Enzymic Activity, Metabolites, and Hematological Responses in High-Risk Newly Received Calves for “Clinical Health” Reference Intervals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
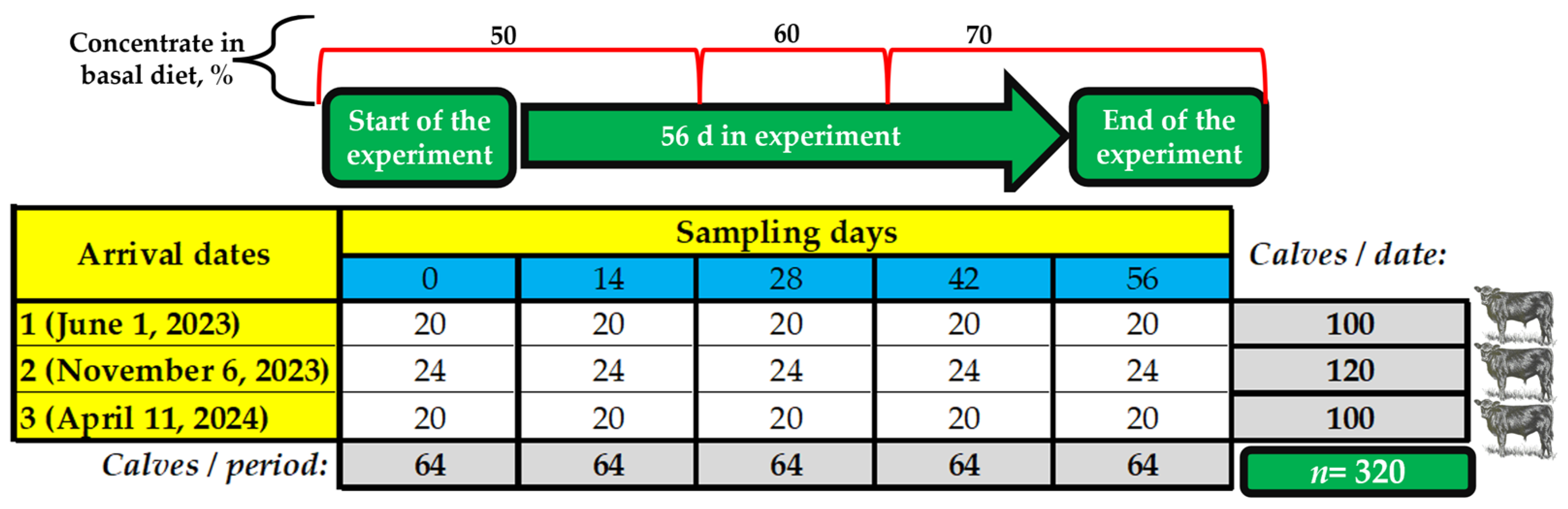
2.1. Location Where the Study Was Performed
2.2. Ethics Statement
2.3. Inclusion Criteria
2.4. Cattle Processing
2.5. Feeding and Health Management
2.6. Assessment of Enzymic Activity, Metabolites, and Hematological Responses
2.7. Calculation of Reference Intervals
3. Results and Discussion
3.1. Enzymic Activity
3.2. Metabolites
3.2.1. Total Protein, Blood Urea Nitrogen, and Creatinine
3.2.2. Total Bilirubin, Total Cholesterol, and Triglycerides
3.2.3. Calcium
3.2.4. Glucose
3.2.5. Electrolytes
3.3. Hematological Responses
3.3.1. White Blood Cells
3.3.2. Platelets
3.3.3. Red Blood Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carroll, J.A.; Arthington, J.D.; Chase, C.C., Jr. Early weaning alters the acute-phase reaction to an endotoxin challenge in beef calves. J. Anim. Sci. 2009, 87, 4167–4172. [Google Scholar] [CrossRef]
- Richeson, J.T.; Kegley, E.B.; Powell, J.G.; Schaut, R.G.; Sacco, R.E.; Ridpath, J.F. Weaning management of newly received beef calves with or without continuous exposure to a persistently infected bovine viral diarrhea virus pen mate: Effects on rectal temperature and serum proinflammatory cytokine and haptoglobin concentrations. J. Anim. Sci. 2013, 91, 1400–1408. [Google Scholar] [CrossRef]
- Richeson, J.T.; Pinedo, P.J.; Kegley, E.B.; Powell, J.G.; Gadberry, M.S.; Beck, P.A.; Falkenberg, S.M. Association of hematologic variables and castration status at the time of arrival at a research facility with the risk of bovine respiratory disease in beef calves. J. Am. Vet. Med. Assoc. 2013, 243, 1035–1041. [Google Scholar] [CrossRef]
- Stanger, K.J.; Ketheesan, N.; Parker, A.J.; Coleman, C.J.; Lazzaroni, S.M.; Fitzpatrick, L.A. The effect of transportation on the immune status of Bos indicus steers. J. Anim. Sci. 2005, 83, 2632–2636. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, L.L.; Siegmann, S.; Field, N.L.; Sugrue, K.; van Reenen, C.G.; Bokkers, E.A.M.; Sayers, G.; Conneely, M. Effect of source and journey on physiological variables in calves transported by road and ferry between Ireland and the Netherlands. Front. Vet. Sci. 2003, 10, 1238734. [Google Scholar] [CrossRef]
- Gupta, S.; Earley, D.; King, S.T.L.; Crowe, M.A. Effect of repeated regrouping and relocation on the physiological, immunological, and hematological variables and performance of steers. J. Anim. Sci. 2005, 83, 1948–1958. [Google Scholar] [CrossRef]
- Step, D.L.; Krehbiel, C.R.; DePra, H.A.; Cranston, J.J.; Fulton, R.W.; Kirkpatrick, J.G.; Gill, D.R.; Payton, M.E.; Montelongo, M.A.; Confer, A.W. Effects of commingling beef calves from different sources and weaning protocols during a fortytwo-day receiving period on performance and bovine respiratory disease. J. Anim. Sci. 2008, 86, 3146–3158. [Google Scholar] [CrossRef]
- Duff, G.C.; Galyean, M.L. Board-invited review: Recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 2007, 85, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Lynch, E.M.; Earley, B.; McGee, M.; Doyle, S. Characterization of physiological and immunological responses in beef cows to abrupt weaning and subsequent housing. BMC Vet. Res. 2010, 6, 37. [Google Scholar] [CrossRef]
- Earley, B.; Buckham Sporer, K.; Gupta, S. Invited Review: Relationship between cattle transport, immunity and respiratory disease. Animal 2017, 11, 486–492. [Google Scholar] [CrossRef]
- Carroll, J.A.; Forsberg, N.E. Influence of stress and nutrition on cattle immunity. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 105–149. [Google Scholar] [CrossRef]
- Galyean, M.L.; Duff, G.C.; Rivera, J.D. Galyean Appreciation Club Review: Revisiting nutrition and health of newly received cattle—What have we learned in the last 15 years? J. Anim. Sci. 2022, 100, skac067. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, K.L.; Hubbert, M.E.; Galyean, M.L.; Löest, C.A. Nutritional recommendations of feedlot consulting nutritionists: The 2015 New Mexico State and Texas Tech University survey. J. Anim. Sci. 2016, 94, 2648–2663. [Google Scholar] [CrossRef] [PubMed]
- Thrall, M.A.; Baker, D.C.; Lassen, E. Veterinary Haematology and Clinical Chemistry; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2004; ISBN 0781768500. [Google Scholar]
- Jones, M.L.; Allison, R.W. Evaluation of the ruminant complete blood cell count. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 377–402. [Google Scholar] [CrossRef] [PubMed]
- Smock, T.M.; Samuelson, K.L.; Wells, J.E.; Hales, K.E.; Hergenreder, J.E.; Rounds, P.W.; Richeson, J.T. Effects of Bacillus subtilis PB6 and/or chromium propionate supplementation on serum chemistry, complete blood count, and fecal Salmonella spp. count in high-risk cattle during the feedlot receiving and finishing periods. Transl. Anim. Sci. 2020, 4, txaa164. [Google Scholar] [CrossRef]
- Crawford, D.M.; Richeson, J.T.; Perkins, T.L.; Samuelson, K.L. Feeding a high-energy finishing diet upon arrival to high-risk feedlot calves: Effects on health, performance, ruminal pH, rumination, serum metabolites, and carcass traits. J. Anim. Sci. 2022, 100, skac194. [Google Scholar] [CrossRef]
- Scott, M.A.; Woolums, A.R.; Karisch, B.B.; Harvey, K.M.; Capik, S.F. Impact of preweaning vaccination on host gene expression and antibody titers in healthy beef calves. Front. Vet. Sci. 2022, 9, 1010039. [Google Scholar] [CrossRef]
- Smock, T.M.; Broadway, P.R.; Sanchez, N.C.B.; Carroll, J.A.; Hoffman, A.A.; Long, N.S.; Manahan, J.L.; McDaniel, Z.S.; Hales, K.E. Infrared thermography or rectal temperature as qualification for targeted metaphylaxis in newly received beef steers and the effects on growth performance, complete blood count, and serum haptoglobin during a 42-day feedlot receiving period. Appl. Anim. Sci. 2023, 39, 213–226. [Google Scholar] [CrossRef]
- Motta, G.A.; Neto, P.S.M.; Nociti, R.P.; Santana, Á.E. Hematological normality, serum biochemistry, and acute phase proteins in healthy beef calves in the Brazilian Savannah. Animals 2023, 13, 2398. [Google Scholar] [CrossRef]
- Rodriguez-Cordero, D.; Carrillo-Muro, O.; Hernandez-Briano, P.; Rivera-Villegas, A.; Estrada-Angulo, A. Effect of Dietary Calcium Propionate Inclusion Level and Duration in High-Risk Newly Received Stocker Calves: Growth Performance, Body Fat Reserves, and Health. Agriculture 2023, 13, 2062. [Google Scholar] [CrossRef]
- Mohri, M.; Sharifi, K.; Eidi, S. Hematology and Serum Biochemistry of Holstein Dairy Calves: Age Related Changes and Comparison with Blood Composition in Adults. Res. Vet. Sci. 2007, 83, 30–39. [Google Scholar] [CrossRef]
- George, J.W.; Snipes, J.; Lane, V.M. Comparison of bovine hematology reference intervals from 1957 to 2006. Vet. Clin. Pathol. 2010, 39, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Panousis, N.; Siachos, N.; Kitkas, G.; Kalaitzakis, E.; Kritsepi-Konstantinou, M.; Valergakis, G.E. Hematology reference intervals for neonatal Holstein calves. Res. Vet. Sci. 2018, 118, 1–10. [Google Scholar] [CrossRef]
- Roadknight, N.W.; Courtman, N.F.; Mansell, P.D.; Jongman, E.C.; Loh, Z.A.; Fisher, A.D. Biochemistry and hematology reference intervals for neonatal dairy calves aged 5–12 days. Vet. Clin. Pathol. 2021, 50, 278–286. [Google Scholar] [CrossRef]
- Radostits, O.M.; Blood, D.C.; Gay, C.C. Veterinary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses; Bailliere Tindall: London, UK, 1994. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Apendixes. In Clinical Biochemistry of Domestic Animals, 6th ed.; Academic Press: San Diego, CA, USA, 2008; pp. 873–904. ISBN 9780123704917. [Google Scholar]
- NASEM. Nutrient Requirements of Beef Cattle, 8th ed.; National Academies Press: Washington, DC, USA, 2016; ISBN 978-0-309-31702-3. [Google Scholar] [CrossRef]
- Russell, K.E.; Roussel, A.J. Evaluation of the ruminant serum chemistry profile. Vet. Clin. N. Am. 2007, 23, 403–426. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, K.; Barnhart, K.; Blanco, J.; Freeman, K.; Harr, K.; Szladovits, B.; Walton, R. ASVCP Quality Assurance and Laboratory Standards Committee (QALS) Guidelines for the Determination of Reference Intervals in Veterinary Species and Other Related Topics; American Society for Veterinary Clinical Pathology: Madison, WI, USA, 2011. [Google Scholar]
- Geffre, A.; Concordet, D.; Braun, J.P.; Trumel, C. Reference Value Advisor: A new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet. Clin. Pathol. 2011, 40, 107–112. [Google Scholar] [CrossRef]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Carlson, P.G. Clinical chemistry test. In Large Animal Internal Medicine, 4th ed.; Smith, B.P., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009; pp. 375–398. ISBN 9780323042970. [Google Scholar]
- Macrae, A. Interpreting blood haematology/biochemistry in cattle and sheep in the field. Livest. Sci. 2017, 22, 28–32. [Google Scholar] [CrossRef]
- Otter, A. Diagnostic blood biochemistry and haematology in cattle. In Pract. 2013, 35, 7–16. [Google Scholar] [CrossRef]
- Latimer, K.S. Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology, 4th ed.; Multimedica Vet.: Hoboken, NJ, USA, 2011; ISBN 849634410X/9788496344105. [Google Scholar]
- Pagana, K.D.; Pagana, T.J. Mosby’s Manual of Diagnostic and Laboratory Tests, 3rd ed.; Elsevier: St. Louis, MO, USA, 2006; ISBN 0323100627. [Google Scholar]
- Otto, F.; Baggasse, P.; Bogin, E.; Harun, M.; Vilela, F. Biochemical blood profile of Angoni cattle in Mozambique. Isr. J. Vet. Med. 2000, 55, 95–102. [Google Scholar]
- Abutarbush, S.M.; Radostits, O.M. Congenital nutritional muscular dystrophy in a beef calf. Can. Vet. J. 2003, 44, 738–739. Available online: https://pubmed.ncbi.nlm.nih.gov/14524629 (accessed on 18 April 2024).
- Pambu-Gollah, R.; Cronje, P.B.; Casey, N.H. An evaluation of the use of blood metabolite concentrations as indicators of nutritional status in free-ranging indigenous goats. S. Afr. J. Anim. Sci. 2000, 30, 115–120. [Google Scholar] [CrossRef]
- Chester-Jones, H.; Fontenot, J.P.; Veit, H.P. Physiological and pathological effects of feeding high levels of magnesium to steers. J. Anim. Sci. 1990, 68, 4400–4413. [Google Scholar] [CrossRef]
- Doornenbal, H.; Tong, A.K.; Murray, N.L. Reference values of blood parameters in beef cattle of different ages and stages of lactation. Can. J. Vet. Res. 1988, 52, 99. Available online: https://pubmed.ncbi.nlm.nih.gov/3349406 (accessed on 18 April 2024).
- Gorlov, I.F.; Levakhin, V.I.; Radchikov, V.F.; Tsai, V.P.; Bozhkova, S.E. Effect of feeding with organic microelement complex on blood composition and beef production of young cattle. Mod. Appl. Sci. 2015, 9, 8. [Google Scholar] [CrossRef]
- Tshuma, T.; Fosgate, G.T.; Hamman, R.; Holm, D.E. Effect of different levels of dietary nitrogen supplementation on the relative blood urea nitrogen concentration of beef cows. Trop. Anim. Health Prod. 2019, 51, 1883–1891. [Google Scholar] [CrossRef]
- Kohn, R.; Dinneen, M.; Russek-Cohen, E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef]
- Waggoner, J.W.; Löest, C.A.; Turner, J.L.; Mathis, C.P.; Hallford, D.M. Effects of dietary protein and bacterial lipopolysaccharide infusion on nitrogen metabolism and hormonal responses of growing beef steers. J. Anim. Sci. 2009, 87, 3656–3668. [Google Scholar] [CrossRef]
- Chimonyo, M.; Hamudikuwana, H.; Kusina, N.T.; Ncube, I. Changes in stress-related plasma metabolite concentrations in working Mashona cows on dietary supplementation. Livest. Prod. Sci. 2002, 73, 165–173. [Google Scholar] [CrossRef]
- Adkins, M.L.; Rollin, E.; Heins, B.D.; Berghaus, R.D.; Credille, B.C. Evaluation of serum metabolic parameters as predictors of bovine respiratory disease events in high-risk beef stocker calves. Bov. Pract. 2020, 54, 9–16. [Google Scholar] [CrossRef]
- Richeson, J.T.; Hughes, H.D.; Broadway, P.R.; Carroll, J.A. Vaccination management of beef cattle: Delayed vaccination and endotoxin stacking. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 575–592. [Google Scholar] [CrossRef]
- Eicher, R.; Liesegang, A.; Bouchard, E.; Tremblay, A. Effect of cow-specific factors and feeding frequency of concentrate on diurnal variations of blood metabolites in dairy cows. Am. J. Vet. Res. 1999, 60, 1493–1499. [Google Scholar] [CrossRef]
- Richeson, J.T.; Beck, P.A.; Hughes, H.D.; Hubbell, D.S.; Gadberry, M.S.; Kegley, E.B.; Powel, J.G.; Prouty, F.L. Effect of growth implant regimen on health, performance, and immunity of high-risk, newly received stocker cattle. J. Anim. Sci. 2015, 93, 4089–4097. [Google Scholar] [CrossRef]
- Agenas, S.; Heath, M.F.; Nixon, R.M.; Wilkinson, J.M.; Phillips, C.J.C. Indicators of under nutrition in cattle. Anim. Welf. 2006, 15, 149–160. [Google Scholar] [CrossRef]
- Bossaert, P.; Trevisi, E.; Opsomer, G.; Bertoni, G.; De Vliegher, S.; Leroy, J.L.M.R. The association between indicators of inflammation and liver variables during the transition period in high-yielding dairy cows: An observational study. Vet. J. 2012, 192, 222–225. [Google Scholar] [CrossRef]
- Sejersen, H.; Sørensen, M.T.; Larsen, T.; Bendixen, E.; Ingvartsen, K.L. Liver protein expression in dairy cows with high liver triglycerides in early lactation. J. Dairy Sci. 2012, 95, 2409–2421. [Google Scholar] [CrossRef]
- Ndlovu, T.; Chimonyo, M.; Okoh, A.I.; Muchenje, V.; Dzama, K.; Raats, J.G. Assessing the nutritional status of beef cattle: Current practices and future prospects. Afr. J. Biotechnol. 2007, 6, 2727–2734. [Google Scholar] [CrossRef]
- Church, D.C.; Fontenot, J.P. Nitrogen Metabolism and Requirements 25-DC Church. In Digestive Physiology and Nutrition of Ruminants; O&B Books: Corvallis, OR, USA, 1971. [Google Scholar]
- Fernandes, S.R.; Freitas, J.A.; Souza, D.F.; Kowalski, L.H.; Dittrich, R.L.; Rossi, P., Jr.; Silva, C.J.A. Lipidograma como ferramenta na avaliação do metabolismo energético em ruminantes. Rev. Bras. Agrociência 2012, 18, 21–32. [Google Scholar] [CrossRef]
- De Leeuw, J.A.; Jongbloed, A.W.; Spoolder, H.A.M.; Verstegen, M.W.A. Effects of hindgut fermentation of non-starch polysaccharides on the stability of blood glucose and insulin levels and physical activity in empty sows. Livest. Sci. 2005, 96, 165–174. [Google Scholar] [CrossRef]
- Allen, M.S.; Bradford, B.J.; Oba, M. Board invited review: The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 2009, 87, 3317–3334. [Google Scholar] [CrossRef]
- Hersom, M.J.; Wettemann, R.P.; Krehbiel, C.R.; Horn, G.W.; Keisler, D.H. Effect of live weight gain of steers during winter grazing: III. Blood metabolites and hormones during feedlot finishing. J. Anim. Sci. 2004, 82, 2059–2068. [Google Scholar] [CrossRef]
- Oosthuysen, E.R.; Hubbert, M.E.; Samuelson, K.L.; Scholljegerdes, E.J.; Duff, G.C.; Löest, C.A. Health evaluation of immune-stimulated and hay-supplemented feedlot receiving calves as assessed by blood gas analysis. Proc. Am. Soc. Anim. 2016, 67, 83–87. [Google Scholar] [CrossRef]
- Xuan, N.H.; Loc, H.T.; Ngu, N.T. Blood biochemical profiles of Brahman crossbred cattle supplemented with different protein and energy sources. Vet. World 2018, 11, 1021. [Google Scholar] [CrossRef]
- Reynolds, C.K.; Aikman, P.C.; Lupoli, B.; Humphries, D.J.; Beever, D.E. Splanchnic metabolism of dairy cows during the transition from late gestation through early lactation. J. Dairy Sci. 2003, 86, 1201–1217. [Google Scholar] [CrossRef]
- Preston, R.L. Receiving Cattle Nutrition. Vet. Clin. N. Am. 2007, 23, 193–205. [Google Scholar] [CrossRef]
- Roland, L.; Drillich, M.; Iwersen, M. Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. Investig. 2014, 26, 592–598. [Google Scholar] [CrossRef]
- Craig, A.; Mai, J.; Cai, S.; Jeyaseelan, S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 2009, 77, 568–575. [Google Scholar] [CrossRef]
- Abramowicz, B.; Kurek, Ł.; Lutnicki, K. Haematology in the early diagnosis of cattle diseases—A review. Vet. Arh. 2019, 89, 579–590. [Google Scholar] [CrossRef]
- Davies, A.O.; Lefkowitz, R.J. Corticosteroidinduced differential regulation of β-adrenergic receptors in circulating human polymorphonuclear leukocytes and mononuclear leukocytes. J. Clin. Endocrinol. Metab. 1980, 51, 599–605. [Google Scholar] [CrossRef]
- Richeson, J.T.; Kegley, E.B.; Gadberry, M.S.; Beck, P.A.; Powell, J.G.; Jones, C.A. Effects of on-arrival versus delayed clostridial or modified live respiratory vaccinations on health, performance, bovine viral diarrhea virus type I titers, and stress and immune measures of newly received beef calves. J. Anim. Sci. 2009, 87, 2409–2418. [Google Scholar] [CrossRef]
- Lopez, F.A.; Oosthuysen, E.R.; Duff, G.C.; Richeson, J.T.; Samuelson, K.L.; Hubbert, M.E.; Löest, C.A. Health, performance, and complete blood counts of newly received feedlot heifers in response to an oral drench of water and crude glycerin. Transl. Anim. Sci. 2018, 2, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Kraft, W.; Dürr, U.M. Clinical Laboratory Diagnostics in Veterinary Medicine, 6th ed.; Schattauer: Stuttgart, Germany, 2005; ISBN 3794523083. [Google Scholar]
- Tornquist, S.J.; Rigas, J. Interpretation of ruminant leukocyte responses. In Schalm’s Veterinary Hematology, 6th ed.; Wiley: Ames, IA, USA, 2010; pp. 307–313. ISBN 9780813817989/0813817986. [Google Scholar]
- Russell, K.E. Platelet kinetics and laboratory evaluation of thrombocytopenia. In Schalm’s Veterinary Hematology, 6th ed.; Wiley: Ames, IA, USA, 2010; pp. 307–313. ISBN 9780813817989/0813817986. [Google Scholar]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The epidemiology of bovine respiratory disease: What is the evidence for predisposing factors? Can. Vet. J. 2010, 10, 1095–1102. Available online: https://pubmed.ncbi.nlm.nih.gov/21197200 (accessed on 8 April 2024).
- Kneipp, J.; Balakrishnan, G.; Chen, R.; Shen, T.J.; Sahu, S.C.; Ho, N.T.; Giovannelli, J.L.; Simplaceanu, V.; Ho, C.; Spiro, T.G. Dynamics of allostery in hemoglobin: Roles of the penultimate tyrosine H bonds. J. Mol. Biol. 2006, 356, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.C. Comparative hematology of common domestic animals. In Essentials of Veterinary Hematology; Wiley: Hoboken, NJ, USA, 1993; Volume 1, pp. 19–53. [Google Scholar]
- Oosthuysen, E.R.; Hubbert, M.E.; Graves, J.R.; Löest, C.A.; Scholljegerdes, E.J. The effects of delayed processing on hydration status, health, and performance of newly received feedlot heifers. ASAS West. Sect. 2017, 69, 299. [Google Scholar] [CrossRef]
- Tomczak, D.J.; Samuelson, K.L.; Jennings, J.S.; Richeson, J.T. Oral hydration therapy with water affects health and performance of high-risk, newly received feedlot cattle. Appl. Anim. Sci. 2018, 35, 30–38. [Google Scholar] [CrossRef]
| Item 2 | Descriptive Statistics | 95% Reference Intervals (RIs) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD 3 | CV 4 | Median | Min | Max | Lower Limit of RIs (90% CI) 5 | Upper Limit of RIs (90% CI) | |||
| ALP, UI | 209.70 | 107.00 | 51.03 | 197.00 | 14.00 | 562.00 | 14.40 | 14.00–57.00 | 469.60 | 427.00–539.00 |
| GGT, UI | 17.00 | 8.90 | 52.35 | 14.00 | 10.00 | 62.00 | 10.00 | 10.00–10.00 | 47.90 | 33.00–61.00 |
| AST, UI | 68.80 | 21.90 | 31.83 | 65.00 | 32.00 | 168.00 | 38.90 | 37.30–40.60 | 124.20 | 115.50–133.00 |
| ALT, UI | 26.50 | 7.30 | 27.55 | 25.00 | 16.00 | 65.00 | 16.60 | 16.20–17.20 | 44.40 | 42.00–47.20 |
| TP, g/dL | 6.22 | 0.83 | 13.34 | 6.30 | 4.00 | 8.00 | 4.40 | 4.18–4.61 | 7.71 | 7.59–7.83 |
| ALB, g/dL | 2.97 | 0.50 | 16.84 | 3.00 | 1.50 | 4.40 | 1.90 | 1.60–2.10 | 3.70 | 3.70–4.30 |
| GLO, g/dL | 3.18 | 0.50 | 15.72 | 3.20 | 2.00 | 4.30 | 2.20 | 2.10–2.30 | 4.11 | 4.10–4.30 |
| ALB/GLO ratio | 0.94 | 0.17 | 18.09 | 0.92 | 0.58 | 1.65 | 0.68 | 0.64–0.70 | 1.32 | 1.25–1.53 |
| BUN, mg/dL | 11.08 | 2.31 | 20.85 | 10.80 | 6.30 | 17.40 | 6.91 | 6.50–7.20 | 16.10 | 15.60–17.10 |
| CRE, mg/dL | 0.81 | 0.20 | 24.69 | 0.78 | 0.48 | 1.42 | 0.52 | 0.49–0.55 | 1.35 | 0.49–1.41 |
| TBIL, mg/dL | 0.34 | 0.29 | 85.29 | 0.20 | 0.12 | 2.00 | 0.20 | 0.20–0.20 | 1.30 | 1.10–1.80 |
| TCHO, mg/dL | 78.60 | 22.00 | 27.99 | 75.00 | 50.00 | 148.00 | 50.00 | 50.00–50.00 | 127.70 | 122.00–129.00 |
| TG, mg/dL | 17.00 | 6.90 | 40.59 | 16.00 | 10.00 | 36.00 | 10.00 | 10.00–10.00 | 33.00 | 31.00–35.00 |
| Ca, mg/dL | 10.28 | 1.42 | 13.81 | 10.50 | 6.60 | 13.40 | 7.12 | 6.54–7.38 | 12.50 | 12.54–12.88 |
| GLU, mg/dL | 89.00 | 22.50 | 25.28 | 92.00 | 20.00 | 146.00 | 26.10 | 20.00–39.00 | 126.00 | 121.00–140.00 |
| Na+, mEq/L | 126.30 | 12.10 | 9.58 | 128.00 | 89.00 | 149.00 | 98.20 | 94.00–104.00 | 143.00 | 141.00–149.00 |
| K+, mEq/L | 4.93 | 1.21 | 24.54 | 4.70 | 2.80 | 9.20 | 3.11 | 3.00–3.40 | 8.59 | 7.90–8.80 |
| Cl−, mEq/L | 90.80 | 9.80 | 10.79 | 91.00 | 70.00 | 119.00 | 71.10 | 70.00–74.00 | 109.00 | 107.00–115.00 |
| Item 1 | Current Research 2 | Means of Different Publications 3 | ||
|---|---|---|---|---|
| Rodríguez-Cordero et al. [21] | Crawford et al. [17] | Smock et al. [19] | ||
| ALP, UI | 14.40–469.60 (209.70 ± 107.00) | 379.40 ± 37.33 *** | - | 111.36 ± 7.81 *** |
| GGT, UI | 10.00–47.90 (17.00 ± 8.90) | 16.60 ± 2.06 | - | - |
| AST, UI | 38.90–124.20 (68.80 ± 21.90) | 74.70 ± 7.61 *** | - | 69.38 ± 3.20 |
| ALT, UI | 16.6–44.4 (26.50 ± 7.30) | - | 16.92 ± 1.39 *** | 19.55 ± 0.76 *** |
| TP, g/dL | 4.40–7.71 (6.22 ± 0.83) | 5.90 ± 0.22 *** | - | 6.96 ± 0.09 *** |
| ALB, g/dL | 1.90–3.70 (2.97 ± 0.50) | 3.90 ± 0.50 *** | - | 2.45 ± 0.05 *** |
| GLO, g/dL | 2.20–4.11 (3.18 ± 0.50) | 3.10 ± 0.20 * | - | 4.50 ± 0.10 *** |
| BUN, mg/dL | 6.91–16.10 (11.08 ± 2.31) | 11.30 ± 0.49 | 8.67 ± 0.54 *** | 8.22 ± 0.30 *** |
| CRE, mg/dL | 0.52–1.35 (0.81 ± 0.20) | 0.75 ± 0.028 *** | 1.20 ± 0.07 *** | 1.19 ± 0.04 *** |
| TBIL, mg/dL | 0.20–1.30 (0.34 ± 0.29) | 0.23 ± 0.018 *** | - | 0.29 ± 0.008 ** |
| TCHO, mg/dL | 50.00–127.70 (78.60 ± 22.00) | 65.50 ± 4.02 | - | - |
| TG, mg/dL | 10.00–33.00 (17.00 ± 6.90) | 35.10 ± 5.97 *** | - | - |
| Ca, mg/dL | 7.12–12.50 (10.28 ± 1.42) | 10.10 ± 1.18 * | - | 10.27 ± 0.09 |
| GLU, mg/dL | 26.10–126.00 (89.00 ± 22.50) | 106.40 ± 7.31 *** | 90.17 ± 3.38 | 94.74 ± 3.06 *** |
| Na+, mEq/L | 98.20–143.00 (126.30 ± 12.10) | 121.20 ± 1.90 *** | 140.75 ± 0.72 *** | 135.82 ± 0.31 *** |
| K+, mEq/L | 3.11–8.59 (4.93 ± 1.21) | 4.4 ± 0.10 *** | 5.70 ± 0.13 *** | 5.20 ± 0.07 *** |
| Cl−, mEq/L | 71.10–109.00 (90.80 ± 9.80) | 85.7 ± 1.65 *** | 96.88 ± 0.69 *** | 94.81 ± 0.28 *** |
| Item 2 | Descriptive Statistics | 95% Reference Intervals (RIs) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD 3 | CV 4 | Median | Min | Max | Lower limit of RIs (90% CI) 5 | Upper limit of RIs (90% CI) | |||
| WBC, ×103 cells/μL | 9.65 | 2.62 | 27.15 | 9.70 | 3.50 | 15.70 | 4.60 | 4.20–5.20 | 15.20 | 14.20–15.60 |
| LYM, ×103 cells/μL | 5.87 | 1.60 | 27.26 | 5.90 | 1.60 | 9.80 | 2.60 | 2.10–3.10 | 9.00 | 8.60–9.50 |
| LYM, % | 59.20 | 9.48 | 16.01 | 59.90 | 13.20 | 84.50 | 33.60 | 27.30–41.70 | 74.61 | 72.90–84.50 |
| MON, ×103 cells/μL | 0.83 | 0.26 | 31.33 | 0.80 | 0.20 | 1.40 | 0.30 | 0.20–0.40 | 1.40 | 1.30–1.40 |
| MON, % | 8.28 | 1.50 | 18.12 | 8.10 | 3.50 | 13.50 | 5.54 | 5.40–6.40 | 12.00 | 11.10–13.10 |
| GRA, ×103 cells/μL | 3.33 | 1.42 | 42.64 | 3.10 | 0.20 | 7.90 | 1.10 | 0.70–1.50 | 6.74 | 6.00–7.10 |
| GRA, % | 35.30 | 14.82 | 41.98 | 32.10 | 10.10 | 86.80 | 17.51 | 14.70–20.30 | 85.57 | 80.30–86.30 |
| PLT, ×103 cells/μL | 239.80 | 90.70 | 37.82 | 224.00 | 69.00 | 470.00 | 91.20 | 70.00–107.00 | 444.90 | 414.00–470.00 |
| MPV, fL | 7.22 | 0.97 | 13.43 | 7.10 | 6.00 | 15.20 | 6.14 | 6.10–6.20 | 9.08 | 8.30–10.20 |
| RBC, ×106 cells/μL | 9.77 | 1.07 | 10.95 | 9.75 | 4.97 | 12.42 | 7.88 | 7.37–8.08 | 11.90 | 11.56–12.20 |
| RDW, % | 26.40 | 2.54 | 9.62 | 26.80 | 13.70 | 31.30 | 19.11 | 17.70–20.20 | 30.13 | 29.30–30.70 |
| HGB, g/100 mL | 12.05 | 1.31 | 10.87 | 12.00 | 7.20 | 15.70 | 9.40 | 9.10–9.90 | 14.40 | 14.10–15.20 |
| HCT, % | 34.27 | 3.92 | 11.44 | 34.30 | 17.50 | 49.90 | 26.65 | 25.80–28.70 | 41.93 | 40.90–43.40 |
| MCV, fL | 35.12 | 3.10 | 8.83 | 34.80 | 27.50 | 44.90 | 29.10 | 27.50–29.90 | 41.80 | 40.30–42.80 |
| MCH, pg | 12.36 | 1.03 | 8.33 | 12.40 | 10.50 | 15.70 | 10.60 | 10.60–10.80 | 14.40 | 14.10–15.00 |
| MCHC, g/dL | 35.35 | 1.98 | 5.60 | 35.50 | 29.00 | 41.50 | 30.58 | 30.00–31.10 | 38.93 | 38.30–42.00 |
| Item 1 | Current Research 2 | Means of Different Publications 3 | |||
|---|---|---|---|---|---|
| Motta et al. [20] | Rodríguez-Cordero et al. [21] | Scott et al. [18] | Smock et al. [19] | ||
| WBC, ×103 cells/μL | 4.60–15.20 (9.65 ± 2.62) | - | 8.90 ± 0.54 *** | 7.32 ± 1.29 *** | 13.03 ± 0.72 *** |
| LYM, ×103 cells/μL | 2.60–9.00 (5.87 ± 1.60) | - | 5.10 ± 0.31 *** | - | 6.79 ± 0.53 *** |
| LYM, % | 33.60–74.61 (59.20 ± 9.48) | - | 54.00 ± 1.77 *** | 51.63 ± 11.92 *** | 53.17 ± 1.19 *** |
| MON, ×103 cells/μL | 0.30–1.40 (0.83 ± 0.26) | - | 0.70 ± 0.06 *** | - | 1.70 ± 0.05 *** |
| MON, % | 5.54–12.00 (8.28 ± 1.50) | - | 7.90 ± 0.34 | 8.36 ± 4.5 | 13.43 ± 1.15 *** |
| GRA, ×103 cells/μL | 1.10–6.74 (3.33 ± 1.42) | - | 3.10 ± 0.27 | - | - |
| GRA, % | 17.51–85.57 (35.30 ± 14.82) | - | 38.00 ± 1.70 ** | - | - |
| PLT, ×103 cells/μL | 91.20–444.90 (239.80 ± 90.70) | 263.81 ± 120.09 *** | 243.00 ± 21.08 | - | 513.70 ± 33.84 *** |
| MPV, fL | 6.14–9.08 (7.22 ± 0.97) | - | 7.2 ± 0.25 | - | - |
| RBC, ×106 cells/μL | 7.88–11.90 (9.77 ± 1.07) | 9.14 ± 1.02 *** | 10.10 ± 0.25 *** | 9.03 ± 0.68 *** | 8.97 ± 0.35 *** |
| RDW, % | 19.11–30.13 (26.40 ± 2.54) | - | 26.00 ± 0.48 | 27.56 ± 3.02 * | - |
| HGB, g/100 ml | 9.40–14.40 (12.05 ± 1.31) | 11.26 ± 0.99 * | 11.60 ± 0.25 * | 12.49 ± 0.91 *** | 12.37 ± 0.36 *** |
| HCT, % | 26.65–41.93 (34.27 ± 3.92) | 33.35 ± 2.92 * | 34.30 ± 0.70 | 35.0 ± 2.53 ** | 36.08 ± 0.95 *** |
| MCV, fL | 29.10–41.80 (35.12 ± 3.10) | 36.68 ± 2.79 *** | 34.10 ± 0.78 *** | 38.84 ± 2.85 *** | 40.39 ± 0.64 *** |
| MCH, pg | 10.60–14.40 (12.36 ± 1.03) | 12.37 ± 0.88 | 11.50 ± 0.26 *** | 13.85 ± 0.81 *** | 13.86 ± 0.62 *** |
| MCHC, g/dL | 30.58–38.93 (35.35 ± 1.98) | 33.74 ± 0.89 *** | 34.00 ± 0.64 *** | 35.69 ± 0.98 * | 34.38 ± 1.27 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Muro, O.; Rodríguez-Cordero, D.; Hernández-Briano, P.; Correa-Aguado, P.I.; Medina-Flores, C.A.; Huerta-López, L.A.; Rodríguez-Valdez, F.J.; Rivera-Villegas, A.; Plascencia, A. Enzymic Activity, Metabolites, and Hematological Responses in High-Risk Newly Received Calves for “Clinical Health” Reference Intervals. Animals 2024, 14, 2342. https://doi.org/10.3390/ani14162342
Carrillo-Muro O, Rodríguez-Cordero D, Hernández-Briano P, Correa-Aguado PI, Medina-Flores CA, Huerta-López LA, Rodríguez-Valdez FJ, Rivera-Villegas A, Plascencia A. Enzymic Activity, Metabolites, and Hematological Responses in High-Risk Newly Received Calves for “Clinical Health” Reference Intervals. Animals. 2024; 14(16):2342. https://doi.org/10.3390/ani14162342
Chicago/Turabian StyleCarrillo-Muro, Octavio, Daniel Rodríguez-Cordero, Pedro Hernández-Briano, Paola Isaira Correa-Aguado, Carlos Aurelio Medina-Flores, Luis Arturo Huerta-López, Francisco Javier Rodríguez-Valdez, Alejandro Rivera-Villegas, and Alejandro Plascencia. 2024. "Enzymic Activity, Metabolites, and Hematological Responses in High-Risk Newly Received Calves for “Clinical Health” Reference Intervals" Animals 14, no. 16: 2342. https://doi.org/10.3390/ani14162342








