Morphology, Glycan Pattern, Heat Shock Proteins, and Sex Steroid Receptors Expression in the Tubal Fimbria Epithelium of the Baboon Papio hamadryas during the Menstrual Cycle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Preparation
2.3. Conventional Histochemistry
2.4. Lectin Histochemistry
2.5. Immunolocalization of HSPs, ERs, and PRs
3. Results
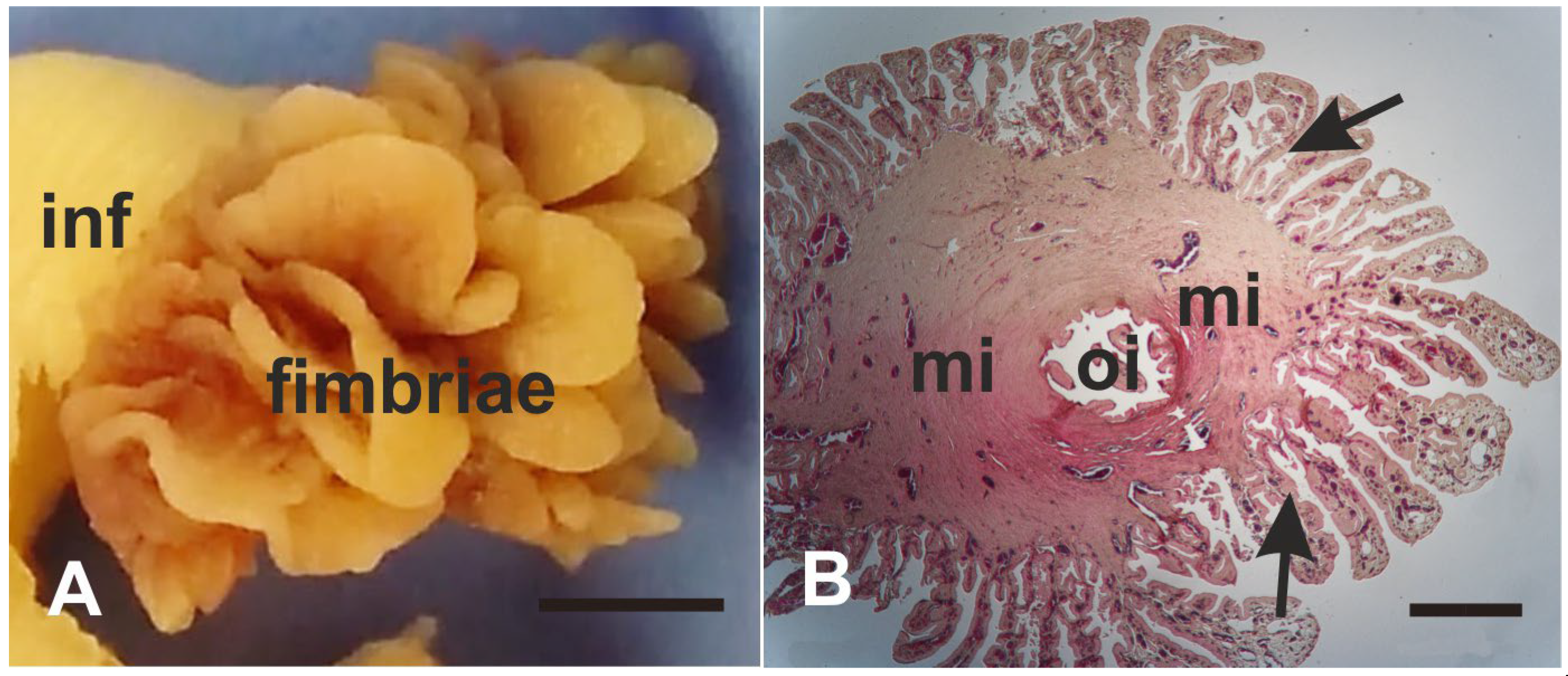
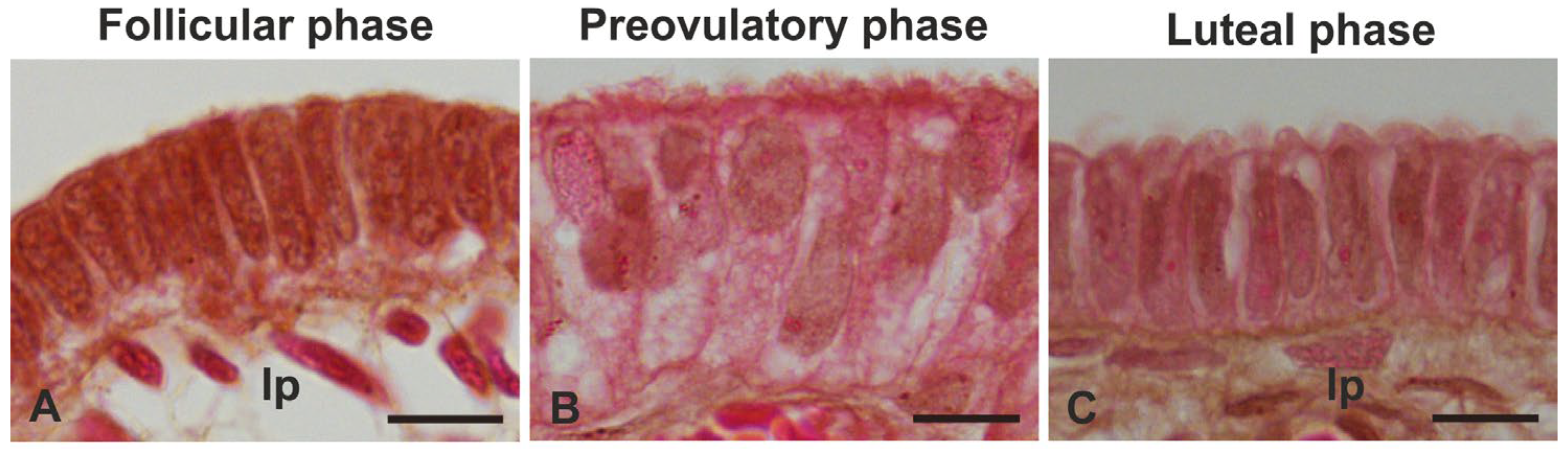
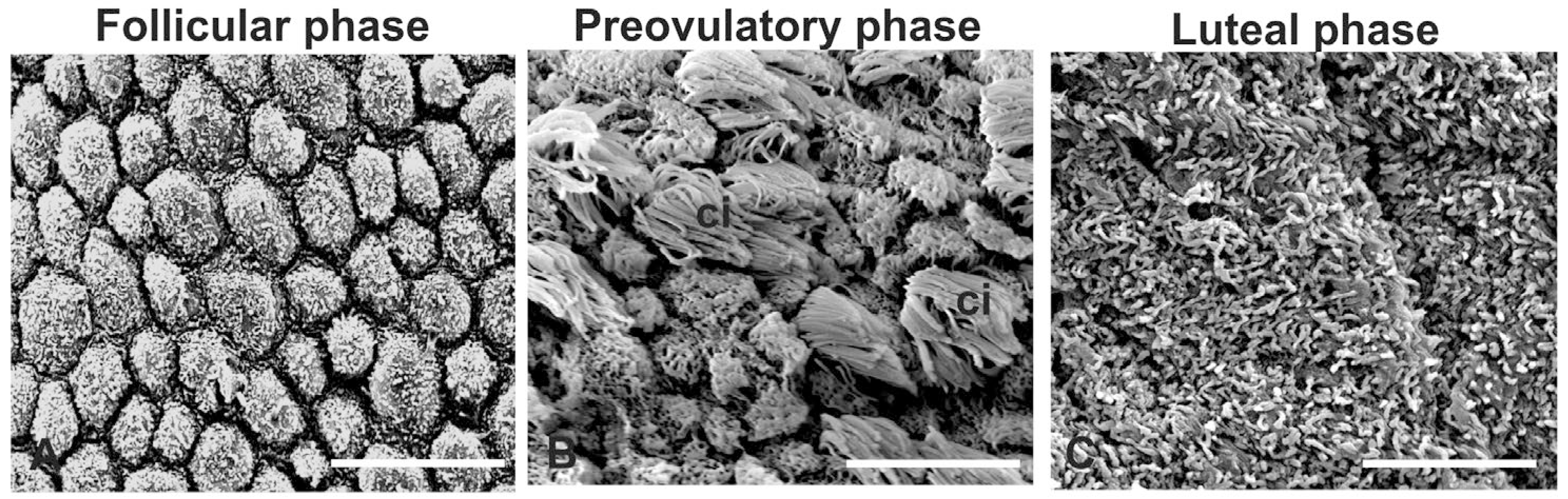
3.1. Morphology
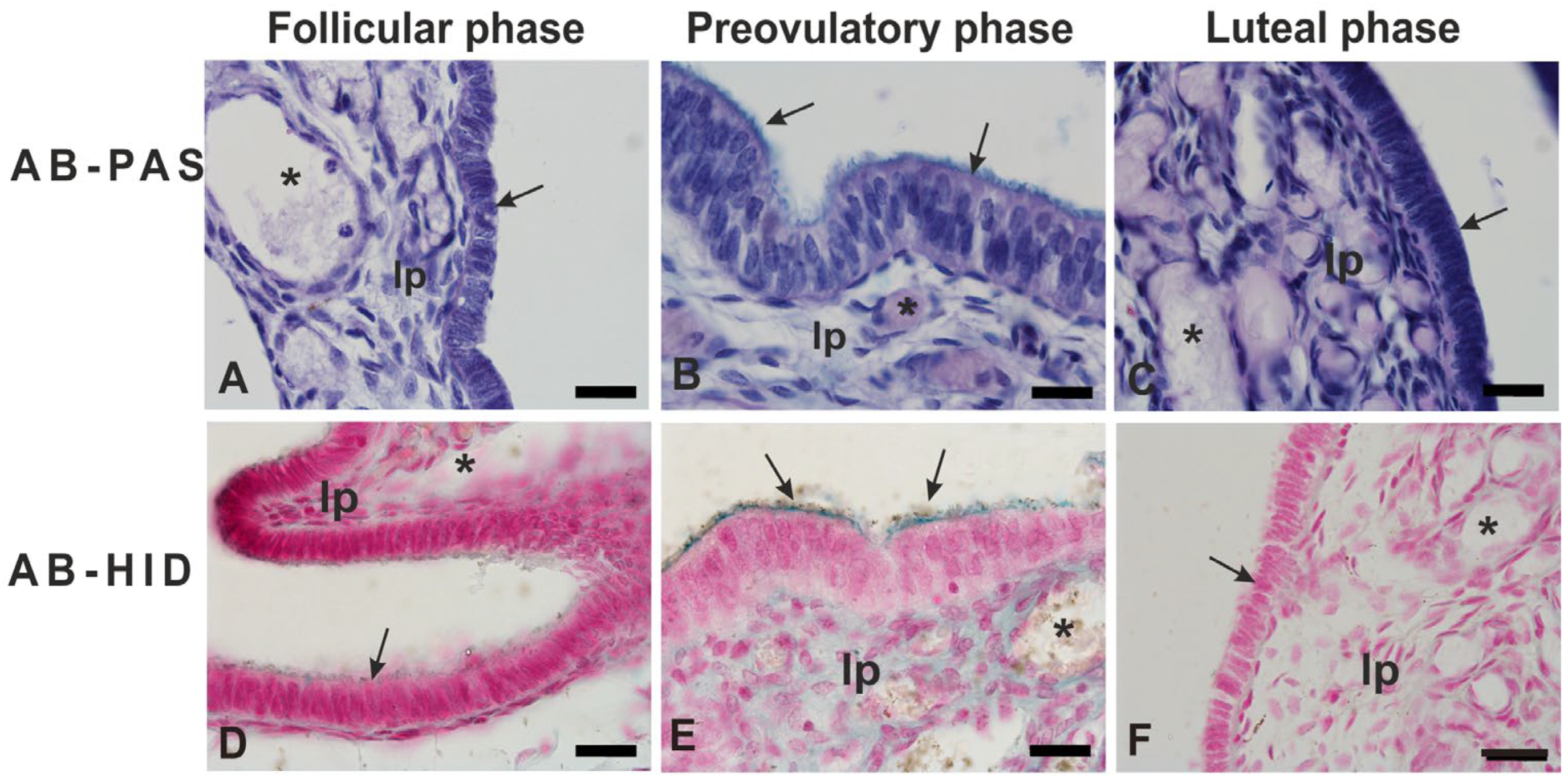
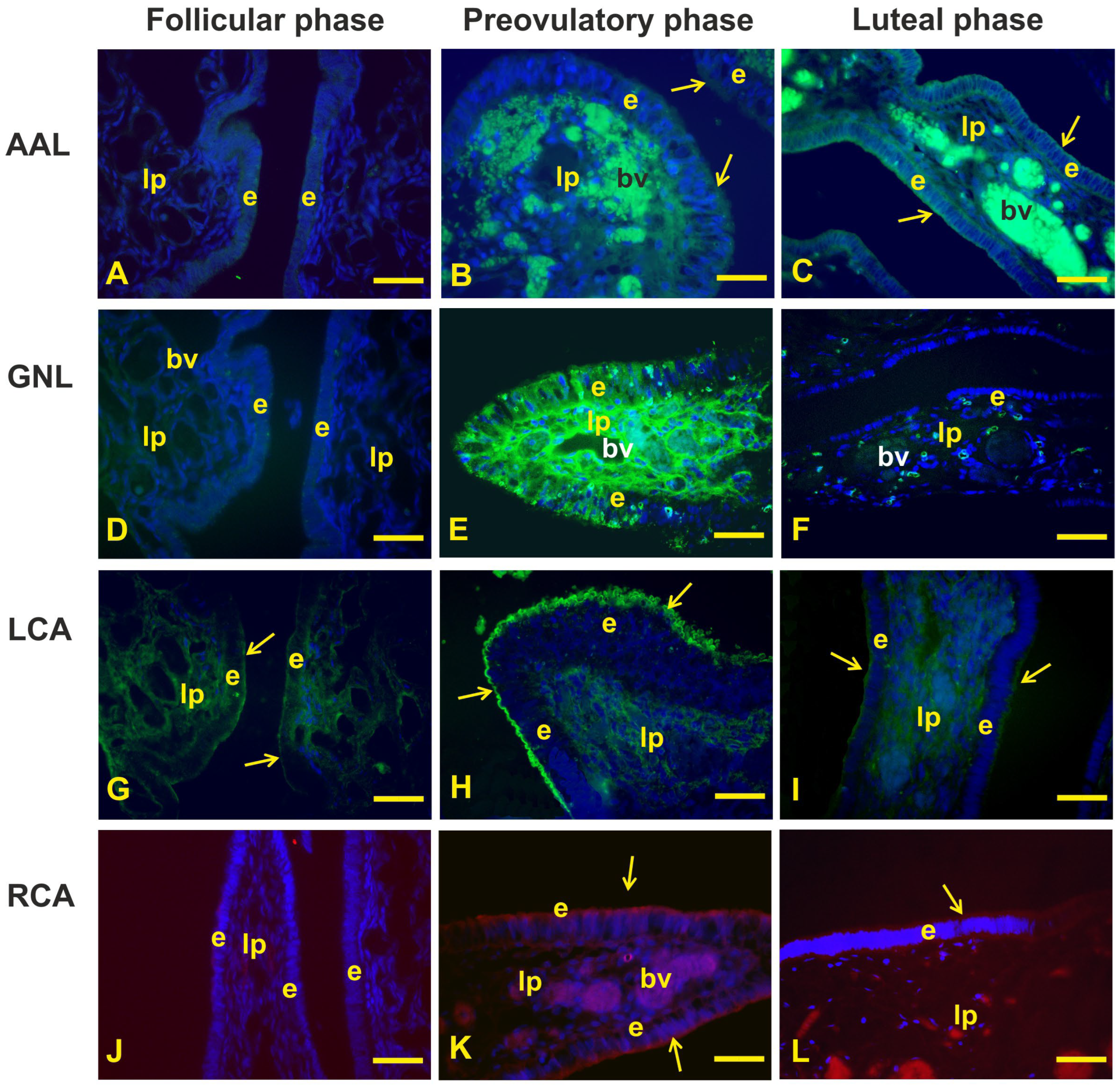
3.2. Glycohistochemistry
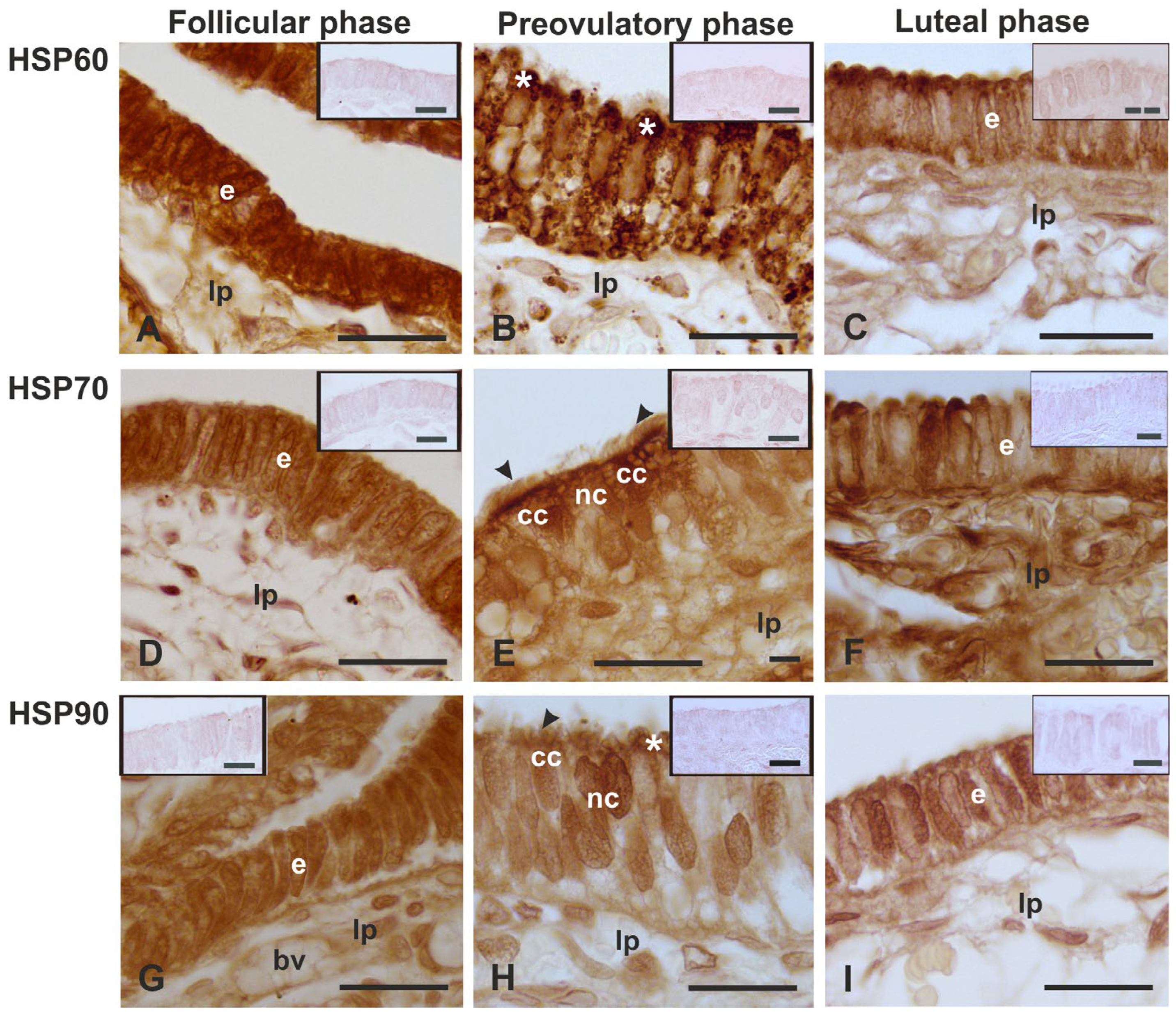
3.3. Immunolocalization of HSPs
3.4. Immunolocalization of ER and PR
4. Discussion
4.1. Morphology
4.2. Glycohistochemistry
4.3. Immunolocalization of the HSPs
4.4. Immunolocalization of ERs and PRs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barton, B.E.; Herrera, G.G.; Anamthathmakula, P.; Rock, J.K.; Willie, A.M.; Harris, E.A.; Takemaru, K.-I.; Winuthayanon, W. Roles of steroid hormones in oviductal function. Reproduction 2020, 159, R125–R137. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, Z.; Peng, H.; Ward, S.M.; Hennig, G.W.; Zheng, H.; Yan, W. Oviductal motile cilia are essential for oocyte pickup but dispensable for sperm and embryo transport. Proc. Natl. Acad. Sci. USA 2021, 118, e2102940118. [Google Scholar] [CrossRef] [PubMed]
- Abe, H. The mammalian oviductal epithelium: Regional variations in cytological and functional aspects of the oviductal secretory cells. Histol. Histopathol. 1996, 11, 743–768. [Google Scholar]
- Mazur, E.C.; Large, M.J.; DeMayo, F.J. Human Oviduct and Endometrium: Changes over the Menstrual Cycle. In The Physiology of Reproduction; Knobil, E., Neill, J., IV, Eds.; Raven Press: New York, NY, USA, 2015; pp. 1077–1097. [Google Scholar]
- Verhage, H.G.; Fazleabas, A.T. The in vitro synthesis of estrogen-dependent proteins by the baboon (Papio anubis) oviduct. Endocrinology 1988, 123, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-H.; Sugimura, M.; Kudo, N. Segmentation of the rat oviduct. Jpn. J. Vet. Res. 1976, 24, 77–86. [Google Scholar] [PubMed]
- Neuer, A.; Spandorfer, S.D.; Giraldo, P.; Dieterle, S.; Rosenwaks, Z.; Witkin, S.S. The role of heat shock proteins in reproduction. Hum. Reprod. Update 2000, 6, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Cinone, M.; Albrizio, M.; Guaricci, A.C.; Lacitignola, L.; Desantis, S. Testicular expression of heat shock proteins 60, 70, and 90 in cryptorchid horses. Theriogenology 2024, 217, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Lone, R. Heat shock proteins with an emphasis on HSP 60. Mol. Biol. Rep. 2021, 48, 6959–6969. [Google Scholar] [CrossRef]
- Kopecek, P.; Altmannova, K.; Weigl, E. Stress proteins: Nomenclature, division and functions. Biomed. Pap. 2001, 145, 39–47. [Google Scholar] [CrossRef]
- Sima, S.; Richter, K. Regulation of the Hsp90 system. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Takaki, E.; Fujimoto, M.; Nakahari, T.; Yonemura, S.; Miyata, Y.; Hayashida, N.; Yamamoto, K.; Vallee, R.B.; Mikuriya, T.; Sugahara, K.; et al. Heat shock transcription factor 1 is required for maintenance of ciliary beating in mice. J. Biol. Chem. 2007, 282, 37285–37292. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.L.; Souto, M.; Fanelli, M.A.; Ciocca, D.R. Constitutive expression of heat shock proteins hsp25 and hsp70 in the rat oviduct during neonatal development, the oestrous cycle and early pregnancy. J. Reprod. Fertil. 2000, 120, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Boilard, M.; Reyes-Moreno, C.; Lachance, C.; Massicotte, L.; Bailey, J.L.; Sirard, M.A.; Leclerc, P. Localization of the chaperone proteins GRP78 and HSP60 on the luminal surface of bovine oviduct epithelial cells and their association with spermatozoa. Biol. Reprod. 2004, 71, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Lachance, C.; Bailey, J.L.; Leclerc, P. Expression of HSp60 and Grp78 in the human endometrium and oviduct, and their effect on sperm functions. Hum. Reprod. 2007, 22, 2606–2614. [Google Scholar] [CrossRef] [PubMed]
- Albrizio, M.; Desantis, S.; Lacitignola, L.; Laricchiuta, P.; Guaricci, A.C.; Cinone, M. The abundance and localization of heat shock proteins (HSP)-60, -70, and -90 in the oviductal ampulla of hamadryas baboon (Papio hamadryas) during the menstrual cycle. Vet. Res. Com. 2024, 48, 979–990. [Google Scholar] [CrossRef]
- Amso, N.N.; Crow, J.; Shaw, R.W. Comparative immunohistochemical study of oestrogen and progesterone receptors in the Fallopian tube and uterus at different stages of the menstrual cycle and the menopause. Hum. Reprod. 1994, 9, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; King, A.E.; Shaw, E.; McDonald, S.E.; Williams, A.R.W.; Saunders, P.T.; Critchley, H.O.D. Attenuated sex steroid receptor expression in Fallopian tube of women with ectopic pregnancy. J. Clin. Endocrinol. Metab. 2009, 94, 5146–5154. [Google Scholar] [CrossRef]
- Maclean, A.; Bunni, E.; Makrydima, S.; Withington, A.; Kamal, A.M.; Valentijn, A.J.; Hapangama, D.K. Fallopian tube epithelial cells express androgen receptor and have a distinct hormonal responsiveness when compared with endometrial epithelium. Hum. Reprod. 2020, 35, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Teilmann, S.C.; Clement, C.A.; Thorup, J.; Byskov, A.G.; Christensen, S.T. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J. Endocrinol. 2006, 191, 525–535. [Google Scholar] [CrossRef]
- McClellan, M.C.; West, N.B.; Tacha, D.E.; Greene, G.L.; Brenner, R.M. Immunocytochemical localization of estrogen receptors in the macaque reproductive tract with monoclonal antiestrophilins. Endocrinology 1984, 114, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Syed, S.M.; Kumar, M.; Carpenter, T.J.; Teixeira, J.M.; Houairia, N.; Negi, S.; Tanwar, P.S. In vivo cell fate tracing provides no evidence for mesenchymal to epithelial transition in adult fallopian tube and uterus. Cell Rep. 2020, 31, 107631. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.F.; Ghosh, A.; Blanch, V.; Grupen, C.G.; Aitken, R.J.; Lim, R.; Drury, H.R.; Baker, M.A.; Gibb, Z.; Tanwar, P.S. Establishment and characterization of oviductal organoids from farm and companion animals. Biol. Reprod. 2023, 108, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Park, E.S.; Xiang, D.; Li, Z. Long-term organoid culture reveals enrichment of organoid-forming epithelial cells in the fimbrial portion of mouse fallopian tube. Stem. Cell Res. 2018, 32, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Chu, S.C.; Hsu, C.F.; Chen, P.C.; Ding, D.C.; Chang, M.Y.; Chu, T.Y. Mutagenic, surviving and tumorigenic effects of follicular fluid in the context of p53 loss: Initiation of fimbria carcinogenesis. Carcinogenesis 2015, 36, 1419–1428. [Google Scholar] [CrossRef]
- Medeiros, F.; Muto, M.G.; Lee, Y.; Elvin, J.A.; Callahan, M.J.; Feltmate, C.; Garber, J.E.; Cramer, D.W.; Crum, C.P. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol. 2006, 30, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Tallon, N.; Akbar, G.; Mccomb, P. Congenital ampullary atresia of the fallopian tube and the coexistence of fimbrial tissue. J. Genit. Syst. Disord. 2013, S1, 1–5. [Google Scholar] [CrossRef]
- Bauer, C. The baboon (Papio sp.) as a model for female reproduction studies. Contraception 2015, 92, 120–123. [Google Scholar] [CrossRef]
- Desantis, S.; Albrizio, M.; Lacitignola, L.; Laricchiuta, P.; Cinone, M. Modification of morphology and glycan pattern of the oviductal epithelium of baboon Papio hamadryas during the menstrual cycle. Animals 2022, 12, 2769. [Google Scholar] [CrossRef]
- Spicer, S.S. Diamine methods for differentiating mucosubstances histochemically. J. Histochem. Cytochem. 1965, 13, 211–234. [Google Scholar] [CrossRef]
- Odor, D.L.; Augustine, J.R. Morphological study of changes in the baboon oviductal epithelium during the menstrual cycle. Microsc. Res. Tech. 1995, 32, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Verhage, H.G.; Mavrogianis, P.A.; Boice, M.A.; Li, W.; Fazleabas, A.T. Oviductal epithelium of the baboon: Hormonal control and the immuno-gold localization of oviduct-specific glycoproteins. Am. J. Anat. 1990, 187, 81–90. [Google Scholar] [CrossRef]
- Li, J.; Hsu, H.C.; Mountz, J.D.; Allen, J.G. Unmasking fucosylation: From cell adhesion to immune system regulation and diseases. Cell Chem. Biol. 2018, 25, 499–512. [Google Scholar] [CrossRef]
- Domino, S.E.; Zhang, L.; Gillespie, P.J.; Saunders, T.L.; Lowe, J.B. Deficiency of reproductive tract α(1,2)fucosylated glycans and normal fertility in mice with targeted deletions of the FUT1 or FUT2 α(1,2)fucosyltransferase locus. Mol. Cell. Biol. 2001, 21, 8336–8345. [Google Scholar] [CrossRef]
- Stanley, P.; Taniguchi, N.; Aebi, M. N-glycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 99–111. [Google Scholar]
- Goumenou, A.; Delaunay, N.; Pichon, V. Recent advances in lectin-based affinity sorbents for protein glycosylation studies. Front. Mol. Biosci. 2021, 8, 746822. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, E.V.; Xue, J.; Xia, J.; Khaja, S.D.; Piskorz, C.F.; Locke, R.D.; Neelamegham, S.; Matta, K.L. Novel interactions of complex carbohydrates with peanut (PNA), Ricinus communis (RCA-I), Sambucus nigra (SNA-I) and wheat germ (WGA) agglutinins as revealed by the binding specificities of these lectins towards mucin core-2 O-linked and N-linked glycans and related structures. Glycoconj. J. 2016, 33, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Verhage, H.G.; Fazleabas, A.T.; Mavrogianis, P.A.; O’Day-Bowman, M.B.; Donnelly, K.M.; Arias, E.B.; Jaffe, R.C. The baboon oviduct: Characteristics of an oestradiol-dependent oviduct-specific glycoprotein. Hum. Reprod. Update 1997, 3, 541–552. [Google Scholar] [CrossRef]
- Lyng, R.; Shur, B.D. Mouse oviduct-specific glycoprotein is an egg-associated ZP3-independent sperm-adhesion ligand. J. Cell Sci. 2009, 122, 3894–3906. [Google Scholar] [CrossRef]
- Sangiorgi, C.; Vallese, D.; Gnemmi, I.; Bucchieri, F.; Balbi, B.; Brun, P.; Leone, A.; Giordano, A.; de Macario, E.C.; Macario, A.J.; et al. HSP60 activity on human bronchial epithelial cells. Int. J. Immunopathol. Pharmacol. 2017, 30, 333–340. [Google Scholar] [CrossRef]
- Liman, N. Heat shock proteins are differentially expressed in the domestic cat (Felis catus) testis, epididymis, and vas deferens. Microsc. Microanal. 2023, 29, 713–738. [Google Scholar] [CrossRef]
- Stephens, R.E.; Lemieux, N.A. Molecular chaperones in cilia and flagella: Implications for protein turnover. Cell Motil. Cytoskelet. 1999, 44, 274–283. [Google Scholar] [CrossRef]
- Saint-Dizier, M.; Schoen, J.; Chen, S.; Banliat, C.; Mermillod, P. Composing the early embryonic microenvironment: Physiology and regulation of oviductal secretions. Int. J. Mol. Sci. 2020, 21, 223. [Google Scholar] [CrossRef]
- Suuronen, T.; Ojala, J.; Hyttinen, J.M.T.; Kaarniranta, K.; Thornell, A.; Kyrylenko, S.; Salminen, A. Regulation of ERα signaling pathway in neuronal HN10 cells: Role of protein acetylation and Hsp90. Neurochem. Res. 2008, 33, 1768–1775. [Google Scholar] [CrossRef]
- Olazabal, U.E.; Pfaff, D.W.; Mobbs, C.V. Sex differences in the regulation of heat shock protein 70 kDa and 90 kDa in the rat ventromedial hypothalamus by estrogen. Brain Res. 1992, 596, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Bauer, M. Heat shock proteins in porcine ovary: Synthesis, accumulation and regulation by stress and hormones. Cell Stress Chaperons 2011, 16, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Ohta, Y.; Inoue, S.; Hiroi, H.; Muramatsu, M.; Iguchi, T. Expression of estrogen, progesterone and androgen receptors in the oviduct of developing, cycling and pre-implantation rats. J. Mol. Endocrinol. 2003, 30, 301–315. [Google Scholar] [CrossRef]
- Okada, A.; Ohta, Y.; Brody, S.L.; Watanabe, H.; Krust, A.; Chambon, P.; Iguchi, T. Role of foxj1 and estrogen receptor alpha in ciliated epithelial cell differentiation of the neonatal oviduct. J. Mol. Endocrinol. 2004, 32, 615–625. [Google Scholar] [CrossRef]
- Wessel, T.; Schuchter, U.; Walt, H. Ciliary motility in bovine oviducts for sensing rapid non-genomic reactions upon exposure to progesterone. Horm. Metab. Res. 2004, 36, 136–141. [Google Scholar] [CrossRef]
- Hunter, M.I.; Thies, K.M.; Winuthayanon, W. Hormonal regulation of cilia in the female reproductive tract. Curr. Opin. Endocr. Metab. Res. 2024, 34, 100503. [Google Scholar] [CrossRef]

| Lectin Abbreviation | Source of Lectin | µg/mL | Sugar Specificity | Inhibitory Sugar |
|---|---|---|---|---|
| AAL | Aleuria aurantia | 25 | Fucα1-3/1-4/1-2/l-6 | Fucose |
| GNL | Galanthusnivalis | 25 | Terminal α1-3Man | αMet-Mannose |
| LCA | Lens culinaris | 25 | Man1-2Man1-3Man | αMet-Mannose |
| RCA120 * | Ricinus communis | 25 | Galβ1-4GlcNAcβ | Galactose |
| Primary Antibody | Type | Epitope | Dilution | Supplier | Catalog Number |
|---|---|---|---|---|---|
| HSP60 (H-1) | mouse monoclonal | Amino acids 28–62 near the N-terminus of human HSP60 | 1:200 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | sc-13115 |
| HSP70 (B-6) | mouse monoclonal | Amino acids 580–601 at the C-terminus of human HSP 70 | 1:200 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | sc-7298 |
| HSP90α (F-2) | mouse monoclonal | Amino acids 342–382 within an internal region of human HSP90α | 1:200 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | sc-515081 |
| ERα (F-10) | mouse monoclonal | Amino acids 570–595 at the C-terminus of ERα of human origin | 1:50 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | sc-8002 |
| PR (AB-52) | mouse monoclonal | native human PR-A and PR-B | 1:50 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | sc-810 |
| Follicular Phase | Preovulatory Phase | Luteal Phase | |
|---|---|---|---|
| PAS | − | − | − |
| AB 2.5 | ± | ++as | − |
| HID | − | ±as | − |
| AAL | − | +ac | ±as |
| GNL | − | ++ | − |
| LCA | ±as | +++as | +as |
| RCA120 | − | ++as | ±as |
| Follicular Phase | Preovulatory Phase | Luteal Phase | ||
|---|---|---|---|---|
| Nonciliated cells | Ciliated cells | |||
| HSP60 | +++/+++n | +g/+++ab | − | + */±n |
| HSP70 | +/+n | ++/+n | ++/++ci/+n | + */±n |
| HSP90 | +/+n | ±/+ab/+n | ±/+ci | + */±n |
| Follicular Phase | Preovulatory Phase | Luteal Phase | ||
|---|---|---|---|---|
| Nonciliated cells | Ciliated cells | |||
| ERα | +n * | +n | − | +n * |
| PR | ++cn */+ * | ++n | +ci | ± */++n |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desantis, S.; Cinone, M.; Lacitignola, L.; Laricchiuta, P.; Rossi, R.; Guaricci, A.C.; Resta, L.; Albrizio, M. Morphology, Glycan Pattern, Heat Shock Proteins, and Sex Steroid Receptors Expression in the Tubal Fimbria Epithelium of the Baboon Papio hamadryas during the Menstrual Cycle. Animals 2024, 14, 2321. https://doi.org/10.3390/ani14162321
Desantis S, Cinone M, Lacitignola L, Laricchiuta P, Rossi R, Guaricci AC, Resta L, Albrizio M. Morphology, Glycan Pattern, Heat Shock Proteins, and Sex Steroid Receptors Expression in the Tubal Fimbria Epithelium of the Baboon Papio hamadryas during the Menstrual Cycle. Animals. 2024; 14(16):2321. https://doi.org/10.3390/ani14162321
Chicago/Turabian StyleDesantis, Salvatore, Mario Cinone, Luca Lacitignola, Pietro Laricchiuta, Roberta Rossi, Antonio Ciro Guaricci, Leonardo Resta, and Maria Albrizio. 2024. "Morphology, Glycan Pattern, Heat Shock Proteins, and Sex Steroid Receptors Expression in the Tubal Fimbria Epithelium of the Baboon Papio hamadryas during the Menstrual Cycle" Animals 14, no. 16: 2321. https://doi.org/10.3390/ani14162321





