Simple Summary
Our previous investigation identified a 67-bp variable duplication in the ADIPOQ promoter region, which may impact cow ovarian development. Our researchers looked into the relationship between ADIPOQ promoter variants and ovarian traits and mRNA expression in Chinese Holstein cows in order to gain a better understanding of how to maximize the milk production of these cows while preserving their reproductive efficiency. Three genotypes were found in 2219 samples, and there was a strong correlation between this mutation and ovarian traits. Moreover, the mutant phenotype dramatically decreased the expression of the ADIPOQ gene mRNA in ovarian tissue. To increase dairy cow fertility, it is crucial to identify the genes that govern ovarian features, given the significance of the ovarian in the female reproductive system. In order to enhance the reproductive quality of Chinese Holstein dairy cows, this study offers a theoretical framework and molecular breeding signals.
Abstract
ADIPOQ plays a crucial role in regulating the reproductive system, but there are few reports on the effects of ADIPOQ on ovarian in dairy cows. Previous studies have verified the presence of a 67-bp mutation in the promoter region of the ADIPOQ gene. Hence, we employed ovarian tissues (n = 2111) and blood samples (n = 108) from Chinese Holstein cows as experimental samples to examine the association between ADIPOQ promoter variants and ovarian traits. We extracted DNA from these samples and conducted genetic typing identification on each sample using advanced techniques like PCR and agarose gel electrophoresis. Consequently, the DD, ID, and II genotypes were discovered. and it has been observed that the mutation frequency of this locus is low in the Chinese Holstein cow. Importantly, the correlational analysis unveiled a significant relationship (p < 0.05) between the weight of ovaries in late estrus and the width of ovaries during the estrus interval with the mutation. Result of the RT-PCR revealed that the ID genotype partially diminished the expression of the ADIPOQ gene. The results of this study suggest that the identified variable duplication could serve as a potential genetic marker for enhancing the ovarian traits of Chinese Holstein cows.
1. Introduction
The Chinese Holstein cow, a breed developed through extensive crossbreeding and selection, stands as the only dairy breed in China and occupies a critical economic position due to its superior milk production traits. Furthermore, animal breeding is regarded as one of the most important economic sectors in a nation, with special importance [1]. Milk productivity is substantially increased after the application of various novel techniques for dairy breeding [2]. Similarly, breeders have primarily focused on milk production traits in Chinese Holstein cows. However, this emphasis has inadvertently led to a notable decline in the conception rate of cows, due to adverse genetic correlation between milk yield and production traits [3]. This is because progesterone and estradiol, two reproductive chemicals that create issues in the cow’s estrous cycle, are eliminated by the increased hepatic metabolism of high-producing cows [4]. Therefore, it is essential to prioritize research aimed at improving milk yield while maintaining reproductive efficiency.
The ovaries are vital organ in female reproduction, playing a crucial role in the production of ova and the secretion of estrogen. The primary factors determining fertility in dairy cattle include follicle size, oocyte quality, timely development of the corpus luteum, and embryo quality. Fully developed, large follicles produce significant amounts of estradiol (E2), which is crucial for proper estrous behavior and timely insemination [5]. According to various studies, this primary organ for fertility has morphological variability. These variabilities are mainly associated with hormone levels and hereditary factors [6]. Thus, identifying essential genes regulating the activities of the ovaries, corpus luteum, and associated traits is fundamental for using molecular marker-assisted selection strategies to enhance cow fertility [7,8].
The multifunctional cytokine called adiponectin (ADIPOQ), also referred to as 30 kDa adipocyte complement-related protein (Acrp30), is secreted by adipose tissue and has a molecular weight of 30 kDa [9]. It was first discovered and cloned in 3T3-1 adipocytes by Scherer et al. in 1995 [10]. Since its discovery, ADIPOQ has been shown to increase insulin sensitivity, prevent atherosclerosis, inhibit inflammation, and participate in lipid metabolism [11]. This protein is predominantly expressed in adipose tissue and circulates in the blood, existing in various forms to perform diverse biological roles by binding to two receptors: adiponectin receptor 1 (Adipo R1) and adiponectin receptor 2 (Adipo R2) [12]. In mammalian liver, fat, and skeletal muscle, ADIPOQ regulates sugar and fat metabolism by affecting sugar utilization and insulin sensitivity. Consequently, most studies have focused on fat deposition in livestock. Numerous animal models have been developed to study the downstream effects of adiponectin signaling and function in diverse tissues in order to better understand the metabolic impacts of adiponectin. For instance, in sheep, mutations in the ADIPOQ gene have been found to correlate with growth traits and carcass traits, while in pigs these mutations have been associated with production and reproductive traits [13]. The bovine ADIPOQ gene is located on chromosome 1, near QTLs associated with marbling, eye muscle area, and dorsal marker thickness, and consists of five exons. Additionally, this gene has influence on weight traits and yield grade in cattle [14].
In 2005, the expression of ADIPOQ was identified in pig ovaries, demonstrating for the first time that ADIPOQ might play a role in the regulation of reproductive processes in animals [15]. Prior to this, ADIPOQ and its receptor had been detected in human ovaries, and ADIPOQ SNPs were associated with polycystic ovaries syndrome [16]. Some research has demonstrated that adipokines have an impact on female reproduction both directly and indirectly. Thus, it has been shown that ADIPOQ regulates the function of the gonads and the hypothalamic–pituitary axis [17]. Subsequent studies found that ADIPOQ was also expressed in rodents and livestock, but its level of expression varies among species [18]. ADIPOQ is now widely studied, and some progress has been made in studies in humans, rats, and pigs [14]. However, there are few reports about the effect of ADIPOQ on the ovarian tissue of cows. Since ovarian development is closely related to reproduction, it is crucial to investigate the factors that influence ovarian development in cows.
Our previous study reported a 67-bp variable duplication in the ADIPOQ promoter region that not only reduced the transcriptional activity of ADIPOQ in 3T3_L1 cells but also reduced its expression in adipose tissue [19]. In combination with the function of ADIPOQ, we speculate that it might affect ovarian development in cows. Therefore, the aim of the current study was to examine the correlation between ADIPOQ promoter polymorphism and ovarian development, which is expected to provide a theoretical basis for improving the fertility of Chinese Holstein cows.
2. Material and Methods
2.1. Ethics Statement
The experimental protocols in this study have received approval from the Institutional Animal Care and Use Committee of the Northwest A&F University. The sample collection was carried out in compliance with the Chinese national standard “Guidelines for the Welfare and Ethical Review of Experimental Animals” (GB/T 35892-2018) [20].
2.2. Experimental Animal and Data Collection
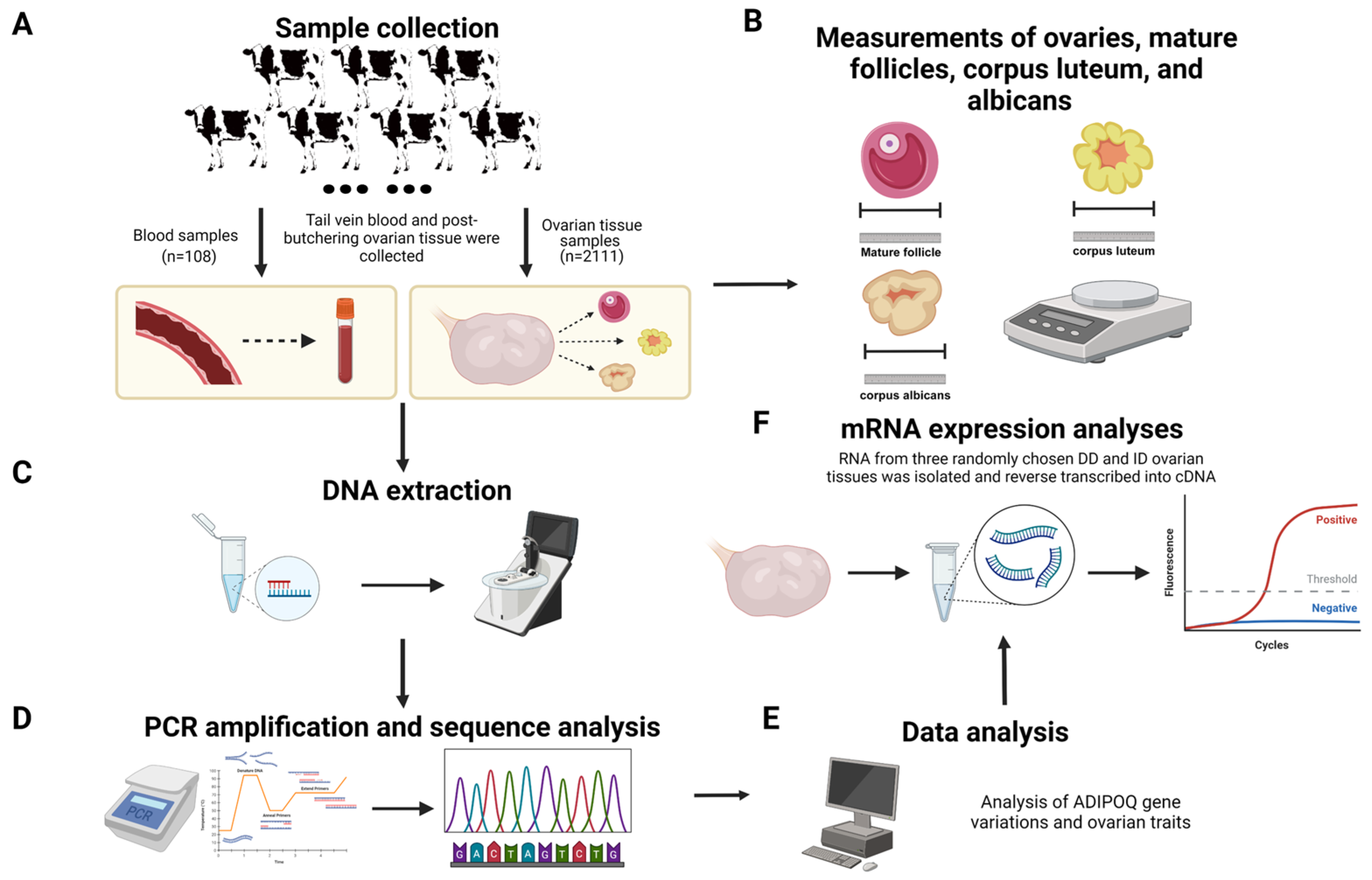
Data and samples were gathered from 2219 healthy adult Chinese Holstein cows (4–5 years old) from well-established dairy farms in Shaanxi Province, China (Figure 1), all of whom received the same nutrition and feeding guidelines and were managed according to the Regulations on Livestock and Poultry Identification and Farming Record Management [21]. The whole-blood samples were collected from the tail veins of 108 cows. The ovaries of the remaining 2111 cows were taken right after they were euthanized. Subsequently, the tissues were put in clean sterile saline solution and sent to the laboratory within two hours. Once the connective and fatty tissues were cut away from the surface of ovaries, measurements were taken of their length, width, height and weight, number and size of mature follicles, number and size of corpus luteum, and number and size of corpus albicans. Only healthy Holstein cows in the same age phase and physiological stage were included in our sample, since follicle size and development, and therefore the dimensions of the ovary, are dependent on the stages of the estrous cycle. Moreover, we identified the estrus stage indirectly by typing the corpus luteum: types 1 and 2 indicated the late stages of estrus; types 3 and 4 indicated the dioestrum; the lack of follicles and corpus luteum on the ovaries indicated pre-estrus; and the absence of corpus luteum and large follicles indicated estrus (types of corpus luteum: conical, type 2: volcanic crater, type 3: mushroom-shaped, type 4: flattened, and type 5: no corpus luteum). After the collection of phenotypic data, the ovarian tissues were rinsed three times with PBS and quickly frozen in a refrigerator set at −80 °C for subsequent experiments [22].
Figure 1.
Research strategies for examining ADIPOQ promoter polymorphisms and ovarian development (created with BioRender.com, accessed on 23 July 2024). (A) Sample collection. (B) Ovarian traits data measurement. (C) DNA extraction. (D) PCR amplification and sequencing. (E) Data analysis. (F) mRNA extraction and RT-PCR.
2.3. DNA Extraction from Different Tissue
The phenol–chloroform method was employed to extract genomic DNA from blood samples and ovarian tissue samples, in accordance with to previous reports [22]. The DNA samples were evaluated for quality by determining the A260/A280 ratio using a NanoDrop 1000 spectrophotometer [23]. The DNA samples were diluted to 50 ng/µL and stored in a refrigerator at −40 °C for subsequent experiments.
2.4. PCR Amplification and Sequence Analysis
The primer sequences, which were in accordance with the findings of earlier investigations (Table 1), were synthesized by Shanghai Bioengineering Biological Co. (Shanghai, China) [19]. The PCR amplification apparatus and protocol followed the methods described by Liu et al. [22]. A volume of 5 μL of PCR product was subjected to electrophoresis on a 3.0% agarose gel at a voltage of 120 V for 40 min (Figure 1). Subsequently, images were captured using a gel imaging system. If the product fragment size met the expected criteria, PCR products of various genotypes were purified by the SanPrep Column PCR Product Purification Kit. The refined product was ligated into a T vector (pMD19-T) and transformed into DH5α-sensitive cells. Subsequently, the recombinant plasmid was amplified using PCR and sent to Xi’an TsingKe Jersey Biotechnology Co. (Xi’an, China) for sequencing [24].

Table 1.
Primer information for the ADIPOQ gene in cattle.
2.5. Molecular Evolutionary Tree Construction
An initial alignment was conducted using ClustalW comparison with default parameters, using the protein sequences of adiponectin from bovine, sheep, goat, pig, horse, dog, Norway rat, chimpanzee, and human sources from the NCBI database. MEGA11 software was then used to generate evolutionary trees [25]. The neighbor-joining method was implemented to construct the tree, with a p-distance, bootstrap procedure with 1000 duplicates, partial deletion, and a 50% site coverage cutoff. The resulting phylogenetic tree was visualized using the Interactive Tree Of Life online tool (https://itol.embl.de/, accessed on 23 July 2024).
2.6. cDNA Synthesis and Quantitative RT-PCR
To identify the effects of different genotypes on ADIPOQ mRNA expression level, a total of six Chinese Holstein cows were randomly selected for RT-PCR analysis, based on the genotyping results (DD = 3, ID = 3). Each sample was subjected to three technical replicates, to ensure robustness in the results. Total RNA was extracted from ovarian tissue using TRIzol total RNA extraction reagent (TaKaRa Biotechnology Co. Ltd., Dalian, China) and stored at −80 °C. According to the manufacturer’s protocol, the First-strand cDNA was synthesized using the PrimeScript TM RT kit (TaKaRa Biotech Co. Ltd.), and then all genome cDNA was stored at −20 °C. According to the ADIPOQ gene sequence in GeneBank, a pair of quantitative polymerase chain reaction (qPCR) primers were designed, as shown in Table 1. A CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA) was used for qPCR; the reaction contained 5 µL SYBR Premix Ex TaqTMII (TaKaRa Biotech Co. Ltd.) and the upstream and downstream primers were each 0.5 µL, cDNA template 1 µL, ddH2O 3 µL. The reaction conditions are as follows: initial denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s and 55 °C for 30 s [26]. qPCR analysis of cDNA derived from each tissue was performed in triplicate, and relative gene expression was normalized using that of β-actin by using the 2−ΔΔCt method, as described previously [27].
2.7. Statistical Analyses
Data on ovarian traits were tallied using the Excel program. Population genotype frequency, allele frequency, and the Hardy–Weinberg equilibrium test (Hardy–Weinberg equilibrium, HWE) were calculated by chi-squared test. The classification results were calculated using Excel. The degree of purity (homozygosity, Ho), heterozygosity (heterozygosity, He), the number of effective (effective number of alleles, Ne), and the polymorphism information content (polymorphism information content, PIC) were calculated using the online software Genetic Diversity Index Calculator (http://www.msrcall.com/Gdicall.aspx, accessed on 15 June 2024) [28]. Correlation analysis of genotypes and ovarian traits was calculated by SPSS 27.0 software (SPSS, Inc., Chicago, IL, USA), and the statistical model is as follows:
Here, Yijk stands for the phenotypic value of each ovarian trait; µ represents the overall population mean; Gi stands for the fixed effect of genotype; and eijk stands for random error [29]. In this model, the impacts of age, sex, sampling season, and rearing environment were not taken into account because they were constant. We utilized the least squares mean with standard deviation for various genotypes and ovarian traits. All data were expressed as mean ± standard error, and p < 0.05 was considered statistically significant [22]. In the meantime, the nonparametric (Kruskal–Wallis) test in SPSS 27.0 software was used to assess the data that did not follow normal distribution and homogeneity of variances [30].
3. Results
3.1. Results of the Variable Duplication Locus Genotyping in the ADIPOQ
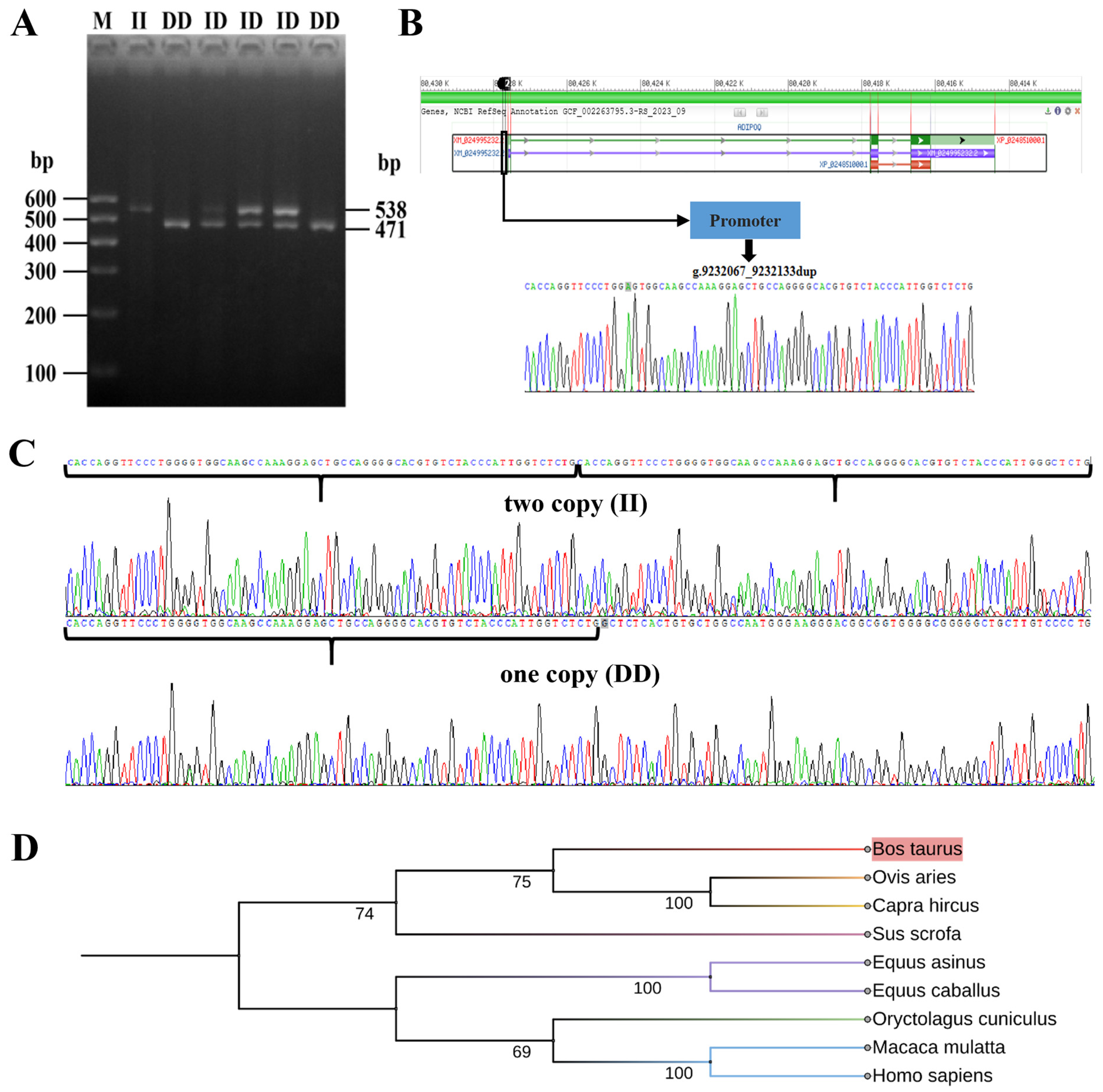
The PCR products were confirmed using agarose gel electrophoresis, and the outcomes are depicted in Figure 2A. The electrophoresis results indicated the presence of ADIPOQ genotypes in the Chinese Holstein cow population, specifically DD (originally named 1D/1D) for the homozygote reference with a product fragment size of 471 bp, ID for the heterozygous genotype (originally named 1D/2D), and II for the homozygote variant genotype (originally named 2D/2D) with two product fragments of 538 bp. These findings align with our anticipated results and provide a basis for conducting additional experiments.

Figure 2.
Identification and analysis of 67-bp duplication in the promoter region of the ADIPOQ gene. (A) The electrophoresis diagrams of the PCR amplification products. DD (471 bp): representing the wild type, contains one copy; ID (471 and 538 bp): defines the heterozygote. (B) Schematic representation of ADIPOQ gene polymorphic loci. (C) The sequence of the 67-bp duplication. II genotype above, DD genotype below. (D) Molecular evolutionary tree of the ADIPOQ gene.
3.2. ADIPOQ Gene Sequence and Molecular Evolution Analysis
PCR results from both genotypes were chosen at random for sequencing confirmation, as depicted in Figure 2B,C. Sequence analysis identified a 67-base pair repetitive segment in both DD and ID genotypes, as well as two repetitive fragments in II genotypes, which aligns with earlier research. As can be seen in Figure 2D, the evolutionary relationships between ADIPOQ genes and species was largely consistent with traditional classification, with cattle showing high affinity with sheep and goats as the closest homologues.
3.3. Estimation of Genetic Parameters of the 67-bp Mutation in the Chinese Holstein Cow Population
For the ADIPOQ promoter region, Table 2 shows the population genetic characterization of the variable repeat sequences. Three different genetic compositions were identified in the samples of ovarian tissue. The frequency of the DD genotype was 0.986, the frequency of the ID genotype was 0.013, and the frequency of the II genotype was 0.0005. The frequency of the “D” allele was 0.993, which was higher than that of the “I” allele (0.007), indicating that the “D” allele was the dominant allele in the population. The polymorphic information content (PIC) value for this locus was 0.014, indicating low polymorphism (PIC < 0.25). In addition, the χ2 test revealed a deviation from Hardy–Weinberg equilibrium for this polymorphic locus (p < 0.05). However, cows with blood samples exhibited only the DD genotype.

Table 2.
Population genetic parameters of the ADIPOQ gene in ovarian tissue samples.
3.4. Correlation Analysis of ADIPOQ Gene Variants with Ovarian Traits
Researchers investigated the relationship between different stages of reproduction and duplication variation in the promoter region of the ADIPOQ gene in Chinese Holstein cows. However, this correlation was not observed during pre-estrus and estrus (Table S1). Yet, according to the data presented in Table 3, there was a significant correlation (p < 0.05) between the weight of the ovaries during late estrus and the width of the ovaries during the estrus interval, with the duplication variation in the promoter region of the ADIPOQ gene. Furthermore, individuals with the DD type had significantly higher weight and greater width of the ovaries compared to individuals with the ID type. Nevertheless, there was no notable link observed for mature follicles, corpus luteum, or corpus albicans.

Table 3.
Relationship between the 67-bp duplication variation within the ADIPOQ gene and the ovarian traits (late estrus and estrus interval) of Chinese Holstein (LSM ± SE).
3.5. ADIPOQ mRNA Expression Analyses in Different Genotypes
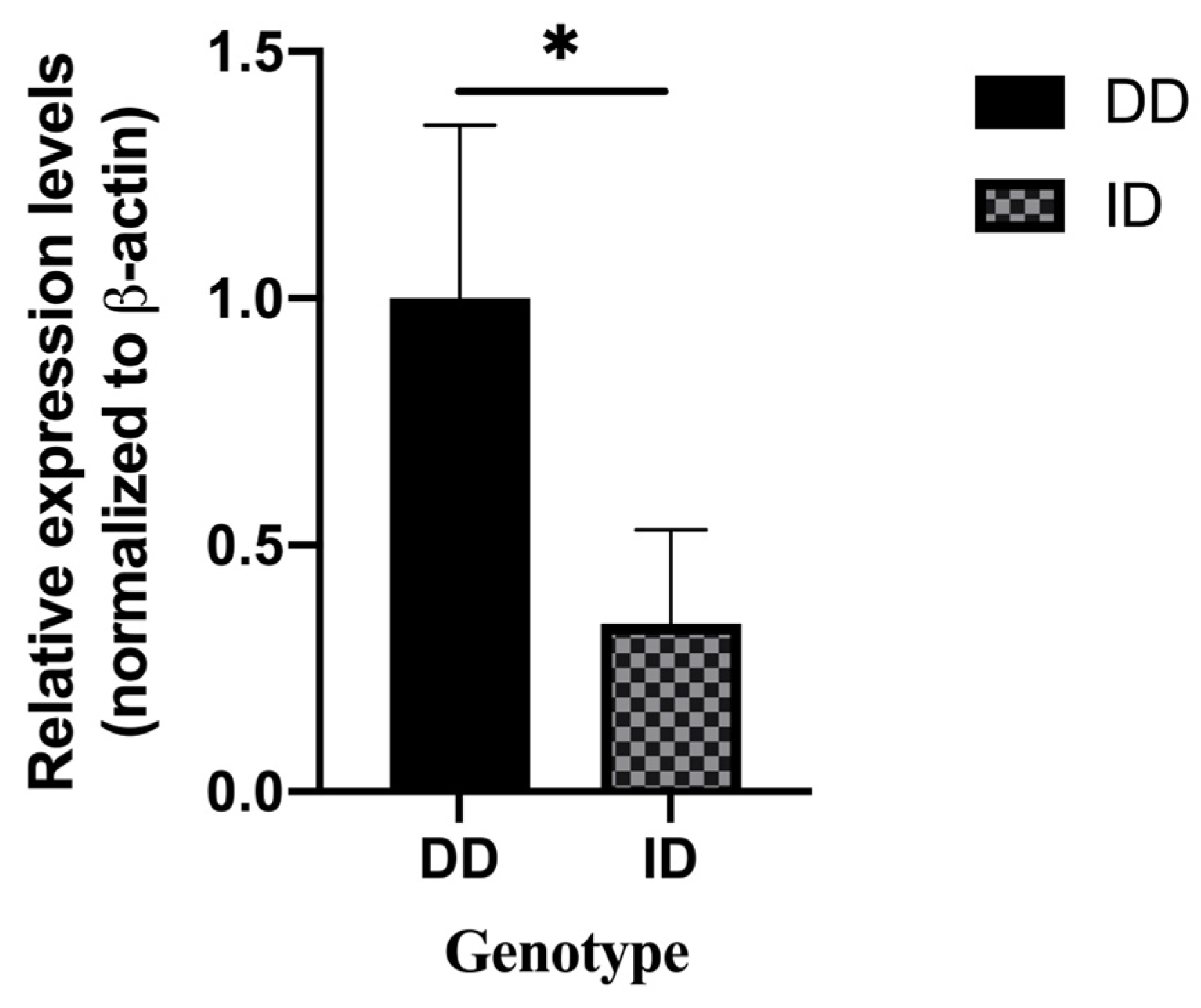
Based on the genotyping results, six ovarian tissues were randomly selected for tissue RNA extraction and reverse transcription to cDNA. qRT-PCR was used to identify the expression of three different genotypes of the ADIPOQ gene in ovarian tissues. The results revealed that the expression levels of ID genotypes were significantly lower than those of the DD genotype (Figure 3). Considering that only one individual had genotype II, we examined the mRNA expression trend of the ADIPOQ gene in this individual and found that its expression was close to that of the ID genotype.

Figure 3.
mRNA expression of different genotypes of the cow ADIPOQ gene in ovarian tissue (DD = 3, ID = 3), “*” means: p < 0.05.
4. Discussion
In Chinese Holstein cow production practices, there is a tendency to over-enhance production traits in order to increase milk production, which has resulted in a significant decline in herd reproductive performance, posing a significant challenge to the long-term development of the Chinese Holstein cow industry [31]. The herd’s reproductive performance is an important driver of production levels, and most reproductive factors exhibit poor heritability. The ovaries are vital for animal reproduction because they produce oocytes and secrete estrogen. Reproductive hormone fluctuations in cows are strongly correlated with the developing state of the ovaries during the estrous cycle. Reproductive success and the stability of subsequent pregnancies in cows are impacted by changes in the size and weight of the ovaries during the estrous cycle, which also directly affects the amount and quality of oocytes [32]. Therefore, research on marker genes linked to ovarian features advances the dairy business by increasing the reproductive efficiency of cows.
Previous studies have shown that the adiponectin system, consisting of adiponectin, its receptors AdipoR1 and AdipoR2, and the APPL1 complex, is found in both the bovine ovaries and embryos. Adiponectin interacts with its receptors and exerts an influence on ovarian function in cattle [33]. The promoter is a component of the gene that acts like a “switch”, which determines gene activity, and mutations in the promoter part of the gene can lead to dysregulation of gene expression [34]. Our previous results have confirmed that a 67-bp variable duplication in the promoter region of the ADIPOQ gene could affect transcriptional activity and the expression of ADIPOQ mRNA [19]. Genetic polymorphism analysis is a measure of the magnitude of genetic variation in a population [35]. Previous testing conducted on Xinjiang Brown cattle revealed the presence of only two genotypes [22]. Our findings revealed the presence of three genotypes—II, DD, and ID—in the Chinese Holstein cow population at this specific locus. This difference might be attributed to different geographical locations and breeding environments. The prevalence of allele “D” in the Chinese Holstein cow population at a relatively high frequency suggests that “D” is the dominant allele. The “DD” genotype was determined to be the prevailing genotype and can serve as a molecular marker for future investigations. This may be the result of natural selection, with the II genotype being gradually eliminated.
Furthermore, research has demonstrated a substantial correlation between ADIPOQ polymorphisms and the risk of developing polycystic ovarian syndrome in people [36]. In this study, we examined the correlation between genetic variants in the core promoter region of the ADIPOQ gene and traits related to bovine ovaries. The weight of the ovary in late estrus (p = 0.012) and the width of the ovary in the estrus interval (p = 0.028) were shown to be significantly correlated with the mutation site of the ADIPOQ gene. Considering the effect of different genotypes on the expression of the ADIPOQ gene, we examined the expression level of the ADIPOQ gene in ovarian tissues of the three genotypes by RT-qPCR, and the results were consistent with expectations. Both homozygote (II) and heterozygotes (ID) suppressed the expression of the ADIPOQ gene. However, a small number of mutant heterozygotes still exist, and these affect the development of bovine embryos by suppressing the expression of the ADIPOQ gene. In summary, the DD genotype can be used as a molecular marker to screen cows for superior traits and further improve herd fertility.
As the most abundantly expressed protein secreted by adipocytes, the ADIPOQ gene has various biological functions, and its role in animal reproduction has been a hotspot in recent studies [35]. The correlation between the ADIPOQ gene promoter polymorphism and ovarian traits was analyzed for the first time in a large sample size of Chinese Holstein cow ovarian tissues in this experiment. The findings verified that the polymorphic locus exhibited a highly significant correlation with ovarian weight and width; however, additional research is required to clarify its specific regulatory mechanism.
5. Conclusions
This study investigated the relationship between ovarian traits and the 67-bp variable duplication in the promoter region of the ADIPOQ gene in Chinese Holstein cows. The DNA from samples of blood and ovarian tissue was extracted and amplified using PCR. Agarose gel electrophoresis was then used to determine the polymorphisms present in the samples. Three genotypes were found to be present in these samples: II, DD, and ID. The DD genotype exhibited a greater frequency compared to the ID and II genotypes. We found that there is a significant relationship between this variant and the width of the estrus-interval ovaries and the weight of the late-estrus ovaries, through the use of correlation analysis. Additionally, we discovered, by using qPCR assays, that the mutant phenotype dramatically reduced the ADIPOQ gene mRNA expression in ovarian tissue. Consequently, our discoveries can serve as a molecular breeding indicator for Chinese Holstein cows and establish a theoretical basis for enhancing their reproductive quality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14162362/s1, Table S1: Relationship between the 67-bp duplication variation within the ADIPOQ gene and the ovarian traits (pre-oestrus and estrus) of Chinese Holstein (LSM ± SE).
Author Contributions
X.L. (Xianyong Lan), C.P. and H.Z. designed the experiment. Y.L. and T.L. carried out all the experiments, and prepared all figures and tables. Y.L. and M.Z. analyzed the data and drafted the manuscript. T.L. and M.Z. designed the methodology. X.L. (Xianyong Lan), Y.L. and T.L. contributed to revising the manuscript. X.L. (Xianyong Lan) and H.Z. assisted in editing the final version of the manuscript. X.L. (Xianyong Lan), H.Z. and X.L. (Xu Liu): project administration. C.P., X.L. (Xu Liu), H.Z. and X.L. (Xianyong Lan): supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Key Research and Development Program for Young Scientists (No:2022YFF1001200); the National Natural Science Foundation of China (No.32372852); the Science Fund for Distinguished Young Scholars of Shaanxi Province (No. 2024JC-JCQN-30); and the Shaanxi Provincial Innovation Leadership Program in Sciences and Technologies for Young and Middle-aged Scientists (No.2023SR205).
Institutional Review Board Statement
All experimental procedures used in this study followed the principle of the International Animal Care and Use Committee of the Northwest A&F University (protocol number: NWAFAC1008).
Informed Consent Statement
We confirm that the genotyping results of this experiment were obtained by the joint efforts of the participating researchers, and the manuscript has not been published in whole or in part, nor has it been considered for publication elsewhere. All authors agreed on the final version of the manuscript.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the Shaanxi Key Laboratory of Molecular Biology for Agriculture, College of Animal Science and Technology, Northwest A&F University. At the same time, we would also like to thank every member who participated in the collection and collation of samples and data, as well as sample extraction and amplification. Li Jie and Zhanerke Akhatayeva collected and organized data. We also want to express our gratitude for the drawing materials provided by BioRender.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Norouzy, A.; Nassiry, M.R.; Eftekhari Shahrody, F.; Javadmanesh, A.; Sulimova, G.E. Identification of bovine leucocyte adhesion deficiency (BLAD) carriers in Holstein and Brown Swiss AI bulls in Iran. Genetika 2005, 41, 1697–1701. [Google Scholar] [CrossRef]
- Butler, W.R. Nutritional Interactions with Reproductive Performance in Dairy Cattle. Anim. Reprod. Sci. 2000, 60–61, 449–457. [Google Scholar] [CrossRef]
- Liu, J.; Xu, L.; Ding, X.; Ma, Y. Genome-Wide Association Analysis of Reproductive Traits in Chinese Holstein Cattle. Genes 2024, 15, 12. [Google Scholar] [CrossRef]
- Sangsritavong, S.; Combs, D.K.; Sartori, R.; Armentano, L.E.; Wiltbank, M.C. High Feed Intake Increases Liver Blood Flow and Metabolism of Progesterone and Estradiol-17β in Dairy Cattle. J. Dairy Sci. 2002, 85, 2831–2842. [Google Scholar] [CrossRef]
- Diskin, M.G.; Waters, S.M.; Parr, M.H.; Kenny, D.A. Pregnancy Losses in Cattle: Potential for Improvement. Reprod. Fertil. Dev. 2016, 28, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Gershon, E.; Dekel, N. Newly Identified Regulators of Ovarian Folliculogenesis and Ovulation. Int. J. Mol. Sci. 2020, 21, 4565. [Google Scholar] [CrossRef]
- Devoto, L.; Henríquez, S.; Kohen, P.; Strauss, J.F. The Significance of Estradiol Metabolites in Human Corpus Luteum Physiology. Steroids 2017, 123, 50–54. [Google Scholar] [CrossRef]
- Lande, R.; Thompson, R. Efficiency of Marker-Assisted Selection in the Improvement of Quantitative Traits. Genetics 1990, 124, 743–756. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Liu, M.; Sweeney, G. Adiponectin Synthesis, Secretion and Extravasation from Circulation to Interstitial Space. Physiology 2021, 36, 134–149. [Google Scholar] [CrossRef]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Lv, Y.; Peng, Y.; Wu, Y.; Feng, Y.; Jia, T.; Xu, S.; Li, S.; Wang, W.; Tian, J.; et al. GCKR and ADIPOQ Gene Polymorphisms in Women with Gestational Diabetes Mellitus. Acta Diabetol. 2023, 60, 1709–1718. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and Adiponectin Receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Hou, S.H.; Zhao, Y.Y.; Wang, Y.Y.; Wang, H.J.; Qin, B.Y.; Zhang, N.F.; Le, B.Y.; Cheng, Z.M.; Gao, P.F.; Guo, X.H.; et al. Polymorphism of Adiponectin Gene Exon 2 in Different Pig Breeds and Its Relationship with Body Weights and Body Measurements in Shanxi White Pig. China Anim. Husb. Vet. Med. 2018, 45, 3497–3504. [Google Scholar]
- Morsci, N.S.; Schnabel, R.D.; Taylor, J.F. Association Analysis of Adiponectin and Somatostatin Polymorphisms on BTA1 with Growth and Carcass Traits in Angus Cattle. Anim. Genet. 2006, 37, 554–562. [Google Scholar] [CrossRef]
- Lord, E.; Ledoux, S.; Murphy, B.D.; Beaudry, D.; Palin, M.F. Expression of Adiponectin and Its Receptors in Swine. J. Anim. Sci. 2005, 83, 565–578. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.X.; Cao, W.W.; Zuo, M.; Zhang, W.Q. Meta-analysis of the rs1501299 and rs2241766 polymorphisms of the lipofuscin gene in association with polycystic ovary syndrome. Evid.-Based Med. 2019, 19, 299–308. [Google Scholar]
- Nikanfar, S.; Oghbaei, H.; Rastgar Rezaei, Y.; Zarezadeh, R.; Jafari-Gharabaghlou, D.; Nejabati, H.R.; Bahrami, Z.; Bleisinger, N.; Samadi, N.; Fattahi, A.; et al. Role of Adipokines in the Ovarian Function: Oogenesis and Steroidogenesis. J. Steroid Biochem. Mol. Biol. 2021, 209, 105852. [Google Scholar] [CrossRef]
- Tabandeh, M.R.; Hosseini, A.; Saeb, M.; Kafi, M.; Saeb, S. Changes in the Gene Expression of Adiponectin and Adiponectin Receptors (AdipoR1 and AdipoR2) in Ovarian Follicular Cells of Dairy Cow at Different Stages of Development. Theriogenology 2010, 73, 659–669. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, M.; Li, C.; Xu, Y.; Sun, J.; Lei, C.; Lan, X.; Zhang, C.; Chen, H. Identification and Genetic Effect of a Variable Duplication in the Promoter Region of the Cattle ADIPOQ Gene. Anim. Genet. 2014, 45, 171–179. [Google Scholar] [CrossRef]
- MacArthur Clark, J.A.; Sun, D. Guidelines for the Ethical Review of Laboratory Animal Welfare People’s Republic of China National Standard GB/T 35892-2018 [Issued 6 February 2018 Effective from 1 September 2018]. Anim. Models Exp. Med. 2020, 3, 103–113. [Google Scholar] [CrossRef]
- Regulations on Livestock and Poultry Identification and Farming Record Management. Chin. Poult. 2006, 57–58. Available online: https://kns.cnki.net/kcms2/article/abstract?v=sxrP1m9hSI8ZR0FF9TJaDKFqDjM4gkLXZoaO6Cjorhxuw88oSsipSQl02o5Gz4DZ0eIkgJXzrsZd_qFy5ErXwezoYvqHd4xF7qOnsg2MXvOseiTlyQCMZ92J2jq7KZ2zmFqRtnWQE-m-nGjjobhSE5k1bBcBmfnsAsDpZNdpRhsUTrKSZcBHxgFg9y3lX_l22jbDNPMNlf8=&uni (accessed on 23 July 2024).
- Liu, T.; Ju, X.; Zhang, M.; Wei, C.; Wang, D.; Wang, Z.; Lan, X.; Huang, X.-X. A 67-Bp Variable Duplication in the Promoter Region of the ADIPOQ Is Associated with Milk Traits in Xinjiang Brown Cattle. Anim. Biotechnol. 2022, 33, 1738–1745. [Google Scholar] [CrossRef]
- Zhang, S.; Dang, Y.; Zhang, Q.; Qin, Q.; Lei, C.; Chen, H.; Lan, X. Tetra-Primer Amplification Refractory Mutation System PCR (T-ARMS-PCR) Rapidly Identified a Critical Missense Mutation (P236T) of Bovine ACADVL Gene Affecting Growth Traits. Gene 2015, 559, 184–188. [Google Scholar] [CrossRef]
- Li, J.; Zhu, X.; Ma, L.; Xu, H.; Cao, X.; Luo, R.; Chen, H.; Sun, X.; Cai, Y.; Lan, X. Detection of a New 20-Bp Insertion/Deletion (Indel) within Sheep PRND Gene Using Mathematical Expectation (ME) Method. Prion 2017, 11, 143–150. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jia, W.; Zhang, J.; Li, X.; Pan, C.; Lei, C.; Chen, H.; Dang, R.; Lan, X. Determination of the novel genetic variants of goat stat5a, gene and their effects on body measurement traits in two Chinese native breeds. Small Rumin. Res. 2016, 121, 232–243. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nei, M.; Roychoudhury, A.K. Sampling Variances of Heterozygosity and Genetic Distance. Genetics 1974, 76, 379–390. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; He, Z.; Tang, W.; Li, T.; Zeng, Z.; He, L.; Shi, Y. A Partition-Ligation-Combination-Subdivision EM Algorithm for Haplotype Inference with Multiallelic Markers: Update of the SHEsis (http://Analysis.Bio-x.Cn). Cell Res. 2009, 19, 519–523. [Google Scholar] [CrossRef]
- Pan, C.; Wu, C.; Jia, W.; Xu, Y.; Lei, C.; Hu, S.; Lan, X.; Chen, H. A Critical Functional Missense Mutation (H173R) in the Bovine PROP1 Gene Significantly Affects Growth Traits in Cattle. Gene 2013, 531, 398–402. [Google Scholar] [CrossRef]
- Lv, S.J.; Chen, F.Y.; Zhang, Z.J.; Wang, L.H.; Zhang, S.S.; Wang, E.Y.; Xu, Z.X.; Shi, Q.T. Identification of Candidate Genes for Cattle Reproductive Traits Using Selective Sweep Method. Henan Agric. Sci. 2020, 49, 133–138. [Google Scholar]
- Hu, J.; Li, Q.; Feng, T.; Ding, H.; Yang, Z.; Dong, W. The research on the relation between weight of ovary and quantity of the porcine oocytes. J. Northwest AF Univ. 2005, 12–15+20. [Google Scholar] [CrossRef]
- Maillard, V.; Uzbekova, S.; Guignot, F.; Perreau, C.; Ramé, C.; Coyral-Castel, S.; Dupont, J. Effect of Adiponectin on Bovine Granulosa Cell Steroidogenesis, Oocyte Maturation and Embryo Development. Reprod. Biol. Endocrinol. RBE 2010, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Gan, W.; Zhu, M. Enhancer-Promoter Interaction Prediction Based on Multi-feature Fusion. Comput. Sci. 2020, 47, 64–71. [Google Scholar]
- Zhao, X.Z.; Zhang, H.L.; Xu, S.F.; Liu, G.L. Analysis of the weight of reproductive traits in the breeding value assessment system of dairy cows. Chin. Dairy Cows 2019, 8–11. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Hao, C.; Tian, Y.; Fu, J. Effects of ADIPOQ Polymorphisms on PCOS Risk: A Meta-Analysis. Reprod. Biol. Endocrinol. RBE 2018, 16, 120. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).