Dynamic Changes in the Nutrient Digestibility, Rumen Fermentation, Serum Parameters of Perinatal Ewes and Their Relationship with Rumen Microbiota
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Sample Collection and Chemical Analysis
2.3. DNA Extraction and Sequencing
2.4. Bioinformatics Analysis
2.5. Statistical Analyses
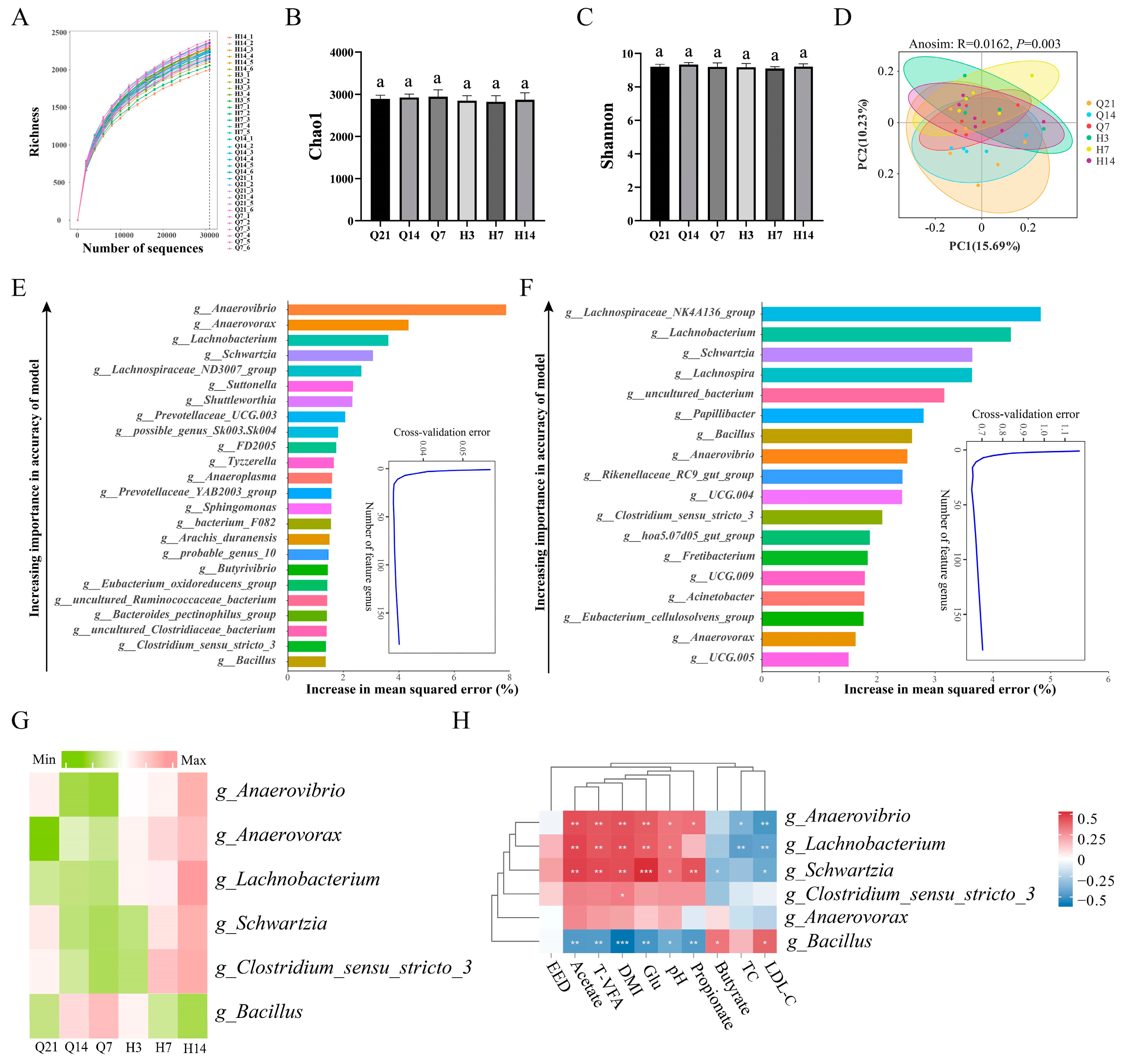
3. Results
3.1. Changes in DMI and Nutrient Digestibility during the Perinatal Period of Ewes
3.2. Changes in Rumen Fluid Parameters during the Perinatal Period of Ewes
3.3. Changes in Serum Biochemical Indexes during the Perinatal Period of Ewes
3.4. Correlation of the Rumen Microbiota with DMI, Nutrient Digestibility, Rumen Fermentation Parameters, and Serum Biochemical Indices during the Perinatal Period of Ewes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Overton, T.R.; McArt, J.A.A.; Nydam, D.V. A 100-Year Review: Metabolic Health Indicators and Management of Dairy Cattle. J. Dairy Sci. 2017, 100, 10398–10417. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, L.S.; Omontese, B.O. Monitoring and Improving the Metabolic Health of Dairy Cows during the Transition Period. Animals 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Kalscheur, K.F.; Drackley, J.K. Symposium Review: Nutrition Strategies for Improved Health, Production, and Fertility during the Transition Period. J. Dairy Sci. 2020, 103, 5684–5693. [Google Scholar] [CrossRef] [PubMed]
- Kok, A.; Chen, J.; Kemp, B.; Van Knegsel, A.T.M. Review: Dry Period Length in Dairy Cows and Consequences for Metabolism and Welfare and Customised Management Strategies. Animal 2019, 13, S42–S51. [Google Scholar] [CrossRef]
- Goselink, R.M.A.; van Baal, J.; Widjaja, H.C.A.; Dekker, R.A.; Zom, R.L.G.; de Veth, M.J.; van Vuuren, A.M. Effect of Rumen-Protected Choline Supplementation on Liver and Adipose Gene Expression during the Transition Period in Dairy Cattle. J. Dairy Sci. 2013, 96, 1102–1116. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between Negative Energy Balance, Metabolic Diseases, Uterine Health and Immune Response in Transition Dairy Cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, H.; Tun, H.M.; Cardoso, F.C.; Plaizier, J.C.; Khafipour, E.; Loor, J.J. Linking Peripartal Dynamics of Ruminal Microbiota to Dietary Changes and Production Parameters. Front. Microbiol. 2017, 7, 2143. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Lecchi, C.; Urh, C.; Sauerwein, H. Proteomics and Metabolomics Characterizing the Pathophysiology of Adaptive Reactions to the Metabolic Challenges during the Transition from Late Pregnancy to Early Lactation in Dairy Cows. J. Proteom. 2018, 178, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Indugu, N.; Vecchiarelli, B.; Baker, L.D.; Ferguson, J.D.; Vanamala, J.K.P.; Pitta, D.W. Comparison of Rumen Bacterial Communities in Dairy Herds of Different Production. BMC Microbiol. 2017, 17, 190. [Google Scholar] [CrossRef]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Liu, J.X.; Guan, L.L. Multi-Omics Reveals That the Rumen Microbiome and Its Metabolome Together with The Host Metabolome Contribute to Individualized Dairy Cow Performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef]
- Krause, D.O.; Denman, S.E.; Mackie, R.I.; Morrison, M.; Rae, A.L.; Attwood, G.T.; McSweeney, C.S. Opportunities to Improve Fiber Degradation in The Rumen: Microbiology, Ecology, And Genomics. FEMS Microbiol. Rev. 2003, 27, 663–693. [Google Scholar] [CrossRef] [PubMed]
- Sarah, M.; Itzhak, M. Islands in The Stream: From Individual to Communal Fiber Degradation in The Rumen Ecosystem. FEMS Microbiol. Rev. 2019, 43, 362–379. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Rahman, A.; Chen, M.; Li, N.; Wu, T.; Qi, Y.; Zheng, N.; Zhao, S.; Wang, J. Rumen Microbiota Succession throughout The Perinatal Period and Its Association with Postpartum Production Traits in Dairy Cows: A Review. Anim. Nutr. 2024, 18, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Kristensen, L.; Difford, G.F.; Poulsen, M.; Noel, S.J.; Abu Al-Soud, W.; Sørensen, S.J.; Lassen, J.; Løvendahl, P.; Højberg, O. Changes in Rumen Bacterial and Archaeal Communities over the Transition Period in Primiparous Holstein Dairy Cows. J. Dairy Sci. 2018, 101, 9847–9862. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.S.; Oikonomou, G.; Lima, S.F.; Bicalho, M.L.S.; Ganda, E.K.; Filho, J.C.d.O.; Lorenzo, G.; Trojacanec, P.; Bicalho, R.C. Prepartum and Postpartum Rumen Fluid Microbiomes: Characterization and Correlation with Production Traits in Dairy Cows. Appl. Environ. Microbiol. 2015, 81, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; White, B.A.; Mizrahi, I. Potential Role of the Bovine Rumen Microbiome in Modulating Milk Composition and Feed Efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef]
- Pitta, D.W.; Kumar, S.; Vecchiarelli, B.; Baker, L.D.; Ferguson, J.D.; Thomsen, N.; Shirley, D.J.; Bittinger, K. Temporal Dynamics in the Ruminal Microbiome of Dairy Cows during the Transition Period. J. Anim. Sci. 2014, 92, 4014–4022. [Google Scholar] [CrossRef]
- Giurgiu, O.; Berean, D.; Ionescu, A.; Ciupe, M.; Cimpean, C.; Radu, C.; Bitica, D.; Bogdan, S.; Bogdan, M. The Effect of Oral Administration of Zeolite on The Energy Metabolism and Reproductive Health of Romanian Spotted Breed in Advanced Gestation and Postpartum Period. Vet. Anim. Sci. 2023, 23, 100333. [Google Scholar] [CrossRef] [PubMed]
- NRC (National Research Council). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New World Camelids; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 17th ed.; AOAC: Arlington, TX, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Guo, Y.-X.; Yang, R.-C.; Duan, C.-H.; Wang, Y.; Hao, Q.-H.; Ji, S.-K.; Yan, H.; Zhang, Y.-J.; Liu, Y.-Q. Effect of Dioscorea Opposite Waste on Growth Performance, Blood Parameters, Rumen Fermentation and Rumen Bacterial Community in Weaned Lambs. Sci. Agric. Sin. 2023, 22, 1833–1846. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, F.; Kong, W.; Wang, R.; Liu, X.; Ding, H.; Ma, Y.; Guo, Y. Dynamic Changes of Rumen Bacteria and Their Fermentative Ability in High-Producing Dairy Cows During the Late Perinatal Period. Front. Microbiol. 2023, 25, 1269123. [Google Scholar] [CrossRef]
- Gao, J.; Sun, Y.; Bao, Y.; Zhou, K.; Kong, D.; Zhao, G. Effects of Different Levels of Rapeseed Cake Containing High Glucosinolates in Steer Ration on Rumen Fermentation, Nutrient Digestibility and The Rumen Microbial Community. Br. J. Nutr. 2021, 125, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Houlahan, K.; Schenkel, F.S.; Hailemariam, D.; Lassen, J.; Kargo, M.; Cole, J.B.; Connor, E.E.; Wegmann, S.; Junior, O.; Miglior, F.; et al. Effects of Incorporating Dry Matter Intake and Residual Feed Intake into a Selection Index for Dairy Cattle Using Deterministic Modeling. Animals 2021, 11, 1157. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.-W.; Wang, Z.-Y.; Ma, T.-W.; Deng, M.-T.; Wang, F.; Zhang, Y.-L. Energy and Protein Requirements for Maintenance of Hu Sheep during Pregnancy. J. Integr. Agric. 2018, 17, 173–183. [Google Scholar] [CrossRef]
- Koong, L.J.; Garrett, W.N.; Rattray, P.V. A Description of the Dynamics of Fetal Growth in Sheep. J. Anim. Sci. 2018, 41, 1065–1068. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: New York, NY, USA, 1994. [Google Scholar]
- Dijkstra, J.; Van Gastelen, S.; Dieho, K.; Nichols, K.; Bannink, A. Review: Rumen Sensors: Data and Interpretation for Key Rumen Metabolic Processes. Animal 2020, 14, S176–S186. [Google Scholar] [CrossRef] [PubMed]
- Firkins, J.L.; Yu, Z.; Morrison, M. Ruminal Nitrogen Metabolism: Perspectives for Integration of Microbiology and Nutrition for Dairy. J. Dairy Sci. 2007, 90, E1–E16. [Google Scholar] [CrossRef]
- Yoshida, H.; Ishii, M.; Akagawa, M. Propionate Suppresses Hepatic Gluconeogenesis via GPR43/AMPK Signaling Pathway. Arch. Biochem. Biophys. 2019, 672, 108057. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.Z.; Xue, M.; Wang, D.; Guan, L.; Liu, J. Days-in-Milk and Parity Affected Serum Biochemical Parameters and Hormone Profiles in Mid-Lactation Holstein Cows. Animals 2019, 9, 230. [Google Scholar] [CrossRef]
- Harmon, D.L.; Yamka, R.M.; Elam, N.A. Factors Affecting Intestinal Starch Digestion in Ruminants: A Review. Can. J. Anim. Sci. 2004, 84, 309–318. [Google Scholar] [CrossRef]
- Mongini, A.; Van Saun, R.J. Pregnancy Toxemia in Sheep and Goats. Vet. Clin. N. Am.—Food Anim. Pract. 2023, 39, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Khan, M.Z.; Abbas, Z.; Hu, L. Major Nutritional Metabolic Alterations Influencing the Reproductive System of Postpartum Dairy Cows. Metabolites 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Tang, S.; Zhou, C.; Han, X.; Tan, Z. Effects of Free Fatty Acids with Different Chain Lengths and Degrees of Saturability on the Milk Fat Synthesis in Primary Cultured Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2019, 67, 8485–8492. [Google Scholar] [CrossRef]
- Pérez-Báez, J.; Risco, C.; Chebel, R.; Gomes, G.; Greco, L.; Tao, S.; Thompson, I.; Amaral, B.D.; Zenobi, M.; Martinez, N.; et al. Association of Dry Matter Intake and Energy Balance Prepartum and Postpartum with Health Disorders Postpartum: Part II. Ketosis and Clinical Mastitis. J. Dairy Sci. 2019, 102, 9151–9164. [Google Scholar] [CrossRef]
- Sun, F.; Cao, Y.; Cai, C.; Li, S.; Yu, C.; Yao, J. Regulation of Nutritional Metabolism in Transition Dairy Cows: Energy Homeostasis and Health in Response to Post-Ruminal Choline and Methionine. PLoS ONE 2016, 11, e0160659. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, C.F.; Pimentel, T.C.; Ferrão, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep Milk: Physicochemical Characteristics and Relevance for Functional Food Development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Arís, A.; Bach, A. Associations between Subclinical Hypocalcemia and Postparturient Diseases in Dairy Cows. J. Dairy Sci. 2017, 100, 7427–7434. [Google Scholar] [CrossRef]
- Megahed, A.A.; Hiew, M.W.H.; El Badawy, S.A.; Constable, P.D. Plasma Calcium Concentrations Are Decreased at Least 9 Hours before Parturition in Multiparous Holstein-Friesian Cattle in a Herd Fed an Acidogenic Diet during Late Gestation. J. Dairy Sci. 2018, 101, 1365–1378. [Google Scholar] [CrossRef]
- Martinez, N.; Risco, C.A.; Lima, F.S.; Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Maunsell, F.; Galvao, K.; Santos, J.E.P. Evaluation of Peripartal Calcium Status, Energetic Profile, and Neutrophil Function in Dairy Cows at Low or High Risk of Developing Uterine Disease. J. Dairy Sci. 2012, 95, 7158–7172. [Google Scholar] [CrossRef]
- Spor, A.; Koren, O.; Ley, R. Unravelling the Effects of the Environment and Host Genotype on the Gut Microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef]
- Liu, H.; Hou, C.; Li, N.; Zhang, X.; Zhang, G.; Yang, F.; Zeng, X.; Liu, Z.; Qiao, S. Microbial and Metabolic Alterations in Gut Microbiota of Sows during Pregnancy and Lactation. FASEB J. 2019, 33, 4490–4501. [Google Scholar] [CrossRef]
- Deusch, S.; Camarinha-Silva, A.; Conrad, J.; Beifuss, U.; Rodehutscord, M.; Seifert, J. A Structural and Functional Elucidation of the Rumen Microbiome Influenced by Various Diets and Microenvironments. Front. Microbiol. 2017, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; López-García, A.; González-Recio, O.; Elcoso, G.; Fàbregas, F.; Chaucheyras-Durand, F.; Castex, M. Changes in the Rumen and Colon Microbiota and Effects of Live Yeast Dietary Supplementation During the Transition from the Dry Period to Lactation of Dairy Cows. J. Dairy Sci. 2019, 102, 6180–6198. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ji, S.; Suen, G.; Wang, F.; Li, S. The Rumen Bacterial Community in Dairy Cows Is Correlated to Production Traits During Freshening Period. Front. Microbiol. 2021, 12, 630605. [Google Scholar] [CrossRef] [PubMed]
- Tröscher-Mußotter, J.; Deusch, S.; Borda-Molina, D.; Frahm, J.; Dänicke, S.; Camarinha-Silva, A.; Huber, K.; Seifert, J. Cow’s Microbiome from Antepartum to Postpartum: A Long-Term Study Covering Two Physiological Challenges. Front. Microbiol. 2022, 13, 1000750. [Google Scholar] [CrossRef] [PubMed]
- Privé, F.; Kaderbhai, N.N.; Girdwood, S.; Worgan, H.J.; Pinloche, E.; Scollan, N.D.; Huws, S.A.; Newbold, C.J. Identification and Characterization of Three Novel Lipases Belonging to Families II and V from Anaerovibrio Lipolyticus 5ST. PLoS ONE 2013, 8, e69076. [Google Scholar] [CrossRef] [PubMed]
- Whitford, M.F.; Yanke, L.J.; Forster, R.J.; Teather, R.M.; Canada, A. Novel Bacterium Isolated from the Rumen and Faeces of Cattle. Int. J. Syst. Evolut. Microbiol. 2001, 51, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhuo, Y.; Liu, Y.; Chen, Y.; Ning, Y.; Yao, J. Association between Premature Ovarian Insufficiency and Gut Microbiota. BMC Pregnancy Childbirth 2021, 21, 418. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Martín-Núñez, G.M.; Roca-Rodríguez, M.M.; Cardona, F.; Coin-Aragüez, L.; Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Muñoz-Garach, A.; Fernández-García, J.C.; Moreno-Indias, I.; et al. H. pylori Eradication Treadtment Alters Gut Microbiota and GLP-1 Secretion in Humans. J. Clin. Med. 2019, 8, 451. [Google Scholar] [CrossRef]
- Gylswyk, N.V.A.N.; Hippe, H.; Rainey, F.A. Bacterium Utilizing Succinate as the Sole Energy Source. Int. J. Syst. Bacteriol. 1997, 47, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus Cereus Group: Bacillus Species with Pathogenic Potential. Gram-Posit. Pathog. 2019, 7, 875–902. [Google Scholar] [CrossRef] [PubMed]
| Item | Content (%) |
|---|---|
| Ingredient | |
| Corn stalk silage | 38.46 |
| Peanut vine | 15.38 |
| Green hay | 15.38 |
| Corn | 14.38 |
| Bran | 3.13 |
| Soybean meal | 11.65 |
| CaHPO4 | 0.13 |
| Premix (1) | 0.67 |
| Sodium bicarbonate | 0.40 |
| NaCl | 0.42 |
| Total | 100.00 |
| Nutritional Indicator | |
| Metabolic energy, ME/(MJ kg−1) (2) | 9.40 |
| Crude protein, CP | 13.49 |
| Ether extract, EE | 2.60 |
| Neutral detergent fiber, NDF | 43.16 |
| Acid detergent fiber, ADF | 18.91 |
| Crude ash | 11.43 |
| Calcium, Ca | 0.66 |
| Phosphorus, P | 0.33 |
| Item (1) | Prepartum | Postpartum | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Q21 | Q14 | Q7 | H3 | H7 | H14 | |||
| DMI (Kg/d) | 1.53 b | 1.43 c | 1.39 c | 1.55 b | 1.82 a | 1.88 a | 0.031 | <0.001 |
| DMD | 68.86 bc | 70.19 b | 74.27 a | 62.52 c | 65.75 c | 70.94 ab | 0.789 | <0.001 |
| CPD | 69.77 b | 74.04 ab | 76.58 a | 69.50 b | 65.09 b | 74.11 ab | 0.877 | <0.001 |
| EED | 74.95 b | 78.44 b | 82.20 ab | 73.51 c | 82.23 ab | 83.40 a | 0.861 | <0.001 |
| NDFD | 52.76 b | 54.48 b | 58.10 ab | 53.71 b | 52.86 b | 59.99 a | 0.752 | 0.011 |
| ADFD | 43.89 b | 49.15 a | 48.93 a | 46.54 ab | 47.67 ab | 47.41 a | 0.727 | 0.224 |
| CaD | 43.46 bc | 44.13 bc | 47.78 b | 39.41 c | 42.31 c | 53.07 a | 0.962 | <0.001 |
| PD | 61.14 b | 64.58 b | 68.89 a | 59.92 b | 60.60 b | 63.66 b | 0.748 | 0.001 |
| Item (1) | Prepartum | Postpartum | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Q21 | Q14 | Q7 | H3 | H7 | H14 | |||
| pH | 6.53 b | 6.54 b | 6.75 a | 6.47 b | 6.33 c | 6.30 c | 0.026 | <0.001 |
| NH3-N (mg/dL) | 6.30 c | 6.42 b | 8.53 a | 7.46 b | 6.75 b | 7.78 b | 0.178 | <0.001 |
| MCP (mg/dL) | 42.15 ab | 41.56 ab | 38.59 b | 40.48 b | 40.68 b | 43.49 a | 0.411 | 0.012 |
| Acetate (mmol/L) | 30.79 c | 31.56 c | 26.65 d | 34.49 b | 42.46 a | 43.41 a | 1.362 | <0.001 |
| Propionate (mmol/L) | 10.74 c | 11.91 bc | 9.42 c | 12.53 b | 16.63 a | 18.17 a | 0.685 | <0.001 |
| Butyrate (mmol/L) | 4.62 b | 4.18 b | 3.55 c | 4.05 b | 5.12 a | 5.60 a | 0.165 | <0.001 |
| A/P | 2.88 a | 2.64 ab | 2.50 ab | 2.77 ab | 2.56 ab | 2.40 b | 0.064 | 0.129 |
| T-VFA (mmol/L) | 46.45 c | 45.65 c | 34.62 d | 51.07 b | 64.69 a | 66.71 a | 2.113 | <0.001 |
| Item (1) | Prepartum | Postpartum | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Q21 | Q14 | Q7 | H3 | H7 | H14 | |||
| Glu (mmol/L) | 2.14 b | 1.49 c | 1.42 c | 1.66 c | 2.63 a | 3.01 a | 0.120 | <0.001 |
| BHBA (mmol/L) | 0.56 b | 0.61 a | 0.68 a | 0.64 a | 0.66 a | 0.81 a | 0.027 | 0.439 |
| NEFA (mmol/L) | 0.28 b | 0.53 a | 0.59 a | 0.57 a | 0.59 a | 0.72 a | 0.041 | 0.064 |
| TG (mmol/L) | 0.35 b | 0.42 b | 0.50 a | 0.44 ab | 0.38 b | 0.36 b | 0.107 | 0.179 |
| TC (mmol/L) | 2.28 a | 2.18 a | 1.74 b | 1.49 bc | 1.17 c | 1.17 c | 0.094 | <0.001 |
| HDL-C (mmol/L) | 1.01 a | 0.84 ab | 0.63 b | 0.63 b | 0.62 b | 0.55 b | 0.034 | 0.002 |
| LDL-C (mmol/L) | 0.73 a | 0.75 a | 0.77 a | 0.59 ab | 0.38 b | 0.36 b | 0.047 | 0.015 |
| Ca (mmol/L) | 2.28 b | 2.25 b | 2.51 a | 2.50 a | 2.51 a | 2.38 ab | 0.024 | <0.001 |
| P (mmol/L) | 2.24 a | 1.94 b | 2.09 ab | 1.96 b | 2.19 ab | 1.84 b | 0.043 | 0.056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, S.; Yin, X.; Duan, C.; Li, J.; Liu, Y.; Zhang, Y. Dynamic Changes in the Nutrient Digestibility, Rumen Fermentation, Serum Parameters of Perinatal Ewes and Their Relationship with Rumen Microbiota. Animals 2024, 14, 2344. https://doi.org/10.3390/ani14162344
Chen J, Wang S, Yin X, Duan C, Li J, Liu Y, Zhang Y. Dynamic Changes in the Nutrient Digestibility, Rumen Fermentation, Serum Parameters of Perinatal Ewes and Their Relationship with Rumen Microbiota. Animals. 2024; 14(16):2344. https://doi.org/10.3390/ani14162344
Chicago/Turabian StyleChen, Jiaxin, Siwei Wang, Xuejiao Yin, Chunhui Duan, Jinhui Li, Yueqin Liu, and Yingjie Zhang. 2024. "Dynamic Changes in the Nutrient Digestibility, Rumen Fermentation, Serum Parameters of Perinatal Ewes and Their Relationship with Rumen Microbiota" Animals 14, no. 16: 2344. https://doi.org/10.3390/ani14162344
APA StyleChen, J., Wang, S., Yin, X., Duan, C., Li, J., Liu, Y., & Zhang, Y. (2024). Dynamic Changes in the Nutrient Digestibility, Rumen Fermentation, Serum Parameters of Perinatal Ewes and Their Relationship with Rumen Microbiota. Animals, 14(16), 2344. https://doi.org/10.3390/ani14162344




