Comparative Proteomic Identification of Ram Sperm before and after In Vitro Capacitation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Workflow
2.2. Animal and Sample Collection
2.3. Sperm Capacitation
2.4. Protein Extraction and Digestion
2.5. TMT Labeling
2.6. High-pH Liquid Chromatographic (HPLC) Separation of Peptides
2.7. LC-MS/MS Analysis
2.8. Data Analysis
2.9. Bioinformatic Analysis
2.10. Parallel Reaction Monitoring (PRM) for Verification
2.11. Statistical Analysis
3. Results
3.1. Evaluation of Capacitation, Tyrosine Phosphorylation Level, and Kinetic Parameters of Sperm before and after Capacitation
3.2. Identification of Differentially Abundant Proteins
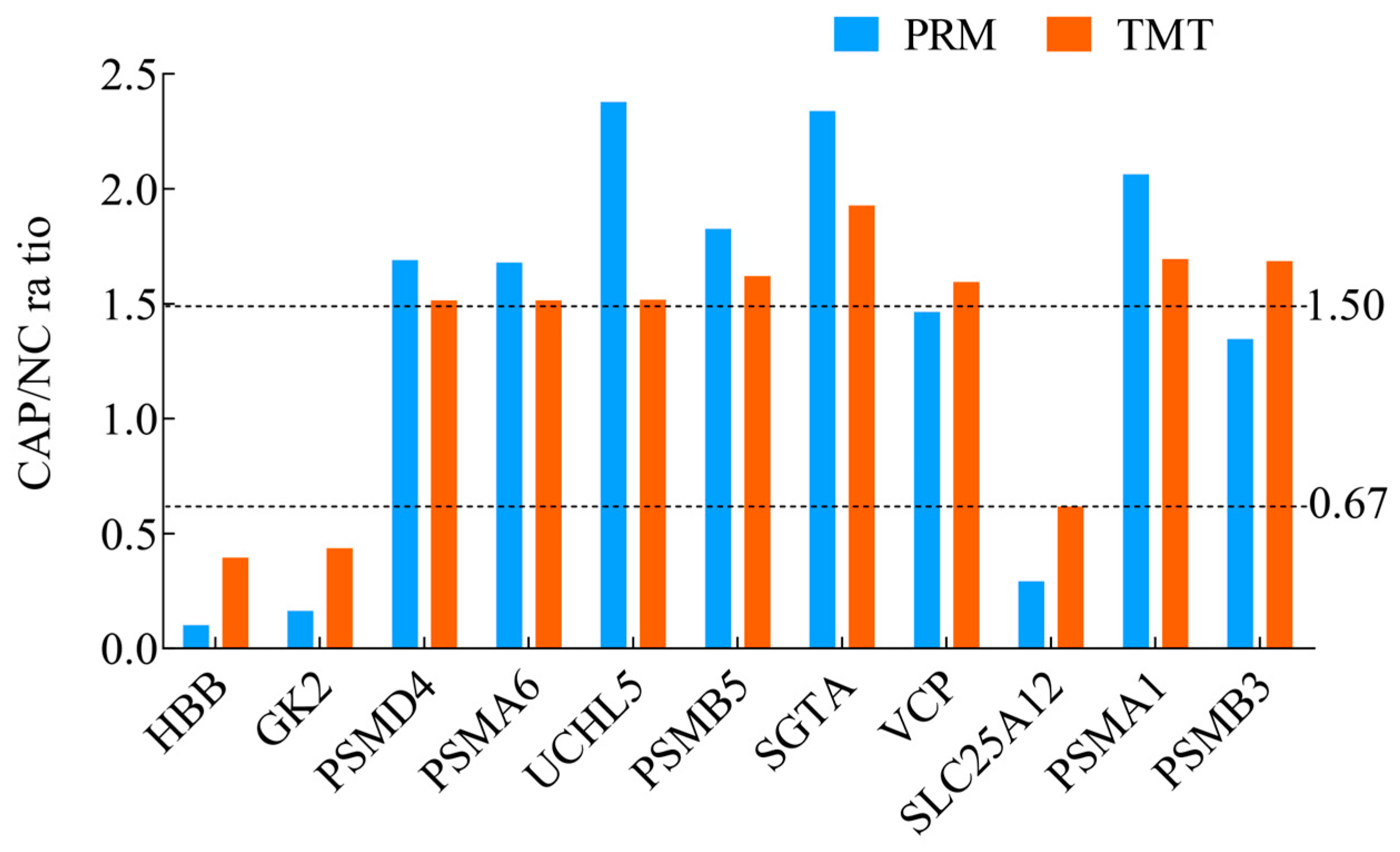
3.3. Validation of Selected DAPs by PRM
3.4. Functional Enrichment Analysis Based on DAPs
3.5. Protein Network Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen-Dayag, A.; Eisenbach, M. Potential assays for sperm capacitation in mammals. Am. J. Physiol. 1994, 267 Pt 1, C1167–C1176. [Google Scholar] [CrossRef]
- Mitra, K.; Rangaraj, N.; Shivaji, S. Novelty of the pyruvate metabolic enzyme dihydrolipoamide dehydrogenase in spermatozoa: Correlation of its localization, tyrosine phosphorylation, and activity during sperm capacitation. J. Biol. Chem. 2005, 280, 25743–25753. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Chen, L.; Ellis, M.; Li, M.; Wang, J.; Zhang, Y.; Fu, P.; Wang, K.; Li, X.; et al. microRNA profiling in three main stages during porcine spermatogenesis. J. Assist. Reprod. Genet. 2015, 32, 451–460. [Google Scholar] [CrossRef]
- Ross, P.; Kan, F.W.; Antaki, P.; Vigneault, N.; Chapdelaine, A.; Roberts, K.D. Protein synthesis and secretion in the human epididymis and immunoreactivity with sperm antibodies. Mol. Reprod. Dev. 1990, 26, 12–23. [Google Scholar] [CrossRef]
- Gur, Y.; Breitbart, H. Protein synthesis in sperm: Dialog between mitochondria and cytoplasm. Mol. Cell. Endocrinol. 2008, 282, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Jagan Mohanarao, G.; Atreja, S.K. Identification of NO induced and capacitation associated tyrosine phosphoproteins in buffalo (Bubalus bubalis) spermatozoa. Res. Vet. Sci. 2012, 93, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Urner, F.; Leppens-Luisier, G.; Sakkas, D. Protein tyrosine phosphorylation in sperm during gamete interaction in the mouse: The influence of glucose. Biol. Reprod. 2001, 64, 1350–1357. [Google Scholar] [CrossRef]
- Hou, Z.; Fu, Q.; Huang, Y.; Zhang, P.; Chen, F.; Li, M.; Xu, Z.; Yao, S.; Chen, D.; Zhang, M. Comparative proteomic identification buffalo spermatozoa during in vitro capacitation. Theriogenology 2019, 126, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ficarro, S.; Chertihin, O.; Westbrook, V.A.; White, F.; Jayes, F.; Kalab, P.; Marto, J.A.; Shabanowitz, J.; Herr, J.C.; Hunt, D.F.; et al. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J. Biol. Chem. 2003, 278, 11579–11589. [Google Scholar] [CrossRef]
- Secciani, F.; Bianchi, L.; Ermini, L.; Cianti, R.; Armini, A.; La Sala, G.B.; Focarelli, R.; Bini, L.; Rosati, F. Protein profile of capacitated versus ejaculated human sperm. J. Proteome Res. 2009, 8, 3377–3389. [Google Scholar] [CrossRef]
- Arcelay, E.; Salicioni, A.M.; Wertheimer, E.; Visconti, P.E. Identification of proteins undergoing tyrosine phosphorylation during mouse sperm capacitation. Int. J. Dev. Biol. 2008, 52, 463–472. [Google Scholar] [CrossRef]
- Baker, M.A.; Reeves, G.; Hetherington, L.; Aitken, R.J. Analysis of proteomic changes associated with sperm capacitation through the combined use of IPG-strip pre-fractionation followed by RP chromatography LC-MS/MS analysis. Proteomics 2010, 10, 482–495. [Google Scholar] [CrossRef]
- Bailey, J.L.; Tardif, S.; Dubé, C.; Beaulieu, M.; Reyes-Moreno, C.; Lefièvre, L.; Leclerc, P. Use of phosphoproteomics to study tyrosine kinase activity in capacitating boar sperm. Kinase activity and capacitation. Theriogenology 2005, 63, 599–614. [Google Scholar] [CrossRef]
- Wright, P.C.; Noirel, J.; Ow, S.Y.; Fazeli, A. A review of current proteomics technologies with a survey on their widespread use in reproductive biology investigations. Theriogenology 2012, 77, 738–765.e52. [Google Scholar]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrin. 2018, 16, 41. [Google Scholar] [CrossRef]
- Darszon, A.; Acevedo, J.J.; Galindo, B.E.; Hernandez-Gonzalez, E.O.; Nishigaki, T.; Trevino, C.L.; Wood, C.; Beltran, C. Sperm channel diversity and functional multiplicity. Reproduction 2006, 131, 977–988. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Nixon, B.; Aitken, R.J. Analysis of chaperone proteins associated with human spermatozoa during capacitation. Mol. Hum. Reprod. 2007, 13, 605–613. [Google Scholar] [CrossRef]
- Dey, S.; Brothag, C.; Vijayaraghavan, S. Signaling Enzymes Required for Sperm Maturation and Fertilization in Mammals. Front. Cell. Dev. Biol. 2019, 7, 341. [Google Scholar] [CrossRef]
- Baker, M.A.; Smith, N.D.; Hetherington, L.; Taubman, K.; Graham, M.E.; Robinson, P.J.; Aitken, R.J. Label-free quantitation of phosphopeptide changes during rat sperm capacitation. J. Proteome Res. 2010, 9, 718–729. [Google Scholar] [CrossRef]
- Kwon, W.S.; Rahman, M.S.; Lee, J.S.; Kim, J.; Yoon, S.J.; Park, Y.J.; You, Y.A.; Hwang, S.; Pang, M.G. A comprehensive proteomic approach to identifying capacitation related proteins in boar spermatozoa. BMC Genom. 2014, 15, 897. [Google Scholar] [CrossRef]
- Miguel-Jimenez, S.; Pina-Beltran, B.; Gimeno-Martos, S.; Carvajal-Serna, M.; Casao, A.; Perez-Pe, R. NADPH Oxidase 5 and Melatonin: Involvement in Ram Sperm Capacitation. Front. Cell. Dev. Biol. 2021, 9, 655794. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free. Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef]
- Scarlata, E.; O’Flaherty, C. Antioxidant Enzymes and Male Fertility: Lessons from Knockout Models. Antioxid. Redox. Signal. 2020, 32, 569–580. [Google Scholar] [CrossRef]
- Efrat, M.; Stein, A.; Pinkas, H.; Breitbart, H.; Unger, R.; Birk, R. Paraoxonase 1 (PON1) attenuates sperm hyperactivity and spontaneous acrosome reaction. Andrology 2019, 7, 24–30. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Mateos-Hernández, L.; Garde, J.J.; Villar, M.; Soler, A.J. Freezing-Thawing Procedures Remodel the Proteome of Ram Sperm before and after In Vitro Capacitation. Int. J. Mol. Sci. 2019, 20, 4596. [Google Scholar] [CrossRef]
- Mejia-Flores, I.; Chiquete-Felix, N.; Palma-Lara, I.; Uribe-Carvajal, S.; de Lourdes Juarez-Mosqueda, M. During capacitation in bull spermatozoa, actin and PLC-zeta undergo dynamic interactions. Zygote 2017, 25, 558–566. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, D.; Salgado-Lucio, M.L.; Roa-Espitia, A.L.; Fierro, R.; Gonzalez-Marquez, H.; Cordero-Martinez, J.; Hernandez-Gonzalez, E.O. Rac1 is necessary for capacitation and acrosome reaction in guinea pig spermatozoa. J. Cell. Biochem. 2020, 121, 2864–2876. [Google Scholar] [CrossRef]
- Majhi, R.K.; Kumar, A.; Yadav, M.; Swain, N.; Kumari, S.; Saha, A.; Pradhan, A.; Goswami, L.; Saha, S.; Samanta, L.; et al. Thermosensitive ion channel TRPV1 is endogenously expressed in the sperm of a fresh water teleost fish (Labeo rohita) and regulates sperm motility. Channels 2013, 7, 483–492. [Google Scholar] [CrossRef]
- Kumar, A.; Majhi, R.K.; Swain, N.; Giri, S.C.; Kar, S.; Samanta, L.; Goswami, C. TRPV4 is endogenously expressed in vertebrate spermatozoa and regulates intracellular calcium in human sperm. Biochem. Biophys. Res. Commun. 2016, 473, 781–788. [Google Scholar] [CrossRef]
- Cordero-Martinez, J.; Reyes-Miguel, T.; Rodriguez-Paez, L.; Garduno-Siciliano, L.; Maldonado-Garcia, D.; Roa-Espitia, A.L.; Hernandez-Gonzalez, E.O. TMEM16A inhibition impedes capacitation and acquisition of hyperactivated motility in guinea pig sperm. J. Cell. Biochem. 2018, 119, 5944–5959. [Google Scholar] [CrossRef]
- Mandal, A.; Naaby-Hansen, S.; Wolkowicz, M.J.; Klotz, K.; Shetty, J.; Retief, J.D.; Coonrod, S.A.; Kinter, M.; Sherman, N.; Cesar, F.; et al. FSP95, a testis-specific 95-kilodalton fibrous sheath antigen that undergoes tyrosine phosphorylation in capacitated human spermatozoa. Biol. Reprod. 1999, 61, 1184–1197. [Google Scholar] [CrossRef]
- Hernández-Silva, G.; Fabián López-Araiza, J.E.; López-Torres, A.S.; Larrea, F.; Torres-Flores, V.; Chirinos, M. Proteomic characterization of human sperm plasma membrane-associated proteins and their role in capacitation. Andrology 2020, 8, 171–180. [Google Scholar] [CrossRef]
- Dube, C.; Leclerc, P.; Baba, T.; Reyes-Moreno, C.; Bailey, J.L. The proacrosin binding protein, sp32, is tyrosine phosphorylated during capacitation of pig sperm. J. Androl. 2005, 26, 519–528. [Google Scholar] [CrossRef]
- Naaby-Hansen, S.; Mandal, A.; Wolkowicz, M.J.; Sen, B.; Westbrook, V.A.; Shetty, J.; Coonrod, S.A.; Klotz, K.L.; Kim, Y.H.; Bush, L.A.; et al. CABYR, a novel calcium-binding tyrosine phosphorylation-regulated fibrous sheath protein involved in capacitation. Dev. Biol. 2002, 242, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Lee, R.K.K.; Hwu, Y.M.; Chu, S.L.; Chen, Y.J.; Chang, W.C.; Lin, S.P.; Li, S.H. SERPINE2, a Serine Protease Inhibitor Extensively Expressed in Adult Male Mouse Reproductive Tissues, May Serve as a Murine Sperm Decapacitation Factor. Biol. Reprod. 2011, 84, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, Y.; Zhou, T.; Zhang, R.; Chen, H.; Chen, S.; Zhao, H. Mechanisms of ROS-induced mitochondria-dependent apoptosis underlying liquid storage of goat spermatozoa. Aging 2019, 11, 7880–7898. [Google Scholar] [CrossRef]
- Bubenickova, F.; Postlerova, P.; Simonik, O.; Sirohi, J.; Sichtar, J. Effect of Seminal Plasma Protein Fractions on Stallion Sperm Cryopreservation. Int. J. Mol. Sci. 2020, 21, 6415. [Google Scholar] [CrossRef] [PubMed]
- Saez-Espinosa, P.; Huerta-Retamal, N.; Robles-Gomez, L.; Aviles, M.; Aizpurua, J.; Velasco, I.; Romero, A.; Gomez-Torres, M.J. Influence of in vitro capacitation time on structural and functional human sperm parameters. Asian. J. Androl. 2020, 22, 447–453. [Google Scholar] [PubMed]
- Zhang, R.; Chu, M.; Chen, Y.; Yan, P. Heparin-induced and caffeine or ouabain supplemented capacitation of frozen-thawed yak (Bos grunniens) spermatozoa. Reprod. Domest. Anim. 2022, 57, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.C.; Atreja, S.K. Capacitation-associated protein tyrosine phosphorylation starts early in buffalo (Bubalus bubalis) spermatozoa as compared to cattle. Anim. Reprod. Sci. 2009, 110, 319–325. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yang, X.N.; Zhang, X.; Liu, B.J.; Liu, W.; Shen, Y.; Gao, Z.B.; Yin, Q.; Wang, C.L.; Zhou, J. TMT-based quantitative proteome profiles reveal the memory function of a whole heart decellularized matrix for neural stem cell trans-differentiation into the cardiac lineage. Biomater. Sci. 2021, 9, 3692–3704. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Chen, Y.; Tang, J.; Wang, P.; Zhang, Y.; Li, C.; Zhang, Z.; Cheng, X. TMT-Based Comparative Proteomic Analysis of the Spermatozoa of Buck (Capra hircus) and Ram (Ovis aries). Genes 2023, 14, 973. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, Y.; Xu, Q.; Zhu, J.; Lin, Y. TMT-based quantitative proteomics analysis reveals the key proteins related with the differentiation process of goat intramuscular adipocytes. BMC Genom. 2021, 22, 417. [Google Scholar] [CrossRef]
- Guan, K.F.; Li, H.; Chen, H.R.; Qi, X.F.; Wang, R.C.; Ma, Y. TMT-based quantitative proteomics analysis reveals the effect of bovine derived MFG-E8 against oxidative stress on rat L6 cells. Food. Funct. 2021, 12, 7310–7320. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Luo, Z.P.; Wu, X.L.; Zhu, J.; Yu, K.; Jin, Y.; Zhang, Z.W.; Zhao, S.H.; Zhou, L. Proteomic Analyses of Cysteine Redox in High-Fat-Fed and Fasted Mouse Livers: Implications for Liver Metabolic Homeostasis. J. Phys. Chem. Lett. 2018, 17, 129–140. [Google Scholar] [CrossRef]
- Hermo, L.; Pelletier, R.M.; Cyr, D.G.; Smith, C.E. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: Changes in spermatid organelles associated with development of spermatozoa. Microsc. Res. Tech. 2010, 73, 279–319. [Google Scholar] [CrossRef]
- Chauvin, T.; Xie, F.; Liu, T.; Nicora, C.D.; Yang, F.; Camp, D.G., 2nd; Smith, R.D.; Roberts, K.P. A systematic analysis of a deep mouse epididymal sperm proteome. Biol. Reprod. 2012, 87, 141. [Google Scholar] [CrossRef]
- Buffone, M.G.; Calamera, J.C.; Verstraeten, S.V.; Doncel, G.F. Capacitation-associated protein tyrosine phosphorylation and membrane fluidity changes are impaired in the spermatozoa of asthenozoospermic patients. Reproduction 2005, 129, 697–705. [Google Scholar] [CrossRef]
- Mostek, A.; Janta, A.; Majewska, A.; Ciereszko, A. Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins. Int. J. Mol. Sci. 2021, 22, 7903. [Google Scholar] [CrossRef] [PubMed]
- Flesch, F.M.; Colenbrander, B.; van Golde, L.M.; Gadella, B.M. Capacitation induces tyrosine phosphorylation of proteins in the boar sperm plasma membrane. Biochem. Biophys. Res. Commun. 1999, 262, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Sun, Z.; Chen, Y.; Chen, J.; Wang, S.; Liu, Q.; Wang, P.; Cheng, X.; Zhang, Z.; Wang, Q. Identification of Biomarkers Affecting Cryopreservation Recovery Ratio in Ram Spermatozoa Using Tandem Mass Tags (TMT)-Based Quantitative Proteomics Approach. Animals 2023, 13, 2368. [Google Scholar] [CrossRef] [PubMed]
- Ashrafzadeh, A.; Karsani, S.A.; Nathan, S. Mammalian sperm fertility related proteins. Int. J. Med. Sci. 2013, 10, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Kota, V.; Dhople, V.M.; Shivaji, S. Tyrosine phosphoproteome of hamster spermatozoa: Role of glycerol-3-phosphate dehydrogenase 2 in sperm capacitation. Proteomics 2009, 9, 1809–1826. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Q.; Zhu, Z.H.; Yue, S.J.; Tang, Y.P.; Chen, Y.Y.; Pu, Z.J.; Tao, H.J.; Zhou, G.S.; Yang, Y.; Guo, M.J.; et al. Integration of organ metabolomics and proteomics in exploring the blood enriching mechanism of Danggui Buxue Decoction in hemorrhagic anemia rats. J. Ethnopharmacol. 2020, 261, 113000. [Google Scholar] [CrossRef]
- Jofre, I.; Cuevas, M.; de Castro, L.S.; de Agostini Losano, J.D.; Torres, M.A.; Alvear, M.; Scheuermann, E.; Andrade, A.F.C.; Nichi, M.; Assumpcao, M.E.O.; et al. Antioxidant Effect of a Polyphenol-Rich Murtilla (Ugni molinae Turcz.) Extract and Its Effect on the Regulation of Metabolism in Refrigerated Boar Sperm. Oxid. Med. Cell. Longev. 2019, 2019, 2917513. [Google Scholar] [CrossRef] [PubMed]
- Kerns, K.; Morales, P.; Sutovsky, P. Regulation of Sperm Capacitation by the 26S Proteasome: An Emerging New Paradigm in Spermatology. Biol. Reprod. 2016, 94, 117. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Diaz, E.S.; Morales, P. Participation of the human sperm proteasome in the capacitation process and its regulation by protein kinase A and tyrosine kinase. Biol. Reprod. 2009, 80, 1026–1035. [Google Scholar] [CrossRef]
- Zigo, M.; Kerns, K.; Sutovsky, M.; Sutovsky, P. Modifications of the 26S proteasome during boar sperm capacitation. Cell. Tissue Res. 2018, 372, 591–601. [Google Scholar] [CrossRef]
- Li, S.H.; Izumi, T.; Hu, J.; Jin, H.H.; Siddiqui, A.A.A.; Jacobson, S.G.; Bok, D.; Jin, M.H. Rescue of Enzymatic Function for Disease-associated RPE65 Proteins Containing Various Missense Mutations in Non-active Sites. J. Biol. Chem. 2014, 289, 18943–18956. [Google Scholar] [CrossRef]
- George, G.; Singh, S.; Lokappa, S.B.; Varkey, J. Gene co-expression network analysis for identifying genetic markers in Parkinson’s disease—A three-way comparative approach. Genomics 2019, 111, 819–830. [Google Scholar] [CrossRef]
- Zhang, N.; Liang, J.B.; Tian, Y.Q.; Yuan, L.G.; Wu, L.; Miao, S.Y.; Zong, S.D.; Wang, L.F. A Novel Testis-specific GTPase Serves as a Link to Proteasome Biogenesis: Functional Characterization of RhoS/RSA-14-44 in Spermatogenesis. Mol. Biol. Cell. 2010, 21, 4312–4324. [Google Scholar] [CrossRef]
- Delgado-Buenrostro, N.L.; Mujica, A.; Chiquete-Felix, N.; Deciga-Alcaraz, A.; Medina-Reyes, E.I.; Uribe-Carvajal, S.; Chirino, Y.I. Role of Wasp and the small GTPases RhoA, RhoB, and Cdc42 during capacitation and acrosome reaction in spermatozoa of English guinea pigs. Mol. Reprod. Dev. 2016, 83, 927–937. [Google Scholar] [CrossRef]
- Song, K.H.; Kim, J.H.; Lee, Y.H.; Bae, H.C.; Lee, H.J.; Woo, S.R.; Oh, S.J.; Lee, K.M.; Yee, C.; Kim, B.W.; et al. Mitochondrial reprogramming via ATP5H loss promotes multimodal cancer therapy resistance. J. Clin. Investig. 2018, 128, 4098–4114. [Google Scholar] [CrossRef] [PubMed]
- Martin-Jimenez, R.; Faccenda, D.; Allen, E.; Reichel, H.B.; Arcos, L.; Ferraina, C.; Strobbe, D.; Russell, C.; Campanella, M. Reduction of the ATPase inhibitory factor 1 (IF1) leads to visual impairment in vertebrates. Cell. Death. Dis. 2018, 9, 669. [Google Scholar] [CrossRef]
- Bellezza, I.; Minelli, A. Adenosine in sperm physiology. Mol. Aspects. Med. 2017, 55, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cann, M.J.; Litvin, T.N.; Iourgenko, V.; Sinclair, M.L.; Levin, L.R.; Buck, J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000, 289, 625–628. [Google Scholar] [CrossRef]
- D’Angelo, A.; Garzia, L.; Andre, A.; Carotenuto, P.; Aglio, V.; Guardiola, O.; Arrigoni, G.; Cossu, A.; Palmieri, G.; Aravind, L.; et al. Prune cAMP phosphodiesterase binds nm23-H1 and promotes cancer metastasis. Cancer Cell 2004, 5, 137–149. [Google Scholar] [CrossRef]
- Marechal, L.; Guillemette, C.; Goupil, S.; Blondin, P.; Leclerc, P.; Richard, F.J. Cyclic nucleotide phosphodiesterases in human spermatozoa and seminal fluid: Presence of an active PDE10A in human spermatozoa. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 147–156. [Google Scholar] [CrossRef]
- Pamarthy, S.; Kulshrestha, A.; Katara, G.K.; Beaman, K.D. The curious case of vacuolar ATPase: Regulation of signaling pathways. Mol. Cancer 2018, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Soler, N.; Beaulieu, V.; Litvin, T.N.; Da Silva, N.; Chen, Y.Q.; Brown, D.; Buck, J.; Levin, L.R.; Breton, S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J. Biol. Chem. 2003, 278, 49523–49529. [Google Scholar] [CrossRef]
- Vasanthakumar, T.; Rubinstein, J.L. Structure and Roles of V-type ATPases. Trends Biochem. Sci. 2020, 45, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Shur, B.D.; Hall, N.G. A role for mouse sperm surface galactosyltransferase in sperm binding to the egg zona pellucida. J. Cell. Biol. 1982, 95 Pt 1, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Talbot, P.; Shur, B.D.; Myles, D.G. Cell adhesion and fertilization: Steps in oocyte transport, sperm-zona pellucida interactions, and sperm-egg fusion. Biol. Reprod. 2003, 68, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Aquila, S. Arguments raised by the recent discovery that insulin and leptin are expressed in and secreted by human ejaculated spermatozoa. Mol. Cell. Endocrinol. 2005, 245, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Oikari, S.; Makkonen, K.; Deen, A.J.; Tyni, I.; Karna, R.; Tammi, R.H.; Tammi, M.I. Hexosamine biosynthesis in keratinocytes: Roles of GFAT and GNPDA enzymes in the maintenance of UDP-GlcNAc content and hyaluronan synthesis. Glycobiology 2016, 26, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Addou-Klouche, L.; Adelaide, J.; Finetti, P.; Cervera, N.; Ferrari, A.; Bekhouche, I.; Sircoulomb, F.; Sotiriou, C.; Viens, P.; Moulessehoul, S.; et al. Loss, mutation and deregulation of L3MBTL4 in breast cancers. Mol. Cancer 2010, 9, 213. [Google Scholar] [CrossRef]
- Zhang, X.H.; Ozawa, Y.; Lee, H.; Wen, Y.D.; Tan, T.H.; Wadzinski, B.E.; Seto, E. Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Gene Dev. 2005, 19, 827–839. [Google Scholar] [CrossRef]
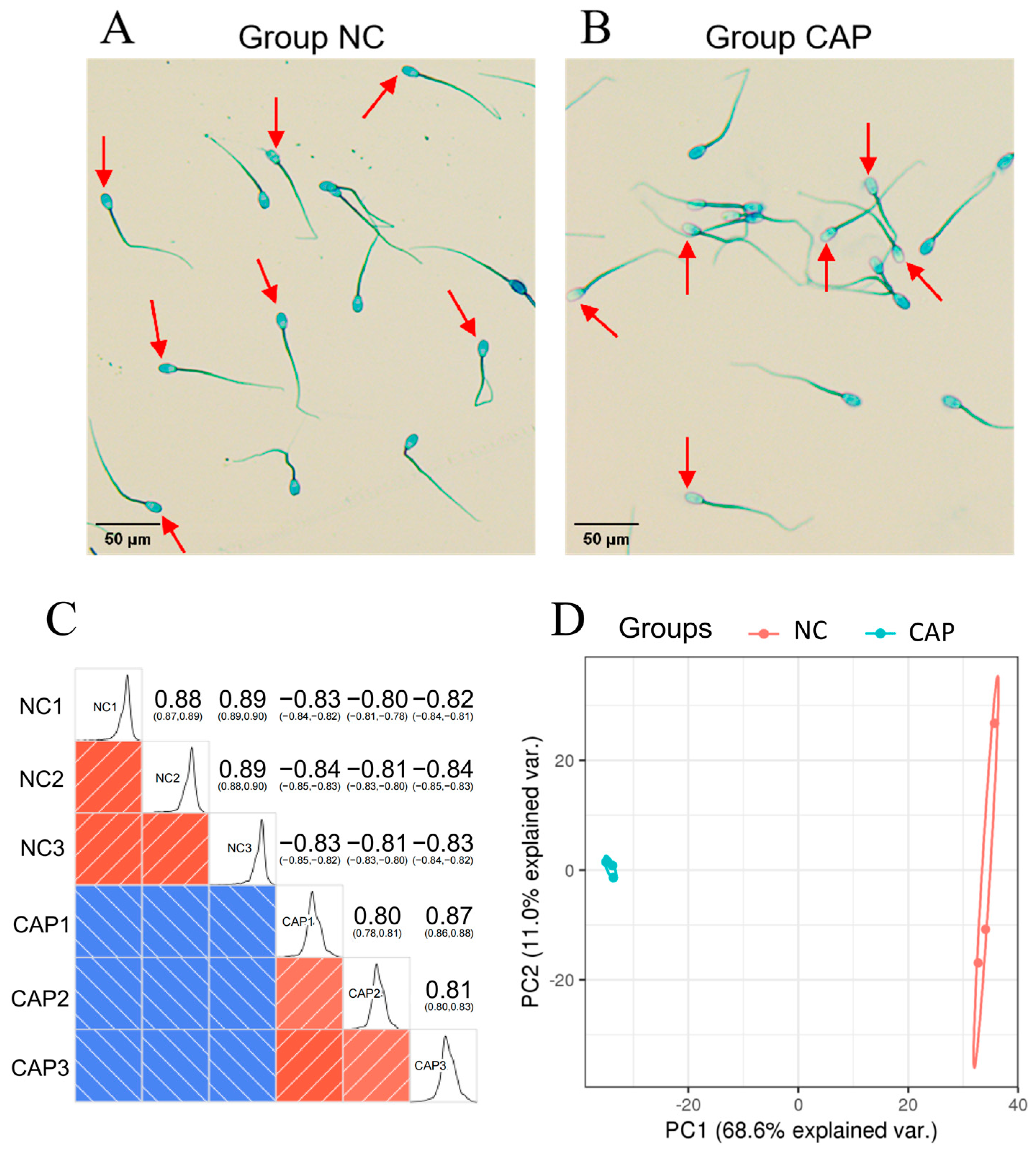



| Sperm Kinematics | Group NC | Group CAP |
|---|---|---|
| MOT (%) | 79.3 ± 6.4 | 83.5 ± 4.3 * |
| VCL (μm/s) | 113.2 ± 11.2 | 123.5 ± 4.0 * |
| VSL (μm/s) | 38.4 ± 8.1 | 68.2 ± 4.9 * |
| VAP (μm/s) | 66.6 ± 10.2 | 96.7 ± 4.4 * |
| ALH (μm) | 4.3 ± 0.5 | 3.2 ± 0.2 * |
| Accession | Gene Name | MW [kDa] | Score | Coverage [%] | Peptides | p-Value | Fold Change |
|---|---|---|---|---|---|---|---|
| P02075 | HBB | 16.07 | 142.24 | 74.5 | 11 | 6.03 × 10−7 | 2.53 |
| W5Q3H2 | DNAL4 | 12.97 | 27.66 | 33.9 | 4 | 3.46 × 10−6 | 2.66 |
| W5NSD8 | TEPP | 30.63 | 87.98 | 29.6 | 10 | 3.72 × 10−5 | 1.57 |
| W5NZ60 | GNPAT | 77.68 | 32.77 | 8.2 | 5 | 0.000324 | 1.57 |
| W5P9X2 | CHCHD3 | 26.76 | 125.87 | 40.9 | 9 | 0.000579 | 1.52 |
| W5PTU5 | SPACA1 | 32.33 | 323.31 | 46.9 | 11 | 0.000644 | 1.87 |
| W5NQT7 | NDUFAB1 | 17.59 | 33.54 | 21 | 4 | 0.001137 | 1.53 |
| B2MVX2 | SLC25A11 | 34.21 | 27.07 | 12.1 | 3 | 0.002582 | 1.634 |
| W5P657 | WDR87 | 331.92 | 35.97 | 2 | 6 | 0.003284 | 2.07 |
| W5P066 | CYB5B | 16.98 | 50.35 | 13.7 | 2 | 0.004602 | 1.61 |
| W5Q3Y4 | ATP2A2 | 106.61 | 35.55 | 5.8 | 6 | 0.004741 | 2.09 |
| W5Q4Y3 | STPG2 | 61.29 | 25.99 | 5.3 | 2 | 0.005075 | 1.57 |
| W5PP37 | ATP5PD | 18.72 | 45.97 | 39.1 | 7 | 0.005245 | 1.63 |
| W5QHI0 | IMMT | 89.27 | 323.31 | 35.5 | 28 | 0.008158 | 1.52 |
| W5PW99 | ADCK5 | 63.79 | 20.70 | 5.5 | 3 | 0.017699 | 2.15 |
| Accession | Gene Name | MW [kDa] | Score | Coverage [%] | Peptides | p-Value | Fold Change |
|---|---|---|---|---|---|---|---|
| P14639 | ALB | 69.19 | 323.31 | 53.7 | 31 | 0.001021 | 7.89 |
| W5P7X5 | WFDC2 | 10.47 | 171.69 | 76 | 11 | 2.3 × 10−5 | 7.05 |
| W5QB42 | CES5A | 64.21 | 119.1 | 21 | 11 | 0.004365 | 5.13 |
| W5P1C2 | NUCB2 | 52.47 | 65.79 | 22.4 | 8 | 2.15 × 10−6 | 4.34 |
| W5Q0Z2 | CTSB | 36.73 | 51.60 | 17.3 | 6 | 2.21 × 10−5 | 4.27 |
| W5Q9A2 | AZGP1 | 35.42 | 61.43 | 23.3 | 5 | 2.47 × 10−5 | 3.86 |
| W5PR14 | FAM3D | 25.39 | 20.86 | 14.3 | 3 | 0.000224 | 3.68 |
| W5Q1M0 | GLB1 | 72.84 | 82.49 | 15.1 | 8 | 3.46 × 10−6 | 3.61 |
| W5NQ55 | DEFB116 | 11.71 | 69.49 | 30.1 | 3 | 4.02 × 10−5 | 3.54 |
| P56283 | NPPC | 13.32 | 38.63 | 34.1 | 4 | 4.42 × 10−5 | 3.36 |
| W5QHU9 | PPIB | 23.73 | 50.51 | 32.4 | 6 | 3.95 × 10−5 | 3.31 |
| H9TN93 | GPx5 | 25.04 | 133.3 | 54.3 | 13 | 1.74 × 10−5 | 3.02 |
| W5PD71 | CRP | 25.27 | 251.4 | 33.5 | 7 | 3.57 × 10−5 | 2.83 |
| W5QIT0 | SORD | 38.27 | 168.92 | 40.2 | 15 | 0.001724 | 2.56 |
| W5PTV7 | ACE | 138.23 | 229.67 | 17 | 21 | 8.97 × 10−7 | 2.55 |
| Accession | Gene Name | Peptides | MW [kDa] | Score | Coverage [%] | CAP/NC | p-Value |
|---|---|---|---|---|---|---|---|
| W5QHM6 | PSMD1 | 22 | 105.95 | 234.400 | 28.5 | 1.511 | 7.48 × 10−3 |
| A7UHZ2 | PSMD4 | 8 | 40.78 | 87.409 | 30.5 | 1.515 | 1.42 × 10−3 |
| W5QHD8 | PSMD2 | 18 | 107.61 | 158.900 | 22.1 | 1.663 | 2.62 × 10−4 |
| W5QD30 | PSMA5 | 8 | 26.41 | 124.850 | 46.5 | 1.545 | 1.98 × 10−4 |
| W5QE19 | PSMB2 | 6 | 22.97 | 43.467 | 27.2 | 1.575 | 2.57 × 10−4 |
| W5QIL2 | PSMB4 | 4 | 28.97 | 54.891 | 25.0 | 1.667 | 2.39 × 10−4 |
| W5QDM2 | PSMB5 | 6 | 28.71 | 47.961 | 24.3 | 1.620 | 4.30 × 10−3 |
| W5PFU1 | PSMD13 | 15 | 42.75 | 122.600 | 38.3 | 1.624 | 2.01 × 10−5 |
| W5PVU5 | PSMB7 | 3 | 29.92 | 25.420 | 8.3 | 2.030 | 5.68 × 10−3 |
| W5P9U4 | PSMA1 | 12 | 29.59 | 101.290 | 41.1 | 1.695 | 7.68 × 10−5 |
| W5PNT5 | PSMD6 | 13 | 45.51 | 130.610 | 32.9 | 1.570 | 3.19 × 10−4 |
| W5QIC3 | PRUNE1 | 7 | 48.85 | 57.096 | 20.1 | 1.580 | 1.00 × 10−3 |
| W5PHB8 | GALT | 7 | 43.34 | 48.862 | 16.9 | 1.567 | 3.94 × 10−3 |
| Q2TCH3 | ACLY | 29 | 120.93 | 236.510 | 30.3 | 1.944 | 2.80 × 10−6 |
| W5PWF2 | ATP6V1H | 10 | 55.80 | 123.990 | 29.4 | 1.614 | 1.50 × 10−5 |
| W5NZZ3 | ATP6V1F | 6 | 13.40 | 36.524 | 42.0 | 1.622 | 3.16 × 10−3 |
| W5PP37 | ATP5PD | 7 | 18.72 | 45.973 | 39.1 | 0.612 | 5.25 × 10−3 |
| W5NYG6 | ATP5IF1 | 4 | 11.37 | 44.214 | 19.8 | 0.631 | 2.41 × 10−2 |
| W5PLZ0 | ATP6V1B2 | 19 | 56.93 | 193.890 | 43.7 | 1.792 | 2.11 × 10−5 |
| W5Q8X9 | PGP | 9 | 25.01 | 129.770 | 41.8 | 1.542 | 1.98 × 10−4 |
| W5NUG3 | GNPDA1 | 8 | 32.56 | 40.061 | 33.2 | 1.513 | 5.18 × 10−4 |
| W5Q0A8 | GNPDA2 | 10 | 31.16 | 109.880 | 43.5 | 1.770 | 1.34 × 10−3 |
| W5QFN9 | UGP2 | 15 | 56.90 | 158.290 | 33.9 | 2.015 | 8.29 × 10−5 |
| W5NSS1 | PPP4R1 | 12 | 105.74 | 111.110 | 17.5 | 1.805 | 1.86 × 10−5 |
| W5NZ60 | GNPAT | 5 | 77.68 | 32.765 | 8.2 | 0.637 | 3.24 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Li, C.-Y.; Wang, P.-H.; Wang, R.; Zhuo, X.; Zhang, Y.; Wang, S.-J.; Sun, Z.-P.; Chen, J.-H.; Cheng, X.; et al. Comparative Proteomic Identification of Ram Sperm before and after In Vitro Capacitation. Animals 2024, 14, 2363. https://doi.org/10.3390/ani14162363
Chen Y-L, Li C-Y, Wang P-H, Wang R, Zhuo X, Zhang Y, Wang S-J, Sun Z-P, Chen J-H, Cheng X, et al. Comparative Proteomic Identification of Ram Sperm before and after In Vitro Capacitation. Animals. 2024; 14(16):2363. https://doi.org/10.3390/ani14162363
Chicago/Turabian StyleChen, Ya-Le, Chun-Yan Li, Peng-Hui Wang, Ru Wang, Xian Zhuo, Yan Zhang, Shi-Jia Wang, Zhi-Peng Sun, Jia-Hong Chen, Xiao Cheng, and et al. 2024. "Comparative Proteomic Identification of Ram Sperm before and after In Vitro Capacitation" Animals 14, no. 16: 2363. https://doi.org/10.3390/ani14162363






