Enhancing the Viability of a Small Giant Panda Population Through Individual Introduction From a Larger Conspecific Group: A Scientific Simulation Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
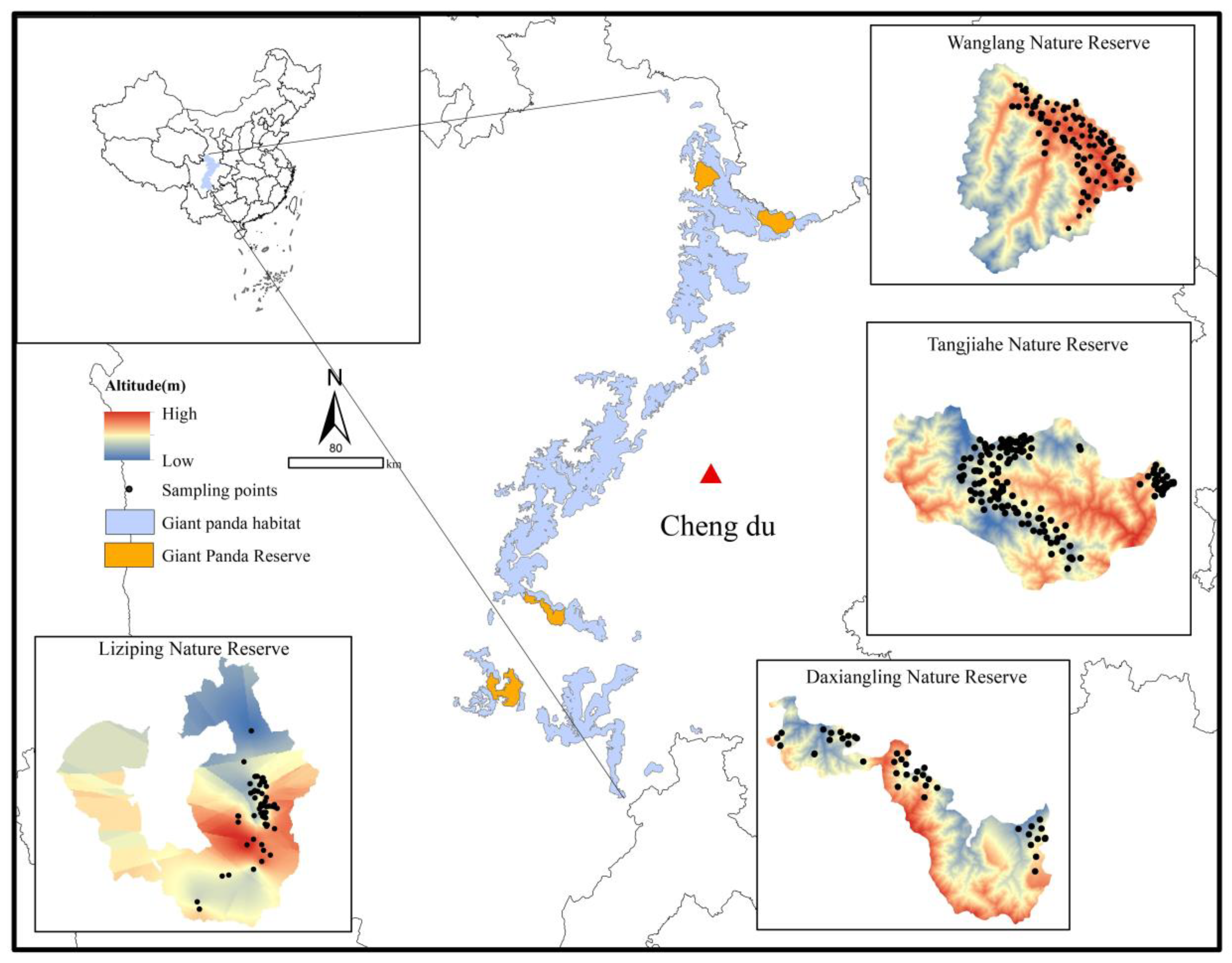
2.1. Study Area and Sample Collection
2.2. DNA Extraction and Microsatellite Amplification
2.3. Data Analysis
2.3.1. Individual Identification
2.3.2. Genetic Diversity and Genetic Structure
2.3.3. Population Viability
3. Results
3.1. Genotyping and Individual Identification
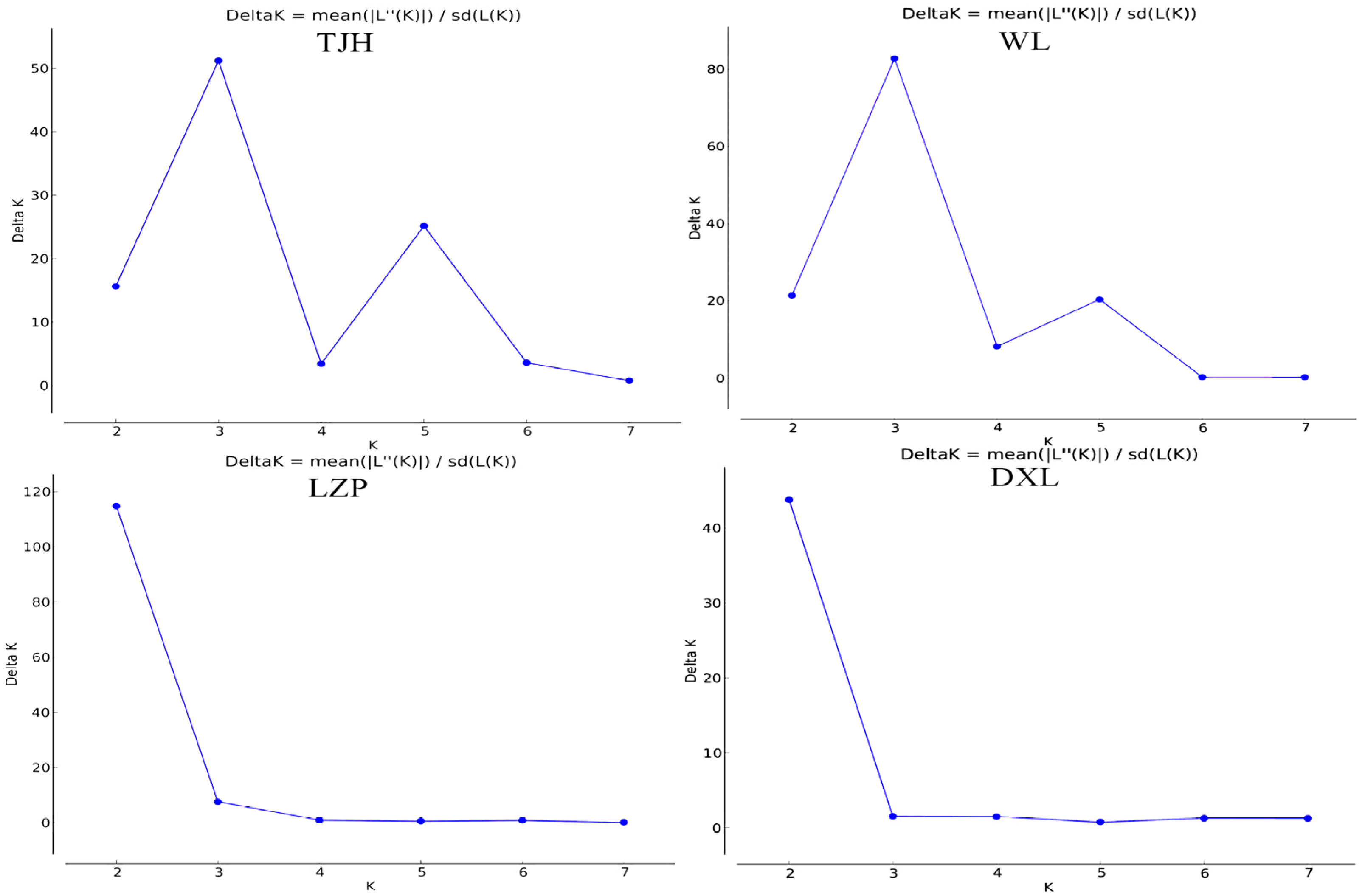
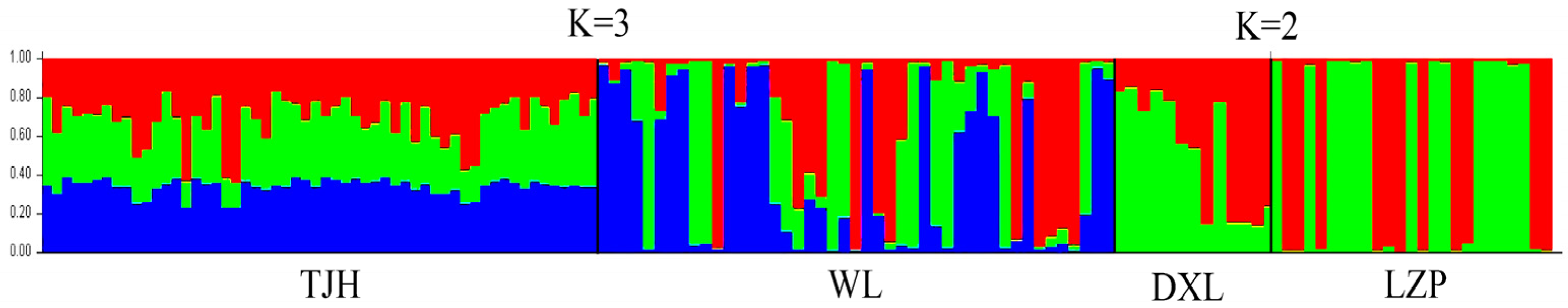
3.2. Genetic Diversity and Genetic Structure
3.3. Population Viability
3.4. Rejuvenation of Small Populations
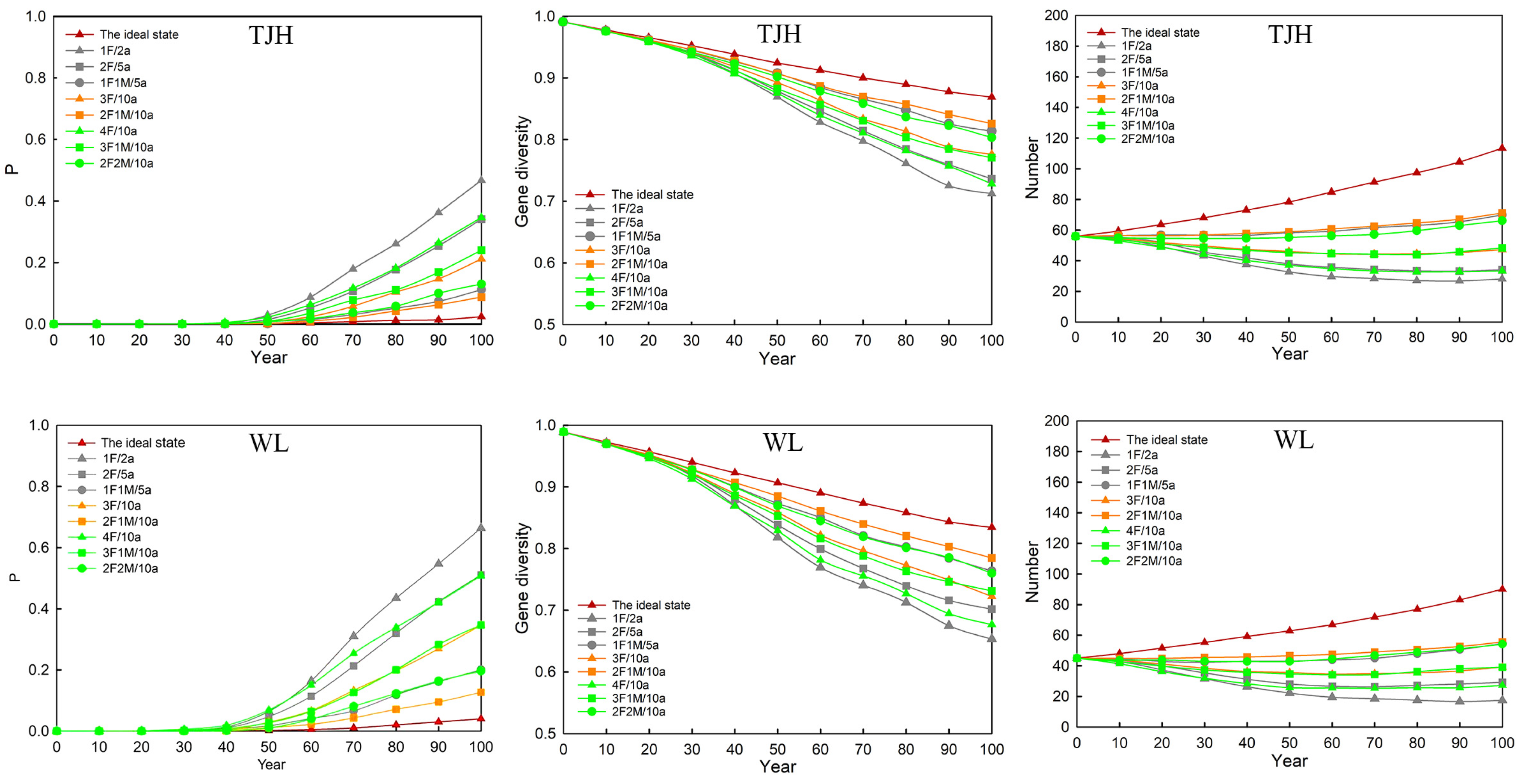
3.4.1. Viability of Small Populations after Introducing Individuals
3.4.2. Viability of Large Populations after Introducing Individuals
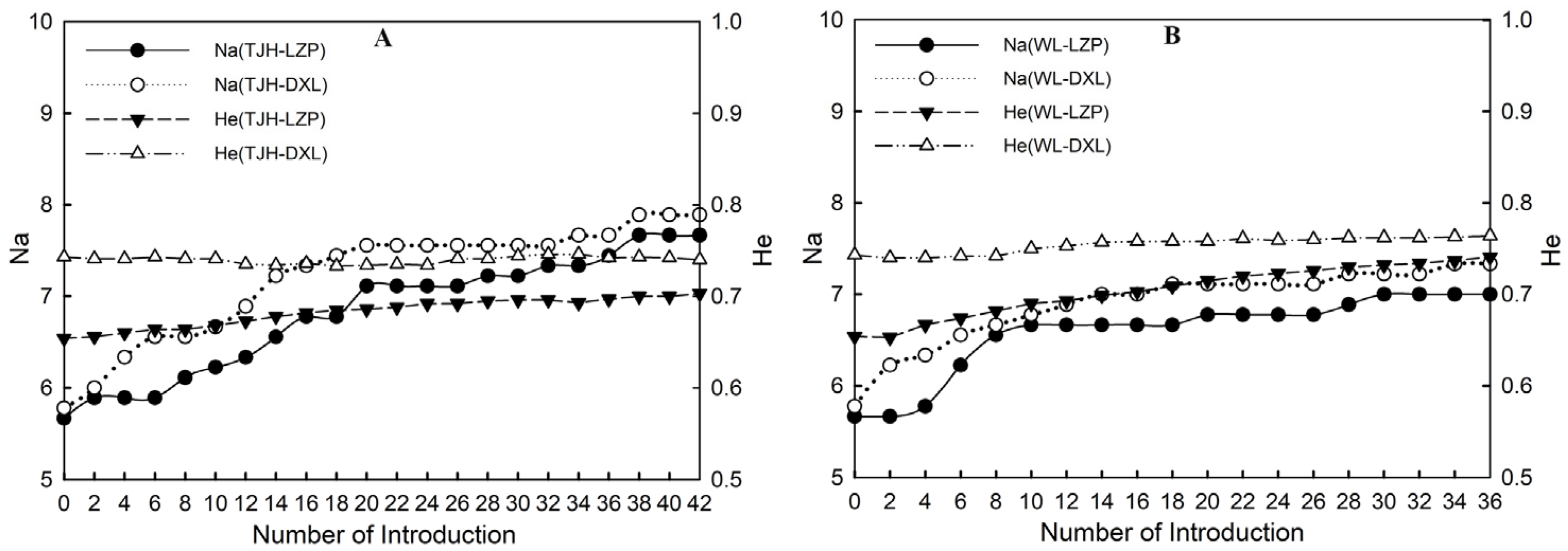
3.4.3. Genetic Level of Small Populations after Introducing Individuals
Estimation of Genetic Level of LZP
Estimation of Genetic Level of DXL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IUCN. The IUCN Red List of Threatened Species. Version 2024-1. Available online: https://www.iucnredlist.org (accessed on 5 August 2024).
- Corlett, R.T. Safeguarding our future by protecting biodiversity. Plant Divers. 2020, 42, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; McShea, W.J.; Wang, D.; Gu, X.; Zhang, X.; Zhang, L.; Shen, X. Retreat of large carnivores across the giant panda distribution range. Nat. Ecol. Evol. 2020, 4, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Li, B.V.; Pimm, S.L. China’s endemic vertebrates sheltering under the protective umbrella of the giant panda. Conserv. Biol. 2015, 30, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Swaisgood, R.R.; Wang, D.; Wei, F. Panda Downlisted but not Out of the Woods. Conserv. Lett. 2017, 11, e12355. [Google Scholar] [CrossRef]
- Li, C.; Connor, T.; Bai, W.; Yang, H.; Zhang, J.; Qi, D.; Zhou, C. Dynamics of the giant panda habitat suitability in response to changing anthropogenic disturbance in the Liangshan Mountains. Biol. Conserv. 2019, 237, 445–455. [Google Scholar] [CrossRef]
- Zhang, J.; Hull, V.; Ouyang, Z.; Li, R.; Connor, T.; Yang, H.; Zhang, Z.; Silet, B.; Zhang, H.; Liu, J. Divergent responses of sympatric species to livestock encroachment at fine spatiotemporal scales. Biol. Conserv. 2017, 209, 119–129. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. The 4th National Survey Report on Giant Panda in China; Science Press: Beijing, China, 2021.
- Swaisgood, R.R.; Martin-Wintle, M.S.; Owen, M.A.; Zhou, X.; Zhang, H. Developmental stability of foraging behavior: Evaluating suitability of captive giant pandas for translocation. Anim. Conserv. 2018, 21, 474–482. [Google Scholar] [CrossRef]
- He, K.; Dai, Q.; Foss-Grant, A.; Gurarie, E.; Fagan, W.F.; Lewis, M.A.; Qing, J.; Huang, F.; Yang, X.; Gu, X.; et al. Movement and activity of reintroduced giant pandas. Ursus 2019, 29, 163–174. [Google Scholar] [CrossRef]
- Hong, M.; Wei, W.; Zhou, H.; Tang, J.; Han, H.; Zhang, Z. Creative conservation in China: Releasing captive giant pandas into the wild. Environ. Sci. Pollut. Res. 2019, 26, 31548–31549. [Google Scholar] [CrossRef]
- Lei, M.; Yuan, S.; Yang, Z.; Hong, M.; Yang, X.; Gu, X.; Huang, F.; Zhang, Z. Comparison of microhabitats and foraging strategies between the captive-born Zhangxiang and wild giant pandas: Implications for future reintroduction. Environ. Sci. Pollut. Res. 2015, 22, 15089–15096. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.G.; Wan, Q.H.; Fujihara, N. Genetic Diversity of the Giant Panda(Ailuropoda melanoleuca) between Big and Small Populations. J. Appl. Anim. Res. 2002, 21, 65–74. [Google Scholar] [CrossRef]
- Mathews, F.; Orros, M.; McLaren, G.; Gelling, M.; Foster, R. Keeping fit on the ark: Assessing the suitability of captive-bred animals for release. Biol. Conserv. 2005, 121, 569–577. [Google Scholar] [CrossRef]
- Peng, J.; Jiang, Z.; Hu, J. Status and conservation of giant panda (Ailuropoda melanoleuca): A review. Folia Zool. 2001, 50, 81–88. [Google Scholar]
- Qi, D.; Zhang, S.; Zhang, Z.; Hu, Y.; Yang, X.; Wang, H.; Wei, F. Measures of giant panda habitat selection across multiple spatial scales for species conservation. J. Wildl. Manag. 2012, 76, 1092–1100. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, J.; Liu, N. The influence of dispersal on the metapopulation viability of Giant Panda (Aliuropoda melanoleuca) in the Minshan Mountains. Acta Zool. Acad. Sci. Hung. 2007, 53, 169–184. [Google Scholar]
- Wei, F.; Swaisgood, R.; Hu, Y.; Nie, Y.; Yan, L.; Zhang, Z.; Qi, D.; Zhu, L. Progress in the ecology and conservation of giant pandas. Conserv. Biol. 2015, 29, 1497–1507. [Google Scholar] [CrossRef]
- Hu, Y.; Qi, D.; Wang, H.; Wei, F. Genetic evidence of recent population contraction in the southernmost population of giant pandas. Genetica 2010, 138, 1297–1306. [Google Scholar] [CrossRef]
- Li, W.; Zhou, C.; Cheng, M.; Tu, H.; Wang, G.; Mao, Y.; Huang, Y.; Chen, M.; Price, M.; Meng, Y.; et al. Large-scale genetic surveys for main extant population of wild giant panda (Ailuropoda melanoleuca) reveals an urgent need of human management. Evol. Appl. 2023, 16, 738–749. [Google Scholar] [CrossRef]
- Qiao, M.; Connor, T.; Shi, X.; Huang, J.; Huang, Y.; Zhang, H.; Ran, J. Population genetics reveals high connectivity of giant panda populations across human disturbance features in key nature reserve. Ecol. Evol. 2019, 9, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, M.; Zhang, Z.; Goossens, B.; Zhu, L.; Zhang, S.; Hu, J.; Bruford, M.W.; Wei, F. Genetic Viability and Population History of the Giant Panda, Putting an End to the “Evolutionary Dead End”? Mol. Biol. Evol. 2007, 24, 1801–1810. [Google Scholar] [CrossRef]
- Yang, B.; Yang, C.; Tu, F.; Du, Y.; Liu, Y.; Li, D.; Zhang, H.; Huang, Y. The Genetics Analysis of Captive Giant Panda Reintroduction. Sichuan J. Zool. 2013, 32, 149–155. [Google Scholar] [CrossRef]
- Allendorf, F.W. Genetic drift and the loss of alleles versus heterozygosity. Zoo Biol. 1986, 5, 181–190. [Google Scholar] [CrossRef]
- Frankham, R.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Boyce, M.S. Population Viability Analysis. Annu. Rev. Ecol. Syst. 1992, 23, 481–497. [Google Scholar] [CrossRef]
- Gong, M.; Song, Y.; Yang, Z.; Lin, C. Important population viability analysis parameters for giant pandas(Aliuropoda melanoleuca). Zool. Res. 2012, 33, 18–24. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Y.; Hu, J. Population viability analysis of giant pandas in the Yele Nature Reserve. J. Nat. Conserv. 2002, 10, 35–40. [Google Scholar] [CrossRef]
- Zhou, Z.; Pan, W. Analysis of the Viability of a Giant Panda Population. J. Appl. Ecol. 1997, 34, 363–374. [Google Scholar] [CrossRef]
- Lu, Z.; Johnson, W.E.; Menotti-Raymond, M.; Yuhki, N.; Martenson, J.S.; Mainka, S.; Shi-Qiang, H.; Zhihe, Z.; Li, G.; Pan, W.; et al. Patterns of Genetic Diversity in Remaining Giant Panda Populations. Conserv. Biol. 2002, 15, 1596–1607. [Google Scholar] [CrossRef]
- Ma, T.; Hu, Y.; Russo, I.R.M.; Nie, Y.; Yang, T.; Xiong, L.; Ma, S.; Meng, T.; Han, H.; Zhang, X.; et al. Walking in a heterogeneous landscape: Dispersal, gene flow and conservation implications for the giant panda in the Qinling Mountains. Evol. Appl. 2018, 11, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Sichuan Provincial Forestry Department. Giant Panda in Sichuan-Report on the Fourth Giant Panda Survey in Sichuan Province; Sichuan Science and Technology Press: Chengdu, China, 2015. [Google Scholar]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. micro-checker: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes. 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Valière, N. gimlet: A computer program for analysing genetic individual identification data. Mol. Ecol. Notes. 2002, 2, 377–379. [Google Scholar] [CrossRef]
- Park, S.D.E. Trypanotolerance in West African Cattle and the Population Genetic Effects of Selection. Ph.D. Thesis, University of Dublin, Dublin, Ireland, 2001. [Google Scholar]
- Peakall, R.O.D.; Smouse, P.E. genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes. 2005, 6, 288–295. [Google Scholar] [CrossRef]
- Raymond, M.; Rousset, F. GENEPOP (Version 1.2): Population Genetics Software for Exact Tests and Ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Mukesh; Kumar, V.P.; Sharma, L.K.; Shukla, M.; Sathyakumar, S. Pragmatic Perspective on Conservation Genetics and Demographic History of the Last Surviving Population of Kashmir Red Deer (Cervus elaphus hanglu) in India. PLoS ONE 2015, 10, e0117069. [Google Scholar] [CrossRef] [PubMed]
- Lacy, R.C. VORTEX: A computer simulation model for population viability analysis. Wildl. Res. 1993, 20, 45–65. [Google Scholar] [CrossRef]
- Wei, F.; Hu, J. Study on Breeding of Wild Giant Panda in Wolong Nature Reserve. Acta Theriol. Sin. 1994, 14, 243–248. [Google Scholar] [CrossRef]
- Wei, F.; Hu, J.; Xu, G.; Jiang, M.; Deng, Q.; Zhong, Z. Preliminary compilation of life table of wild giant panda. Acta Theriol. Sin. 1989, 9, 81–86. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, J. Population Viability Analysis for the Giant Panda in Baoxing County, Sichuan. Sichuan J. Zool. 2010, 29, 161–165. [Google Scholar]
- Zhu, L.; Wu, P.; Zhang, H.; Hu, J. Viability analysis of giant panda population in Xiaoxiangling mountains. J. China West Norm. Univ. (Nat. Sci.) 2008, 29, 112–116. [Google Scholar]
- Ralls, K.; Ballou, J.D.; Templeton, A. Estimates of Lethal Equivalents and the Cost of Inbreeding in Mammals. Conserv. Biol. 1988, 2, 185–193. [Google Scholar] [CrossRef]
- Wei, F.; Fgeng, Z.; Hu, J. Population Viability Analysis Computer Model of Giant Panda Population in Wuyipeng, Wolong Natural Reserve, China. Bears Their Biol. Manag. 1997, 9, 19–23. [Google Scholar] [CrossRef][Green Version]
- Hu, J.; Reid, D.G.; Dong, S.; Wang, W.; Huang, Y. Foraging behavior and carrying capacity of giant pandas after bamboo flowering. J. China West Norm. Univ. (Nat. Sci.) 1990, 11, 103–113. [Google Scholar] [CrossRef]
- Hu, J.; Xia, L. The Giant Panda in Wolong; Sichuan Science and Technology Press: Chengdu, China, 1985. [Google Scholar]
- Rong, Z.; Zhou, H.; Wei, W.; Zhang, J.; Shen, L.; Zhang, Z. Giant panda habitat suitability assessment in Tangjiahe Nature Reserve based on MAXENT model. J. Lanzhou Univ. Nat. Sci. 2017, 53, 269–273. [Google Scholar] [CrossRef]
- Ruan, T.; Han, H.; Wei, W.; Qiu, L.; Hong, M.; Tang, J.; Zhou, H.; Zhang, Z. Habitat suitability evaluation for giant panda in Liziping National Nature Reserve, Sichuan Province. Glob. Ecol. Conserv. 2021, 30, e01780. [Google Scholar] [CrossRef]
- Waits, L.P.; Luikart, G.; Taberlet, P. Estimating the probability of identity among genotypes in natural populations: Cautions and guidelines. Mol. Ecol. 2001, 10, 249–256. [Google Scholar] [CrossRef]
- Fuller, R.A.; McDonald-Madden, E.; Wilson, K.A.; Carwardine, J.; Grantham, H.S.; Watson, J.E.M.; Klein, C.J.; Green, D.C.; Possingham, H.P. Replacing underperforming protected areas achieves better conservation outcomes. Nature 2010, 466, 365–367. [Google Scholar] [CrossRef]
- Li, Y. Research on the habitat status of a population of Giant Pandas in the Minshan Mountain before and after the Wenchuan earthquake. J. Sichuan For. Sci. Technol. 2009, 30, 43–47. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Wu, H.; Hou, W. An analysis on population viability for Giant Panda in Tangjiahe. Acta Ecol. Sin. 2002, 22, 990–998. [Google Scholar]
- Ran, J.; Liu, S.; Wang, H.; Zeng, Z.; Sun, Z.; Liu, S. A survey of disturbance of Giant Panda habitat in the Xiaoxiangling Mountains of Sichuan Province. Acta Theriol. Sin. 2004, 24, 277–281. [Google Scholar] [CrossRef]
- Zhu, L.; Zhan, X.; Wu, H.; Zhang, S.; Meng, T.; Bruford, M.W.; Wei, F. Conservation Implications of Drastic Reductions in the Smallest and Most Isolated Populations of Giant Pandas. Conserv. Biol. 2010, 24, 1299–1306. [Google Scholar] [CrossRef]
- Ran, J.; Zeng, Z.; Liu, S.; Wang, H. A survey of the Giant Panda population and habitats in the Daxiangling Mountains. J. Sichuan Univ. (Nat. Sci. Ed.) 2006, 43, 889–893. [Google Scholar]
- Xu, W.; Ouyang, Z.; Jiang, Z.; Zhen, H.; Liu, J. Assessment of giant panda habitat in the Daxiangling Mountain Range, Sichuan, China. Biodivers. Sci. 2006, 14, 223–231. [Google Scholar]
- Zhao, C.; Yue, B.; Ran, J.; Moermond, T.; Hou, N.; Yang, X.; Gu, X. Relationship between human disturbance and Endangered giant panda Ailuropoda melanoleuca habitat use in the Daxiangling Mountains. Oryx 2016, 51, 146–152. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Wan, Q.; Lou, J.; Li, W.; Ge, Y.; Fang, S. Patterns of Adaptive and Neutral Diversity Identify the Xiaoxiangling Mountains as a Refuge for the Giant Panda. PLoS ONE 2013, 8, e70229. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a Genetic Linkage Map in Man Using Restriction Fragment Length Polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed]
- Shen, F.; Zhang, Z.; He, W.; Yue, B.; Zhang, A.; Zhang, L.; Hou, R.; Wang, C.; Watanabe, T. Microsatellite variability reveals the necessity for genetic input from wild giant pandas (Ailuropoda melanoleuca) into the captive population. Mol. Ecol. 2009, 18, 1061–1070. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Shen, F.; Yang, X.; Zhang, L.; Chen, L.; Zhang, W.; Zhu, Q.; Hou, R. Microsatellite variability reveals high genetic diversity and low genetic differentiation in a critical giant panda population. Curr. Zool. 2011, 57, 717–724. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Clark, T.W.; Lacy, R.C.; Thomas, V.C. Population viability analysis as a tool in wildlife conservation policy: With reference to Australia. Environ. Manag. 1993, 17, 745–758. [Google Scholar] [CrossRef]
- Schenkman, L. Hope for Wild Pandas. Sci. China Ser. C Life Sci. 2010, 328, 553. [Google Scholar] [CrossRef]
- Soulé, M.; Gilpin, M.; Conway, W.; Foose, T. The millenium ark: How long a voyage, how many staterooms, how many passengers? Zoo Biol. 2005, 5, 101–113. [Google Scholar] [CrossRef]
- Ma, B.; Lei, S.; Qing, Q.; Wen, Y. Should the endangered status of the Giant panda really be reduced? The case of Giant panda conservation in Sichuan, China. Animals 2018, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gu, X.; Nie, Y.; Huang, F.; Huang, Y.; Dai, Q.; Hu, Y.; Yang, Y.; Zhou, X.; Zhang, H.; et al. Reintroduction of the giant panda into the wild: A good start suggests a bright future. Biol. Conserv. 2018, 217, 181–186. [Google Scholar] [CrossRef]
- Zhan, X.J.; Zhang, Z.J.; Wu, H.; Goossens, B.; Li, M.; Jiang, S.W.; Bruford, M.W.; Wei, F.W. Molecular analysis of dispersal in giant pandas. Mol. Ecol. 2007, 16, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, W.; Yuan, F.; Cao, D.; Zhang, Z. The Science Underlying Giant Panda Conservation Translocations. Animals. 2023, 13, 3332. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, F.; He, K.; Feng, F.; Gu, X.; Yang, X.; Dai, Q.; Qi, D.; Yang, Z. The genetic structure of small Giant Panda population in the Liziping Nature Reserve after reintroduction. J. Hechi Univ. 2016, 36, 1–7. [Google Scholar]
- Rummel, L.; Martínez–Abraín, A.; Mayol, J.; Ruiz–Olmo, J.; Mañas, F.; Jiménez, J.; Gómez, J.A.; Oro, D. Use of wild–caught individuals as a key factor for success in vertebrate translocations. Anim. Biodivers. Conserv. 2016, 39, 207–219. [Google Scholar] [CrossRef]
- Fox, G.A. Extinction Risk of Heterogeneous Populations. Ecology 2005, 86, 1191–1198. [Google Scholar] [CrossRef]
- Eillisl, S.; Pan, W.; Xie, Z.; Wildt, D. The giant panda as a social, biological and conservation phenomenon. In Giant Pandas: Biology, Veterinary Medicine and Management; Wildt, D.E., Zhang, A., Zhang, H., Janssen, D.L., Ellis, S., Eds.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Wei, F.; Hu, Y.; Yan, L.; Nie, Y.; Wu, Q.; Zhang, Z. Giant Pandas Are Not an Evolutionary cul-de-sac: Evidence from Multidisciplinary Research. Mol. Biol. Evol. 2014, 32, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, S.; Gu, X.; Wei, F. Significant genetic boundaries and spatial dynamics of giant pandas occupying fragmented habitat across southwest China. Mol. Ecol. 2011, 20, 1122–1132. [Google Scholar] [CrossRef]
- Tallmon, D.; Luikart, G.; Waples, R. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 2004, 19, 489–496. [Google Scholar] [CrossRef]


| Loci | Repeat Motif | Primer Sequence (5′–3′) | Size (bp) |
|---|---|---|---|
| Panda-22 | (CAA)12 | F: AGGGGAGAGAACATTGCTCG R: GAAGCCAGCCCAACTTTTCC | 177–186 |
| Ame-μ26 | (CA)11 | F: TTTTCAGGCCTCCGAAAAC R: ATTCCCAATAAAGCAAATCAGA | 114–120 |
| GPL-60 | (TCTT)12 | F: TGCCGGAAAGTTCTAAGCAT R: TTTCTCTCCCTCTCCCCTTC | 218–238 |
| Ame-μ13 | (CA)18 | F: GGAAGCATTAAGGAAAACATGC R: AATGATGACCATTTCAAACGC | 142–171 |
| Ame-μ11 | (CA)12 | F: TATGCCACCTGCCCAGAC R: GATGGAAAGAGTAGAGCCAAGG | 228–236 |
| Ame-μ10 | (CA)16 | F: ACCGTGCTCTTAATCCCCTT R: CCCATGCTTATGAGAAACAGG | 138–160 |
| GPZ-6 | (AAAG)11 | F: CCTGGCAGGGCAAAGTATT R: CCCCGTGAAAACATCAAGAC | 194–222 |
| GPZ-47 | (AATG)20 | F: GACCTCAGTGTACGCCCAGT R: CTGGACAGGCAGGTAGAAGC | 174–210 |
| GPL-47 | (TCTA)20 | F: TCCCCCTCTATGGTAAAAGG R: CCATGTTGGGTGTAGGGATT | 140–172 |
| Reserve | Area (km2) | Suitable and Sub-Suitable Habitats | Coverage Rate of Edible Bamboo | Maximum Capacity |
|---|---|---|---|---|
| TJH | 400.00 | 51.00% [49] | 83.94% | 519 |
| WL | 322.97 | - | 41.81% | 409 |
| DXL | 284.50 | - | 98.58% | 850 |
| LZP | 479.40 | 26.58% [50] | 69.90% | 270 |
| TJH (n = 56) | WL (n = 45) | LZP (n = 25) | DXL (n = 13) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | I | Ho | He | P | Na | I | Ho | He | P | Na | I | Ho | He | P | Na | I | Ho | He | P | |
| Panda-22 | 4.000 | 1.249 | 0.547 | 0.682 | 0.0303 | 5.000 | 1.005 | 0.778 | 0.575 | 0.9990 | 4.000 | 1.204 | 0.250 | 0.666 | 0.0001 | 5.000 | 1.413 | 0.769 | 0.722 | 0.0486 |
| Ame-μ26 | 7.000 | 1.554 | 0.518 | 0.748 | 0 | 4.000 | 1.276 | 0.778 | 0.704 | 0.8261 | 5.000 | 1.201 | 0.560 | 0.594 | 0.0146 | 5.000 | 1.487 | 0.231 | 0.749 | 0 |
| GPL-60 | 5.000 | 1.396 | 0.554 | 0.715 | 0.0564 | 5.000 | 1.534 | 0.659 | 0.767 | 0.0713 | 4.000 | 0.961 | 0.480 | 0.554 | 0.2801 | 5.000 | 1.380 | 0.385 | 0.710 | 0.0023 |
| Ame-μ13 | 11.000 | 1.589 | 0.607 | 0.705 | 0 | 7.000 | 1.347 | 0.452 | 0.684 | 0 | 6.000 | 1.220 | 0.400 | 0.610 | 0.0026 | 9.000 | 2.034 | 0.308 | 0.849 | 0 |
| Ame-μ11 | 6.000 | 1.315 | 0.518 | 0.672 | 0.0005 | 4.000 | 1.354 | 0.682 | 0.735 | 0.0899 | 5.000 | 1.026 | 0.640 | 0.538 | 0.6727 | 5.000 | 1.445 | 0.333 | 0.743 | 0.0007 |
| Ame-μ10 | 8.000 | 1.681 | 0.547 | 0.771 | 0 | 7.000 | 1.675 | 0.300 | 0.787 | 0 | 8.000 | 1.770 | 0.800 | 0.801 | 0.3761 | 6.000 | 1.662 | 0.692 | 0.790 | 0.0120 |
| GPZ-6 | 8.000 | 1.438 | 0.464 | 0.645 | 0 | 6.000 | 1.655 | 0.659 | 0.787 | 0.0057 | 5.000 | 1.127 | 0.320 | 0.570 | 0.0045 | 4.000 | 1.029 | 0.308 | 0.559 | 0.0086 |
| GPL-47 | 10.000 | 1.880 | 0.618 | 0.816 | 0.0001 | 9.000 | 1.953 | 0.548 | 0.838 | 0 | 6.000 | 1.455 | 0.800 | 0.729 | 0.7751 | 7.000 | 1.654 | 0.462 | 0.769 | 0.0270 |
| GPZ-47 | 9.000 | 1.704 | 0.446 | 0.770 | 0 | 10.000 | 1.955 | 0.350 | 0.838 | 0 | 8.000 | 1.880 | 0.520 | 0.827 | 0 | 6.000 | 1.667 | 0.385 | 0.796 | 0 |
| Average | 7.556 | 1.534 | 0.536 | 0.725 | —— | 6.333 | 1.528 | 0.578 | 0.746 | —— | 5.667 | 1.316 | 0.530 | 0.654 | —— | 5.778 | 1.531 | 0.430 | 0.743 | —— |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, J.; Yu, J.; Li, C.; Zhao, X.; Mo, L.; Wu, W.; Gai, Y.; Xu, Q.; Ni, J.; et al. Enhancing the Viability of a Small Giant Panda Population Through Individual Introduction From a Larger Conspecific Group: A Scientific Simulation Study. Animals 2024, 14, 2345. https://doi.org/10.3390/ani14162345
Zhang Y, Liu J, Yu J, Li C, Zhao X, Mo L, Wu W, Gai Y, Xu Q, Ni J, et al. Enhancing the Viability of a Small Giant Panda Population Through Individual Introduction From a Larger Conspecific Group: A Scientific Simulation Study. Animals. 2024; 14(16):2345. https://doi.org/10.3390/ani14162345
Chicago/Turabian StyleZhang, Yuzhen, Jiabin Liu, Jiaojiao Yu, Cheng Li, Xing Zhao, Li Mo, Wei Wu, Yulin Gai, Qiang Xu, Jiubin Ni, and et al. 2024. "Enhancing the Viability of a Small Giant Panda Population Through Individual Introduction From a Larger Conspecific Group: A Scientific Simulation Study" Animals 14, no. 16: 2345. https://doi.org/10.3390/ani14162345
APA StyleZhang, Y., Liu, J., Yu, J., Li, C., Zhao, X., Mo, L., Wu, W., Gai, Y., Xu, Q., Ni, J., Shen, L., Gu, H., Zhang, J., Qi, D., & Gu, X. (2024). Enhancing the Viability of a Small Giant Panda Population Through Individual Introduction From a Larger Conspecific Group: A Scientific Simulation Study. Animals, 14(16), 2345. https://doi.org/10.3390/ani14162345







