Molecular Detection of Anaplasma phagocytophilum in Cats in Europe and Associated Risk Factors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Population
3.2. Submissions to the Laboratory
3.3. Anaplasma Phagocytophilum-Infected Cats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dziegiel, B.; Adaszek, L.; Kalinowski, M.; Winiarczyk, S. Equine granulocytic anaplasmosis. Res. Vet. Sci. 2013, 95, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Chirek, A.; Silaghi, C.; Pfister, K.; Kohn, B. Granulocytic anaplasmosis in 63 dogs: Clinical signs, laboratory results, therapy and course of disease. J. Small Anim. Pr. 2018, 59, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, F.S.; Mandel, E.J.; Krebs, J.W.; Massung, R.F.; McQuiston, J.H. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am. J. Trop. Med. Hyg. 2011, 85, 124–131. [Google Scholar] [CrossRef] [PubMed]
- De Arcangeli, S.; Balboni, A.; Serafini, F.; Battilani, M.; Dondi, F. Anaplasma phagocytophilum infection in thrombocytopenic dogs. Vet. Ital. 2018, 54, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.E.; Birtles, R.J.; Day, M.J. Arthropod-transmitted infectious diseases of cats. J. Feline Med. Surg. 2001, 3, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, A.M.; Bodaan, C.; Postigo, M.; Nieuwenhuijs, H.; Opsteegh, M.; Franssen, L.; Jebbink, F.; Jongejan, F. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis. 2007, 7, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Bown, K.J.; Lambin, X.; Telford, G.R.; Ogden, N.H.; Telfer, S.; Woldehiwet, Z.; Birtles, R.J. Relative importance of Ixodes ricinus and Ixodes trianguliceps as vectors for Anaplasma phagocytophilum and Babesia microti in field vole (Microtus agrestis) populations. Appl. Env. Microbiol. 2008, 74, 7118–7125. [Google Scholar] [CrossRef]
- Diaz-Sanchez, S.; Hernandez-Jarguin, A.; Torina, A.; de Mera, I.G.F.; Blanda, V.; Caracappa, S.; Gortazar, C.; de la Fuente, J. Characterization of the bacterial microbiota in wild-caught Ixodes ventalloi. Ticks Tick. Borne Dis. 2019, 10, 336–343. [Google Scholar] [CrossRef]
- Santos, A.S.; Santos-Silva, M.M.; Sousa, R.; Bacellar, F.; Dumler, J.S. PCR-based survey of Anaplasma phagocytophilum in Portuguese ticks (Acari: Ixodidae). Vector Borne Zoonotic Dis. 2009, 9, 33–40. [Google Scholar] [CrossRef]
- Kruppenbacher, A.S.; Muller, E.; Aardema, M.L.; Schafer, I.; von Loewenich, F.D. Granulocytic anaplasmosis in cats from central Europe and molecular characterization of feline Anaplasma phagocytophilum strains by ankA gene, groEL gene and multilocus sequence typing. Parasit. Vectors 2023, 16, 348. [Google Scholar] [CrossRef]
- Schäfer, I.; Kohn, B.; Muller, E. Anaplasma phagocytophilum in domestic cats from Germany, Austria and Switzerland and clinical/laboratory findings in 18 PCR-positive cats (2008–2020). J. Feline Med. Surg. 2022, 24, 290–297. [Google Scholar] [CrossRef]
- Geisen, V.; Pantchev, N.; Wuelfing, K.; Wurthner, C.; Gierschner, K.; Urban, C.; Lambach, Y.; Hartmann, K.; Bergmann, M. Anaplasma phagocytophilum infection associated with strong inflammatory response in 3 cats. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere 2024, 52, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.R.; Chandrashekar, R.; Stillman, B.; Liu, J.; Mather, T.N. Evidence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in cats after exposure to wild-caught adult Ixodes scapularis. J. Vet. Diagn. Investig. 2015, 27, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, I.; Kohn, B. Anaplasma phagocytophilum infection in cats: A literature review to raise clinical awareness. J. Feline Med. Surg. 2020, 22, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Hofmann-Lehmann, R.; Radford, A.D.; Tasker, S.; Belák, S.; Addie, D.D.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; et al. Anaplasma, Ehrlichia and Rickettsia species infections in cats: European guidelines from the ABCD on prevention and management. J. Feline Med. Surg. 2017, 19, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Englert, T.; Stuetzer, B.; Hawley, J.R.; Lappin, M.R.; Hartmann, K. Prevalence of selected rickettsial infections in cats in Southern Germany. Comp. Immunol. Microbiol. Infect. Dis. 2015, 42, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Hartmann, K. Vector-borne diseases in cats in Germany. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere 2017, 45, 329–335. [Google Scholar] [CrossRef]
- Savidge, C.; Ewing, P.; Andrews, J.; Aucoin, D.; Lappin, M.R.; Moroff, S. Anaplasma phagocytophilum infection of domestic cats: 16 cases from the northeastern USA. J. Feline Med. Surg. 2016, 18, 85–91. [Google Scholar] [CrossRef]
- Spada, E.; Proverbio, D.; Galluzzo, P.; Della Pepa, A.; Perego, R.; Bagnagatti De Giorgi, G.; Ferro, E. Molecular study on selected vector-borne infections in urban stray colony cats in northern Italy. J. Feline Med. Surg. 2014, 16, 684–688. [Google Scholar] [CrossRef]
- Persichetti, M.F.; Solano-Gallego, L.; Serrano, L.; Altet, L.; Reale, S.; Masucci, M.; Pennisi, M.G. Detection of vector-borne pathogens in cats and their ectoparasites in Southern Italy. Parasit. Vectors 2016, 9, 247. [Google Scholar] [CrossRef]
- Ebani, V.V.; Bertelloni, F. Serological evidence of exposure to Ehrlichia canis and Anaplasma phagocytophilum in Central Italian healthy domestic cats. Ticks Tick. Borne Dis. 2014, 5, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Hamel, D.; Bondarenko, A.; Silaghi, C.; Nolte, I.; Pfister, K. Seroprevalence and bacteremia [corrected] of Anaplasma phagocytophilum in cats from Bavaria and Lower Saxony (Germany). Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 163–167. [Google Scholar]
- Morgenthal, D.; Hamel, D.; Arndt, G.; Silaghi, C.; Pfister, K.; Kempf, V.A.; Kohn, B. Prevalence of haemotropic Mycoplasma spp., Bartonella spp. and Anaplasma phagocytophilum in cats in Berlin/Brandenburg (Northeast Germany). Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 418–427. [Google Scholar] [PubMed]
- Elfving, K.; Malmsten, J.; Dalin, A.M.; Nilsson, K. Serologic and molecular prevalence of Rickettsia helvetica and Anaplasma phagocytophilum in wild cervids and domestic mammals in the central parts of Sweden. Vector Borne Zoonotic Dis. 2015, 15, 529–534. [Google Scholar] [CrossRef]
- Ayllon, T.; Diniz, P.P.; Breitschwerdt, E.B.; Villaescusa, A.; Rodriguez-Franco, F.; Sainz, A. Vector-borne diseases in client-owned and stray cats from Madrid, Spain. Vector Borne Zoonotic Dis. 2012, 12, 143–150. [Google Scholar] [CrossRef]
- Ayllon, T.; Villaescusa, A.; Tesouro, M.A.; Sainz, A. Serology, PCR and culture of Ehrlichia/Anaplasma species in asymptomatic and symptomatic cats from central Spain. Clin. Microbiol. Infect. 2009, 15 (Suppl. S2), 4–5. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Hegarty, B.; Espada, Y.; Llull, J.; Breitschwerdt, E. Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet. Microbiol. 2006, 118, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Tabar, M.D.; Altet, L.; Francino, O.; Sanchez, A.; Ferrer, L.; Roura, X. Vector-borne infections in cats: Molecular study in Barcelona area (Spain). Vet. Parasitol. 2008, 151, 332–336. [Google Scholar] [CrossRef]
- Beall, M.J.; Chandrashekar, R.; Eberts, M.D.; Cyr, K.E.; Diniz, P.P.; Mainville, C.; Hegarty, B.C.; Crawford, J.M.; Breitschwerdt, E.B. Serological and molecular prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia species in dogs from Minnesota. Vector Borne Zoonotic Dis. 2008, 8, 455–464. [Google Scholar] [CrossRef]
- Foley, J.E.; Foley, P.; Madigan, J.E. Spatial distribution of seropositivity to the causative agent of granulocytic ehrlichiosis in dogs in California. Am. J. Vet. Res. 2001, 62, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Persichetti, M.F.; Pennisi, M.G.; Vullo, A.; Masucci, M.; Migliazzo, A.; Solano-Gallego, L. Clinical evaluation of outdoor cats exposed to ectoparasites and associated risk for vector-borne infections in Southern Italy. Parasit. Vectors 2018, 11, 136. [Google Scholar] [CrossRef]
- Alves, A.S.; Milhano, N.; Santos-Silva, M.; Santos, A.S.; Vilhena, M.; de Sousa, R. Evidence of Bartonella spp., Rickettsia spp. and Anaplasma phagocytophilum in domestic, shelter and stray cat blood and fleas, Portugal. Clin. Microbiol. Infect. 2009, 15 (Suppl. S2), 1–3. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, H.; Martinez-Diaz, V.L.; Cardoso, L.; Vieira, L.; Altet, L.; Francino, O.; Pastor, J.; Silvestre-Ferreira, A.C. Feline vector-borne pathogens in the north and centre of Portugal. Parasit. Vectors 2013, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.E.; Binns, S.H.; Birtles, R.J.; Day, M.J.; Smithson, R.; Kenny, M.J. Molecular evidence of tick-transmitted infections in dogs and cats in the United Kingdom. Vet. Rec. 2005, 157, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Saz, S.; Martinez, M.; Nijhof, A.M.; Gerst, B.; Gentil, M.; Muller, E.; Fernandez, A.; Gonzalez, A.; Yusuf, M.S.M.; Greco, G.; et al. Molecular survey on vector-borne pathogens in clinically healthy stray cats in Zaragoza (Spain). Parasit. Vectors 2023, 16, 428. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, V.; Pantchev, N.; Balzer, H.J.; Meyersen, A.; Straubinger, R.K. First case of Anaplasma platys infection in a dog from Croatia. Parasit. Vectors 2012, 5, 49. [Google Scholar] [CrossRef]
- Talleklint, L.; Jaenson, T.G. Increasing geographical distribution and density of Ixodes ricinus (Acari: Ixodidae) in central and northern Sweden. J. Med. Entomol. 1998, 35, 521–526. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Talleklint, L.; Lundqvist, L.; Olsen, B.; Chirico, J.; Mejlon, H. Geographical distribution, host associations, and vector roles of ticks (Acari: Ixodidae, Argasidae) in Sweden. J. Med. Entomol. 1994, 31, 240–256. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Jaenson, D.G.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors 2012, 5, 8. [Google Scholar] [CrossRef]
- Omazic, A.; Han, S.; Albihn, A.; Ullman, K.; Choklikitumnuey, P.; Perissinotto, D.; Grandi, G. Ixodid tick species found in northern Sweden—Data from a frontier area. Ticks Tick. Borne Dis. 2023, 14, 102244. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.; Talleklint, L.; Polfeldt, T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 2000, 108, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Perret, J.L.; Guigoz, E.; Rais, O.; Gern, L. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol. Res. 2000, 86, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S. Studies on the dynamics of active populations of the sheep tick, Ixodes ricinus L. in Co. Wicklow, Ireland. Acarologia 1984, 25, 167–178. [Google Scholar]
- Kjellander, P.; Bergvall, U.A.; Chirico, J.; Ullman, K.; Christensson, M.; Lindgren, P.E. Winter activity of Ixodes ricinus in Sweden. Parasit. Vectors 2023, 16, 229. [Google Scholar] [CrossRef]
- Neelakanta, G.; Sultana, H.; Fish, D.; Anderson, J.F.; Fikrig, E. Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J. Clin. Investig. 2010, 120, 3179–3190. [Google Scholar] [CrossRef]
- Jarnemo, A.; Liberg, O. Red fox removal and roe deer fawn survival—A 14-year study. J. Wildl. Manag. 2005, 69, 1090–1098. [Google Scholar] [CrossRef]
- Wallmenius, K.; Pettersson, J.H.; Jaenson, T.G.; Nilsson, K. Prevalence of Rickettsia spp., Anaplasma phagocytophilum, and Coxiella burnetii in adult Ixodes ricinus ticks from 29 study areas in central and southern Sweden. Ticks Tick. Borne Dis. 2012, 3, 100–106. [Google Scholar] [CrossRef]
- Severinsson, K.; Jaenson, T.G.; Pettersson, J.; Falk, K.; Nilsson, K. Detection and prevalence of Anaplasma phagocytophilum and Rickettsia helvetica in Ixodes ricinus ticks in seven study areas in Sweden. Parasit. Vectors 2010, 3, 66. [Google Scholar] [CrossRef]
- Sormunen, J.J.; Penttinen, R.; Klemola, T.; Vesterinen, E.J.; Hanninen, J. Anaplasma phagocytophilum in questing Ixodes ricinus ticks in southwestern Finland. Exp. Appl. Acarol. 2016, 70, 491–500. [Google Scholar] [CrossRef]
- Zakham, F.; Korhonen, E.M.; Puonti, P.T.; Castren, R.S.; Uusitalo, R.; Smura, T.; Kant, R.; Vapalahti, O.; Sironen, T.; Kinnunen, P.M. Molecular detection of pathogens from ticks collected from dogs and cats at veterinary clinics in Finland. Parasit. Vectors 2023, 16, 327. [Google Scholar] [CrossRef]
- Skarphedinsson, S.; Lyholm, B.F.; Ljungberg, M.; Sogaard, P.; Kolmos, H.J.; Nielsen, L.P. Detection and identification of Anaplasma phagocytophilum, Borrelia burgdorferi, and Rickettsia helvetica in Danish Ixodes ricinus ticks. APMIS 2007, 115, 225–230. [Google Scholar] [CrossRef]
- Schreiber, C.; Krucken, J.; Beck, S.; Maaz, D.; Pachnicke, S.; Krieger, K.; Gross, M.; Kohn, B.; von Samson-Himmelstjerna, G. Pathogens in ticks collected from dogs in Berlin/Brandenburg, Germany. Parasit. Vectors 2014, 7, 535. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Kramer, A.; Sachse, S.; Straube, E. Detection of Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus ticks in a region of Middle Germany (Thuringia). Ticks Tick. Borne Dis. 2010, 1, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Tappe, J.; Strube, C. Anaplasma phagocytophilum and Rickettsia spp. infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany): Revisited. Ticks Tick. Borne Dis. 2013, 4, 432–438, Erratum in Ticks Tick. Borne Dis. 2016, 7, 1059–1060. [Google Scholar] [CrossRef] [PubMed]
- Knoll, S.; Springer, A.; Hauck, D.; Schunack, B.; Pachnicke, S.; Strube, C. Regional, seasonal, biennial and landscape-associated distribution of Anaplasma phagocytophilum and Rickettsia spp. infections in Ixodes ticks in northern Germany and implications for risk assessment at larger spatial scales. Ticks Tick. Borne Dis. 2021, 12, 101657. [Google Scholar] [CrossRef]
- Andersson, M.O.; Vichova, B.; Tolf, C.; Krzyzanowska, S.; Waldenstrom, J.; Karlsson, M.E. Co-infection with Babesia divergens and Anaplasma phagocytophilum in cattle (Bos taurus), Sweden. Ticks Tick. Borne Dis. 2017, 8, 933–935. [Google Scholar] [CrossRef]
- Skarphedinsson, S.; Jensen, P.M.; Kristiansen, K. Survey of tickborne infections in Denmark. Emerg. Infect. Dis. 2005, 11, 1055–1061. [Google Scholar] [CrossRef]
- Hirsch, E.N.; Geijer, J.; Andersson, M. Owner perceived behavior in cats and the influence of husbandry practices, housing and owner attitudes in Sweden. Animals 2022, 12, 2664. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Vaux, A.G.C.; Hansford, K.M.; Pietzsch, M.E.; Gillingham, E.L. Ticks in the ecotone: The impact of agri-environment field margins on the presence and intensity of Ixodes ricinus ticks (Acari: Ixodidae) in farmland in southern England. Med. Vet. Entomol. 2020, 34, 175–183. [Google Scholar] [CrossRef]
- Kjaer, L.J.; Soleng, A.; Edgar, K.S.; Lindstedt, H.E.H.; Paulsen, K.M.; Andreassen, A.K.; Korslund, L.; Kjelland, V.; Slettan, A.; Stuen, S.; et al. Predicting and mapping human risk of exposure to Ixodes ricinus nymphs using climatic and environmental data, Denmark, Norway and Sweden, 2016. Eurosurveillance 2019, 24, 1800101. [Google Scholar] [CrossRef]
- Lindstrom, A.; Jaenson, T.G. Distribution of the common tick, Ixodes ricinus (Acari: Ixodidae), in different vegetation types in southern Sweden. J. Med. Entomol. 2003, 40, 375–378. [Google Scholar] [CrossRef]
- Hartelt, K.; Oehme, R.; Frank, H.; Brockmann, S.O.; Hassler, D.; Kimmig, P. Pathogens and symbionts in ticks: Prevalence of Anaplasma phagocytophilum (Ehrlichia sp.), Wolbachia sp., Rickettsia sp., and Babesia sp. in Southern Germany. Int. J. Med. Microbiol. 2004, 293 (Suppl. S37), 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ravicini, S.; Pastor, J.; Hawley, J.; Brewer, M.; Castro-Lopez, J.; Beall, M.; Lappin, M.R. Prevalence of selected infectious disease agents in stray cats in Catalonia, Spain. JFMS Open Rep. 2016, 2, 2055116916634109. [Google Scholar] [CrossRef] [PubMed]
- Granick, J.L.; Armstrong, P.J.; Bender, J.B. Anaplasma phagocytophilum infection in dogs: 34 cases (2000–2007). J. Am. Vet. Med. Assoc. 2009, 234, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Kohn, B.; Galke, D.; Beelitz, P.; Pfister, K. Clinical features of canine granulocytic anaplasmosis in 18 naturally infected dogs. J. Vet. Intern. Med. 2008, 22, 1289–1295. [Google Scholar] [CrossRef]
- Bergmann, M.; Englert, T.; Stuetzer, B.; Hawley, J.R.; Lappin, M.R.; Hartmann, K. Prevalence of Bartonella species infections in cats in Southern Germany. Vet. Rec. 2017, 180, 325. [Google Scholar] [CrossRef]
- Day, M.J. Cats are not small dogs: Is there an immunological explanation for why cats are less affected by arthropod-borne disease than dogs? Parasit. Vectors 2016, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- Gethmann, J.; Hoffmann, B.; Kasbohm, E.; Suss, J.; Habedank, B.; Conraths, F.J.; Beer, M.; Klaus, C. Research paper on abiotic factors and their influence on Ixodes ricinus activity-observations over a two-year period at several tick collection sites in Germany. Parasitol. Res. 2020, 119, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, I.; Kohn, B.; Silaghi, C.; Fischer, S.; Marsboom, C.; Hendrickx, G.; Muller, E. Molecular and serological detection of Anaplasma phagocytophilum in dogs from Germany (2008–2020). Animals 2023, 13, 720. [Google Scholar] [CrossRef]





| Risk Factor | A. phagocytophilum-Infected/Number of Tested Cats (%) | Constrasts | Multivariable Analyses | ||
|---|---|---|---|---|---|
| OR | 95% CI | p-Value | |||
| Age (n = 781) | Years | 0.92 | 0.86–0.98 | 0.019 | |
| Sex (n = 864) | M: 48/562 (8.5) | F/M | 0.85 | 0.47–1.53 | 0.600 |
| F: 21/302 (6.9) | |||||
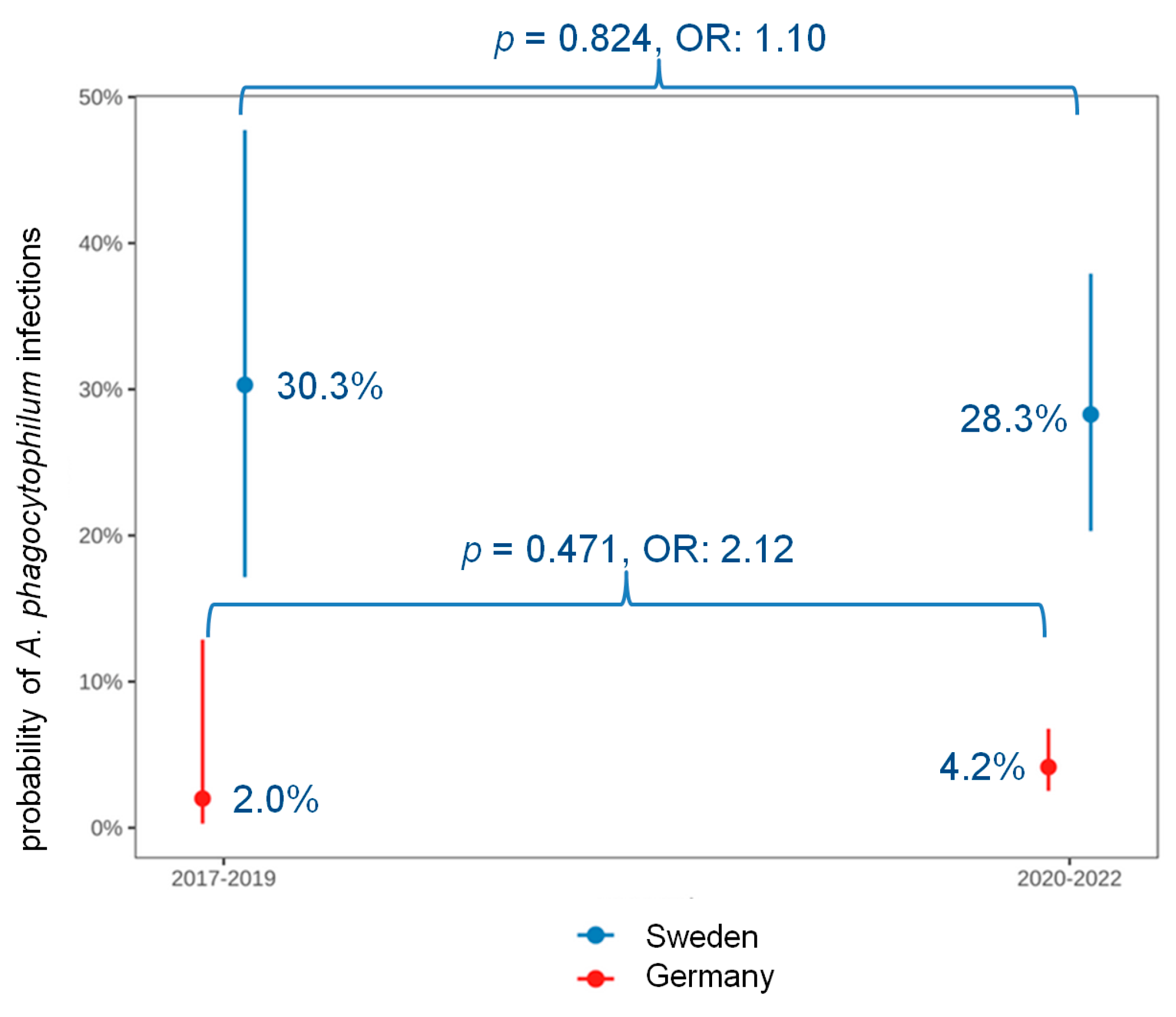
| Period of submission of the sample (n = 1015) | 2017–2019: 22/184 (12.0) | (2017–2019)/(2020–2022) | 2.09 | 0.26–0.88 | 0.017 |
| 2020–2022: 54/831 (6.5) | |||||
| Seasonality | Summer/spring | 4.10 | 1.00–16.9 | 0.051 | |
| spring: 4/178 (2.3) | Autumn/summer | 0.80 | 0.36–1.78 | 0.900 | |
| summer: 37/315 (11.8) | Winter/summer | 0.32 | 0.10–0.99 | 0.047 | |
| autumn: 27/286 (9.4) | Autumn/spring | 3.30 | 0.78–13.9 | 0.140 | |
| winter: 8/236 (3.4) | Winter/autumn | 0.40 | 0.12–1.27 | 0.200 | |
| Winter/spring | 1.31 | 0.25–6.78 | >0.900 | ||
| Univariable analysis | |||||
| Origin | NE: 49/195 (25.1) | NE/CE | 8.70 | 4.74–16.00 | <0.001 |
| CE: 26/702 (3.70) | NE/SE | 39.94 | 3.68–433.70 | <0.001 | |
| SE: 1/118 (0.85) | CE/SE | 4.59 | 0.42–50.60 | 0.296 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geisen, V.; Pantchev, N.; Zablotski, Y.; Kim, O.; Globokar Vrhovec, M.; Hartmann, K.; Bergmann, M. Molecular Detection of Anaplasma phagocytophilum in Cats in Europe and Associated Risk Factors. Animals 2024, 14, 2368. https://doi.org/10.3390/ani14162368
Geisen V, Pantchev N, Zablotski Y, Kim O, Globokar Vrhovec M, Hartmann K, Bergmann M. Molecular Detection of Anaplasma phagocytophilum in Cats in Europe and Associated Risk Factors. Animals. 2024; 14(16):2368. https://doi.org/10.3390/ani14162368
Chicago/Turabian StyleGeisen, Vera, Nikola Pantchev, Yury Zablotski, Olga Kim, Majda Globokar Vrhovec, Katrin Hartmann, and Michéle Bergmann. 2024. "Molecular Detection of Anaplasma phagocytophilum in Cats in Europe and Associated Risk Factors" Animals 14, no. 16: 2368. https://doi.org/10.3390/ani14162368
APA StyleGeisen, V., Pantchev, N., Zablotski, Y., Kim, O., Globokar Vrhovec, M., Hartmann, K., & Bergmann, M. (2024). Molecular Detection of Anaplasma phagocytophilum in Cats in Europe and Associated Risk Factors. Animals, 14(16), 2368. https://doi.org/10.3390/ani14162368







