr. 2013, 190, 214–219. [Google Scholar] [CrossRef]
- Gongruttananun, N. Influence of red light on reproductive performance, eggshell ultrastructure, and eye morphology in Thai-native hens. Poult. Sci. 2011, 90, 2855–2863. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.B.; Yang, H.M.; Wang, Z.Y. Effect of monochromatic lights on egg production, sex hormone levels, and expression of their receptors in pigeons. Livest. Sci. 2018, 216, 233–236. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Zi, C.; Wang, Z. Transcriptomic analysis of the red and green light responses in Columba livia domestica. 3 Biotech 2019, 9, 20. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Liu, Z.; Guo, X.; Sun, Y.; Kang, L.; Jiang, Y. Transcriptomic and proteomic analyses of ovarian follicles reveal the role of VLDLR in chicken follicle selection. BMC Genom. 2020, 21, 486. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.Y.; Zhang, C.; Miao, D.Z.; Mao, X.Y.; Lu, S.M.; Yang, H.M.; Wang, Z.Y. Characterization of ovarian follicles, serum steroid hormone concentration, and steroidogenic gene expression profiles in the developing ovarian follicles in White King pigeons. Poult. Sci. 2023, 102, 102673. [Google Scholar] [CrossRef]
- Brady, K.; Liu, H.C.; Hicks, J.; Long, J.A.; Porter, T.E. Global gene expression analysis of the turkey hen hypothalamo-pituitary-gonadal axis during the preovulatory hormonal surge. Poult. Sci. 2023, 102, 102547. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.P.; Liu, H.; Hu, J.; Han, X.; Qi, J.; Ouyang, Q.; Hu, B.; He, H.; Li, L.; Wang, J.; et al. Transcriptomic analyses of the HPG axis-related tissues reveals potential candidate genes and regulatory pathways associated with egg production in ducks. BMC Genom. 2022, 23, 281. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Shi, Y.; Ye, H.; Luo, W.; Geng, F. Quantitative proteomic analyses during formation of chicken egg yolk. Food Chem. 2022, 374, 131828. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Z.; Yao, Y.; Cao, Z.F.; Gu, T.T.; Xu, Q.; Chen, G.H. Histological characteristics of follicles and reproductive hormone secretion during ovarian follicle development in laying geese. Poult. Sci. 2019, 98, 6063–6070. [Google Scholar] [CrossRef]
- Miao, D.Z.; Liu, C.; Deng, Z.Y.; Zhang, C.; Guo, Z.Y.; Li, W.Q.; Wang, Y.; Yang, H.M.; Wang, Z.Y. Characterization of reproductive hormones and related gene expression in the hypothalamus and pituitary gland in the egg-laying interval in White King pigeon. Poult. Sci. 2024, 103, 103422. [Google Scholar] [CrossRef]
- Gan, X.; Wang, J.W.; Li, Q.; Deng, Y.; Hu, J.W.; Li, L.; Han, C.C. Dynamic development characteristics of mature follicular wall in goose:A new perspective of follicle development and grading. Acta Vet. Zootech. Sin. 2019, 50, 1607–1613. [Google Scholar]
- Tilly, J.L.; Kowalski, K.I.; Johnson, A.L. Stage of ovarian follicular development associated with the initiation of steroidogenic competence in avian granulosa cells. Biol. Reprod. 1991, 44, 305–314. [Google Scholar] [CrossRef]
- Johnson, A.L. Ovarian follicle selection and granulosa cell differentiation. Poult. Sci. 2015, 94, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, J.; Zhou, S.; Mi, Y.; Tan, X.; Zhang, C. Enhancing effect of FSH on follicular development through yolk formation and deposition in the low-yield laying chickens. Theriogenology 2020, 157, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Senior, B.E.; Furr, B.J.A. A preliminary assessment of the source of oestrogen within the ovary of the domestic fowl, Gallus domesticus. Reproduction 1975, 43, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, D.; Gilbert, E.R.; Xiao, Q.; Zhao, X.; Wang, Y.; Yin, H.D.; Zhu, Q. Effect of monochromatic light on expression of estrogen receptor (ER) and progesterone receptor (PR) in ovarian follicles of chicken. PLoS ONE 2015, 10, e0144102. [Google Scholar] [CrossRef] [PubMed]
- Woolveridge, I.; Peddie, M.J. The inhibition of androstenedione production in mature thecal cells from the ovary of the domestic hen (Gallus domesticus): Evidence for the involvement of progestins. Steroids 1997, 62, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Tetsuka, M.; Yamamoto, S.; Hayashida, N.; Hayashi, K.G.; Hayashi, M.; Acosta, T.J.; Miyamoto, A. Expression of 11beta-hydroxysteroid dehydrogenases in bovine follicle and corpus luteum. J. Endocrinol. 2003, 177, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Tetsuka, M.; Nishimoto, H.; Miyamoto, A.; Okuda, K.; Hamano, S. Gene expression of 11b-HSD and glucocorticoid receptor in the bovine (Bos taurus) follicle during follicular maturation and atresia: The role of follicular stimulating hormone. J. Reprod. Dev. 2010, 56, 616–622. [Google Scholar] [CrossRef]
- Latif, S.A.; Pardo, H.A.; Hardy, M.P.; Morris, D.J. Endogenous selective inhibitors of 11beta-hydroxysteroid dehydrogenase isoforms 1 and 2 of adrenal origin. Mol. Cell. Endocrinol. 2005, 243, 43–50. [Google Scholar] [CrossRef]
- Kim, S.O.; Trau, H.A.; Duffy, D.M. Vascular endothelial growth factors C and D may promote angiogenesis in the primate ovulatory follicle. Biol. Reprod. 2017, 96, 389–400. [Google Scholar] [CrossRef]
- Harwood, B.N.; Cross, S.K.; Radford, E.E.; Haac, B.E.; De Vries, W.N. Members of the WNT signaling pathways are widely expressed in mouse ovaries, oocytes, and cleavage stage embryos. Dev. Dyn. 2008, 237, 1099–1111. [Google Scholar] [CrossRef]
- Ahmadi, S.; Nemoto, Y.; Ohkubo, T. Impact of In Ovo Leptin Injection and Dietary Protein Levels on Ovarian Growth Markers and Early Folliculogenesis in Post-Hatch Chicks (Gallus domesticus). Biology 2024, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, M.; Sancho, E.; Verweij, C.; de Lau, W.; Oving, I.; Hurlstone, A.; van der Horn, K.; Batlle, E.; Coudreuse, D.; Haramis, A.P.; et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002, 111, 241–250. [Google Scholar] [CrossRef]
- Hermey, G.; Methner, A.; Schaller, H.C.; Hermans-Borgmeyer, I. Identification of a novel seven-transmembrane receptor with homology to glycoprotein receptors and its expression in the adult and developing mouse. Biochem. Biophys. Res. Commun. 1999, 254, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Rastetter, R.H.; Bernard, P.; Palmer, J.S.; Chassot, A.A.; Chen, H.; Western, P.S.; Ramsay, R.G.; Chaboissier, M.C.; Wilhelm, D. Marker genes identify three somatic cell types in the fetal mouse ovary. Dev. Biol. 2014, 394, 242–252. [Google Scholar] [CrossRef]
- Kaivo-oja, N.; Jeffery, L.A.; Ritvos, O.; Mottershead, D.G. Smad signalling in the ovary. Reprod Biol Endocrin. 2006, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Glister, C.; Kemp, C.F.; Knight, P.G. Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: Actions of BMP-4,-6 and-7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction 2004, 127, 239–254. [Google Scholar] [CrossRef]
- Domingues, R.R.; Andrade, F.S.; Andrade, J.P.N.; Moghbeli, S.M.; Gomez-Leon, V.; Madureira, G.; Mello, M.R.B.; Kirkpatrick, B.W.; Wiltbank, M.C. SMAD6 inhibits granulosa cell proliferation and follicle growth rate in carrier and noncarrier heifers of the Trio allele. Reproduction 2023, 165, 269–279. [Google Scholar] [CrossRef]


| Gene | Primer Sequence (5′→3′) | Length |
|---|---|---|
| STMN1 | F: ATGCTGAATATCTGTTACACGTC R: CCATTTTGTTCCGCGTGTC | 130 bp |
| WNT6 | F: CCAGCAGTTCATGGATGCCAA R: AAACGTCCGGCTTCATTGTTG | 169 bp |
| CD36 | F: CCCAAAGAAAATATCACGGAA R: ATATCAGGTTCAAAACGAGCAA | 80 bp |
| AOCX2 | F: AAGTGAACGCCACACGTCT R: CGGTTACCACTCAGCATCGCTTG | 169 bp |
| ANGPT4 | F: TCTACACCCTGCACATCACC R: TCCATGTCGCAGTACGCCTT | 58 bp |
| CACNB2 | F: GACGCTGATACCATTAACCAC R: TACATCAAACATTTCGGGAGG | 171 bp |
| GAPDH | F: CTCTACTCATGGCCACTTCCG R: ACAACGTATTCAGCACCAGC | 138 bp |
| Group 1 | Granulosa Cell Layer (μm) | Theca Cell Layer (μm) | Connective Tissue Layer (μm) |
|---|---|---|---|
| RLF1 2 | 15.200 ± 1.210 a | 41.866 ± 2.571 | 35.664 ± 4.276 |
| WLF1 | 8.767 ± 0.736 b | 38.433 ± 2.444 | 49.267 ± 6.631 |
| RLF2 | 13.633 ± 0.884 | 35.667 ± 2.267 | 49.900 ± 1.513 |
| RLSF1 | 7.100 ± 0.451 | 37.267 ± 4.037 | 32.500 ± 3.843 |
| WLSF1 | 7.133 ± 0.939 | 30.700 ± 3.009 | 40.100 ± 1.815 |
| Samples 1 | Raw Read/M | Clean Reads/M | Clean Bases/G | Mapped Reads/% | Q30/% | GC/% |
|---|---|---|---|---|---|---|
| RO3-1 | 48.95 | 47.01 | 6.87 | 83.33 | 94.45 | 49.92 |
| RO3-2 | 50.38 | 47.88 | 6.94 | 83.92 | 94.36 | 48.95 |
| RO3-3 | 48.20 | 47.22 | 6.95 | 87.11 | 96.04 | 48.85 |
| WO3-1 | 51.24 | 48.39 | 6.99 | 84.26 | 93.39 | 49.31 |
| WO3-2 | 52.81 | 49.15 | 7.01 | 82.07 | 94.07 | 49.16 |
| WO3-3 | 48.66 | 47.23 | 6.90 | 86.57 | 96.34 | 49.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zuo, K.; Zhang, C.; Miao, D.; Chen, J.; Yang, H.; Wang, Z. Histological Characteristics of Follicles, Reproductive Hormones and Transcriptomic Analysis of White King Pigeon Illuminated with Red Light. Animals 2024, 14, 2320. https://doi.org/10.3390/ani14162320
Wang Y, Zuo K, Zhang C, Miao D, Chen J, Yang H, Wang Z. Histological Characteristics of Follicles, Reproductive Hormones and Transcriptomic Analysis of White King Pigeon Illuminated with Red Light. Animals. 2024; 14(16):2320. https://doi.org/10.3390/ani14162320
Chicago/Turabian StyleWang, Ying, Kui Zuo, Chi Zhang, Dongzhi Miao, Jing Chen, Haiming Yang, and Zhiyue Wang. 2024. "Histological Characteristics of Follicles, Reproductive Hormones and Transcriptomic Analysis of White King Pigeon Illuminated with Red Light" Animals 14, no. 16: 2320. https://doi.org/10.3390/ani14162320
APA StyleWang, Y., Zuo, K., Zhang, C., Miao, D., Chen, J., Yang, H., & Wang, Z. (2024). Histological Characteristics of Follicles, Reproductive Hormones and Transcriptomic Analysis of White King Pigeon Illuminated with Red Light. Animals, 14(16), 2320. https://doi.org/10.3390/ani14162320






