Ticks and Tick-Borne Pathogens: Occurrence and Host Associations over Four Years of Wildlife Surveillance in the Liguria Region (Northwest Italy)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Collection and Identification
2.2. Molecular Analysis for Pathogen Detection
2.2.1. DNA/RNA Extraction
2.2.2. PCR Amplification for TBP Screening
2.2.3. PCR Amplification for TBP Confirmation and Sequencing
2.3. Database and Statistical Analysis
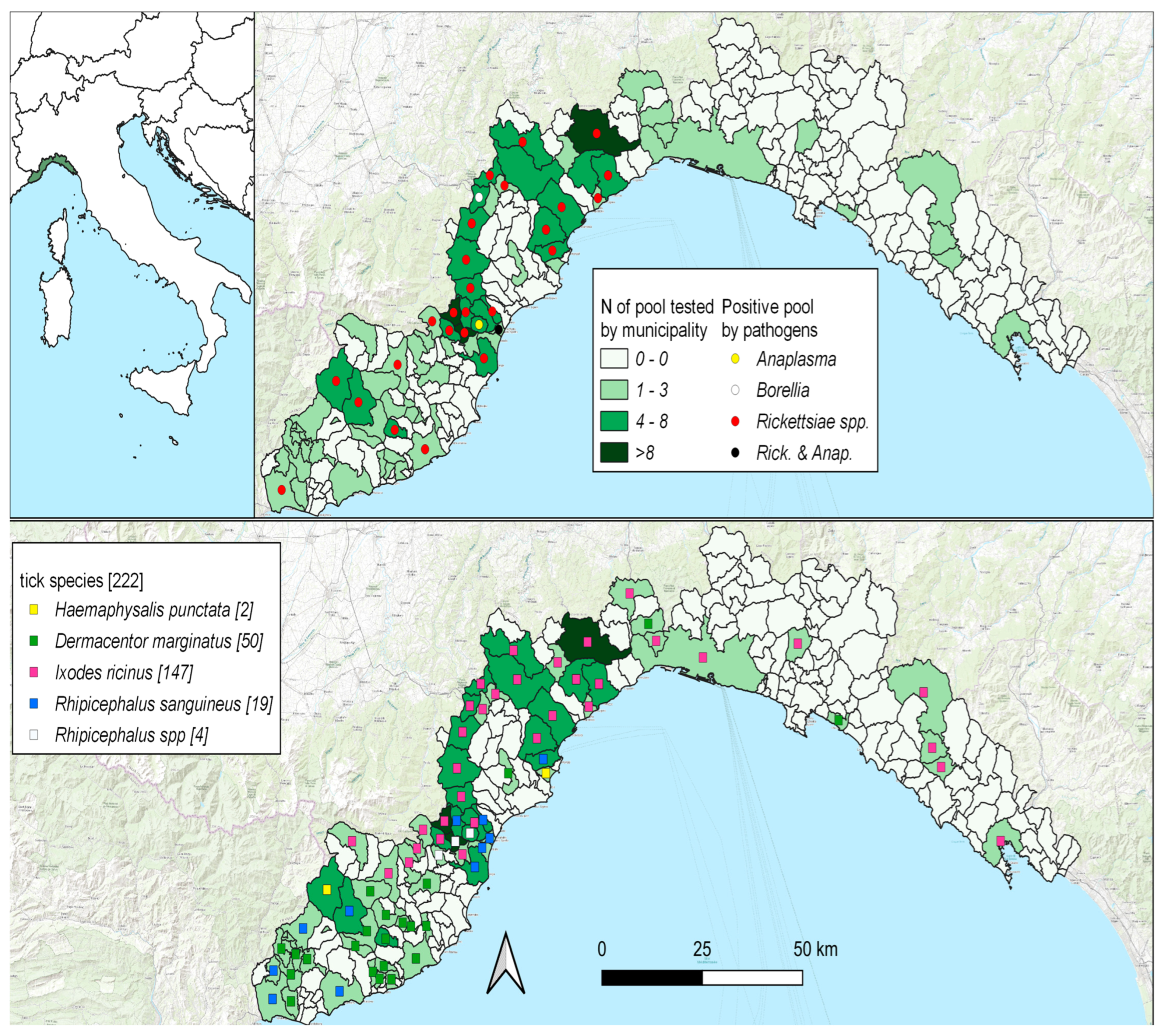
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, B.D.; Grace, D.; Sones, K. Current drivers and future directions of global livestock disease dynamics. Proc. Natl. Acad. Sci. USA 2011, 110, 20871–20877. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.X.; Hansen, A.; Hanson-Easey, S.; Cameron, S.; Xiang, J.; Liu, Q.; Sun, Y.; Weinstein, P.; Han, G.S.; Williams, C.; et al. Infectious diseases, urbanization and climate change: Challenges in future China. Int. J. Environ. Res. Public Health 2015, 12, 11025–11036. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.; Cerri, D.; Fratini, F.; Ampola, M.; Andreani, E. Seroprevalence of Anaplasma phagocytophilum in domestic and wild animals from central Italy. New Microbiol. 2008, 31, 371–375. [Google Scholar] [PubMed]
- Gilbert, L. The Impacts of Climate Change on Ticks and Tick-Borne Disease Risk. Annu. Rev. Entomol. 2021, 66, 373–388. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability; Part A: Global and Sectoral Aspects; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, N.; Boyer, P.; Talagrand-Reboul, E.; Hansmann, Y. Ticks and tick-borne diseases. Med. Mal. Infect. 2019, 49, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Stroffolini, G.; Segala, F.V.; Lupia, T.; Faraoni, S.; Rossi, L.; Tomassone, L.; Zanet, S.; De Rosa, F.G.; Di Perri, G.; Calcagno, A. Serology for Borrelia spp. In Northwest Italy: A Climate-Matched 10-Year Trend. Life 2021, 11, 1310. [Google Scholar] [CrossRef] [PubMed]
- Heyman, P.; Cochez, C.; Hofhuis, A.; Van Der Giessen, J.; Sprong, H.; Porter, S.R.; Losson, B.; Saegerman, C.; Donoso-Mantke, O.; Niedrig, M.; et al. A clear and present danger: Tick-borne diseases in Europe. Expert Rev. Anti-Infect. Ther. 2010, 8, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Drigo, M.; Zelená, H.; Pasotto, D.; Cassini, R.; Mondin, A.; Franzo, G.; Tucciarone, C.M.; Ossola, M.; Vidorin, E.; et al. Wild ungulates as sentinels of flaviviruses and tick-borne zoonotic pathogen circulation: An Italian perspective. BMC Vet. Res. 2023, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vozmediano, A.; Bellato, A.; Rossi, L.; Hoogerwerf, M.N.; Sprong, H.; Tomassone, L. Use of Wild Ungulates as Sentinels of TBEV Circulation in a Naïve Area of the Northwestern Alps, Italy. Life 2022, 12, 1888. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.J.; Hunter, M.S.; Zchori-Fein, E. The emerging diversity of Rickettsia. Proc. R. Soc. B Boil Sci. 2006, 273, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Santibáñez, S.; García-Álvarez, L.; Palomar, A.M.; Oteo, J.A. Rickettsioses in Europe. Microbes Infect. 2015, 17, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Gaudart, J.; Bitam, I.; Huynh, T.P.; Raoult, D.; Parola, P. Why are there so few Rickettsia conorii conorii-infected Rhipicephalus sanguineus ticks in the wild? PLoS Negl. Trop. Dis. 2012, 6, e1697. [Google Scholar] [CrossRef] [PubMed]
- Silva-Pinto, A.; De Lurdes Santos, M.; Sarmento, A. Tick-borne lymphadenopathy, an emerging disease. Ticks Tick Borne Dis. 2014, 5, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Mediannikov, O.; Raoult, D.; Parola, P. The relationship between spotted fever group rickettsiae and ixodid ticks. Vet. Res. 2009, 40, 34. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.A.; Portillo, A. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 2012, 3, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Guccione, C.; Colomba, C.; Tolomeo, M.; Trizzino, M.; Iaria, C.; Cascio, A. Rickettsiales in Italy. Pathogens 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A wide spread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Arricau-Bouvery, N.; Rodolakis, A. Is Q fever an emerging or re-emerging zoonosis? Vet. Res. 2005, 36, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Berri, M.; Souriau, A.; Crosby, M.; Crochet, D.; Lechopier, P.; Rodolakis, A. Relationships between the shedding of Coxiella burnetii, clinical signs and serological responses of 34 sheep. Vet. Rec. 2001, 148, 502–505. [Google Scholar] [CrossRef]
- Rodolakis, A. Q fever State of art, epidemiology, diagnosis and prophylaxis. Small Rumin. Res. 2006, 62, 121–124. [Google Scholar] [CrossRef]
- Muskens, J.; van Maanen, C.; Mars, M.H. Dairy cows with metritis: Coxiella burnetii test results in uterine, blood and bulk milk samples. Vet. Microbiol. 2011, 147, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.S. Coxiella burnetii associated reproductive disorders in domestic animals- a critical review. Acta Vet. Scand. 2013, 55, 13. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Roura, X.; Sainz, A.; Miró, G.; Solano-Gallego, L. Species of ticks and carried pathogens in owned dogs in Spain: Results of a one-year national survey. Ticks Tick-Borne Dis. 2017, 8, 443–452. [Google Scholar] [CrossRef] [PubMed]
- del Cerro, A.; Oleaga, A.; Somoano, A.; Barandika, J.F.; García-Pérez, A.L.; Espí, A. Molecular identification of tick-borne pathogens (Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Coxiella burnetii and piroplasms) in questing and feeding hard ticks from North-Western Spain. Ticks Tick-Borne Dis. 2022, 13, 101961. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Nardoni, S.; Mancianti, F. Arthropod-Borne Pathogens in Wild Canids. Vet. Sci. 2023, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Hrazdilová, K.; Lesiczka, P.M.; Bardoň, J.; Vyroubalová, Š.; Šimek, B.; Zurek, L.; Modrý, D. Wild boar as a potential reservoir of zoonotic tick-borne pathogens. Ticks Tick-Borne Dis. 2021, 12, 101558. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R. Trophic Resource Use and Partitioning in Multispecies Ungulate Communities. Ph.D. Thesis, Swedish University of Agricultural Sciences, Umeå, Sweden, 2019. [Google Scholar]
- Jaenson, T.G.; Jaenson, D.G.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, A.; Schiavetti, I.; Listorti, V.; Dellepiane, M.; Masotti, C.; Ercolini, C.; Guardone, L.; Razzuoli, E. Hard Ticks (Ixodidae) from Wildlife in Liguria, Northwest Italy: Tick Species Diversity and Tick-Host Associations. Insects 2022, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.C.; Murrell, A. Systematics and evolution of ticks with a list of valid genus and species names. Parasitology 2004, 129, S15–S36. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.L.; Walker, A.R. Ticks of Domestic Animals in the Mediterranean Region: A Guide to Identification of Species; University of Zaragoza: Zaragoza, Spain, 2004; pp. 1–131. [Google Scholar]
- Nava, S.; Beati, L.; Venzal, J.M.; Labruna, M.B.; Szabó, M.P.; Petney, T.; Saracho-Bottero, M.N.; Tarragona, E.L.; Dantas-Torres, F.; Silva, M.M.S.; et al. Rhipicephalus sanguineus (Latreille, 1806): Neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick-Borne Dis. 2018, 9, 1573–1585. [Google Scholar] [CrossRef]
- Schwaiger, M.; Cassinotti, P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick-borne encephalitis virus (TBEV) RNA. J. Clin. Virol. 2003, 27, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Nevland, S.; Moum, T. Fatal cases of Tick-borne fever (TBF) in sheep caused by several 16S rRNA gene variants of Anaplasma phagocytophilum. Ann. N. Y. Acad. Sci. 2003, 990, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Skotarczak, B.; Wodecka, B.; Cichocka, A. Coexistence DNA of Borrelia burgdorferi sensu lato and Babesia microti in Ixodes ricinus ticks from north-western Poland. Ann. Agric. Environ. Med. 2002, 9, 25–28. [Google Scholar] [PubMed]
- Berri, M.; Laroucau, K.; Rodolakis, A. The detection of Coxiella burnetii from ovine genital swabs, milk and fecal samples by the use of a single touchdown polymerase chain reaction. Vet. Microbiol. 2000, 72, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.A.; Portillo, A.; Santibáñez, S.; Blanco, J.R.; Pérez-Martínez, L.; Ibarra, V. Cluster of cases of human Rickettsia felis infection from Southern Europe (Spain) diagnosed by PCR. J. Clin. Microbiol. 2006, 44, 2669–2671. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Jang, W.J.; Kim, J.H.; Ryu, J.S.; Lee, S.H.; Park, K.H.; Paik, H.S.; Koh, Y.S.; Choi, M.S.; Kim, I.S. Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg. Infect. Dis. 2005, 11, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.R.; Kimsey, P.J.; Madigan, R.B.; Barlough, J.E.; Dumler, J.E.; Brooks, D.L. Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae). J. Med. Entomol. 1996, 33, 1–5. [Google Scholar] [CrossRef]
- Schets, F.M.; de Heer, L.; de Roda Husman, A.M. Coxiella burnetii in sewage water at sewage water treatment plants in a Q fever epidemic area. Int. J. Hyg. Environ. Health 2013, 216, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Briciu, V.T.; Sebah, D.; Coroiu, G.; Lupşe, M.; Cârstina, D.; Ţăţulescu, D.F.; Mihalca, A.D.; Gherman, C.M.; Leucuţa, D.; Meyer, F.; et al. Immunohistochemistry and real-time PCR as diagnostic tools for detection of Borrelia burgdorferi sensu lato in ticks collected from humans. Exp. Appl. Acarol. 2016, 69, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ivacic, L.; Reed, K.D.; Mitchell, P.D.; Ghebranious, N. A Light Cycler TaqMan assay for detection of Borrelia burgdorferi sensu lato in clinical samples. Diagn. Microbiol. Infect. Dis. 2007, 57, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hofmeester, T.R.; Coipan, E.C.; van Wieren, S.E.; Prins, H.H.T.; Takken, W.; Sprong, H. Few vertebrate species dominate the Borrelia burgdorferi s.l. life cycle. Environ. Res. Lett. 2016, 11, 043001. [Google Scholar] [CrossRef]
- Fabri, N.D.; Sprong, H.; Hofmeester, T.R.; Heesterbeek, H.; Donnars, B.F.; Widemo, F.; Ecke, F.; Cromsigt, J.P.G.M. Wild ungulate species differ in their contribution to the transmission of Ixodes ricinus-borne pathogens. Parasites Vectors 2021, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.A.; Kalmár, Z.; Balea, A.; Mihaiu, M.; Sándor, A.D.; Cocian, A.; Crăciun, S.; Bouari, C.; Briciu, V.T.; Fiț, N. The Role of Wild Boars in the Circulation of Tick-Borne Pathogens: The First Evidence of Rickettsia monacensis Presence. Animals 2023, 13, 1743. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, I.; Antognini, E.; Canonico, C.; Montalbano, M.G.; Necci, A.; di Donato, A.; Moriconi, M.; Morandi, B.; Morganti, G.; Crotti, S.; et al. One health approach to rickettsiosis: A five-year study on spotted fever group rickettsiae in ticks collected from humans, animals and environment. Microorganisms 2021, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Selmi, M.; Ballardini, M.; Salvato, L.; Ricci, E. Rickettsia spp. in Dermacentor marginatus ticks: Analysis of the host-vector-pathogen interactions in a northern Mediterranean area. Exp. Appl. Acarol. 2017, 72, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Petney, T.N.; Pfaeffle, M.P.; Skuballa, J.D. An annotated checklist of the ticks (Acari: Ixodida) of Germany. Syst Appl. Acarol. 2012, 17, 115–170. [Google Scholar] [CrossRef]
- Sevestre, J.; Diarra, A.Z.; Oumarou, H.A.; Durant, J.; Delaunay, P.; Parola, P. Detection of emerging tick-borne disease agents in the Alpes-Maritimes region, southeastern France. Ticks Tick-Borne Dis. 2021, 12, 101800. [Google Scholar] [CrossRef]
- Mysterud, A.; Hügli, C.; Viljugrein, H. Tick infestation on medium–large-sized mammalian hosts: Are all equally suitable to Ixodes ricinus adults? Parasites Vectors 2021, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.; Bournez, L.; Boulanger, N.; Fite, J.; Livoreil, B.; McCoy, K.D.; Quillery, E.; René-Martellet, M.; Bonnet, S.I. The distribution, phenology, host range and pathogen prevalence of Ixodes ricinus in France: A systematic map and narrative review. Peer Community J. 2023, 3, e81. [Google Scholar] [CrossRef]
- Bertola, M.; Montarsi, F.; Obber, F.; Da Rold, G.; Carlin, S.; Toniolo, F.; Porcellato, E.; Falcaro, C.; Mondardini, V.; Ormelli, S.; et al. Occurrence and Identification of Ixodes ricinus Borne Pathogens in Northeastern Italy. Pathogens 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Melis, S.; Batisti Biffignandi, G.; Olivieri, E.; Galon, C.; Vicari, N.; Prati, P.; Moutailler, S.; Sassera, D.; Castelli, M. High-throughput screening of pathogens in Ixodes ricinus removed from hosts in Lombardy, northern Italy. Ticks Tick Borne Dis. 2024, 15, 102285. [Google Scholar] [CrossRef] [PubMed]
- Jore, S.; Vanwambeke, S.O.; Viljugrein, H.; Isaksen, K.; Kristoffersen, A.B.; Woldehiwet, Z.; Johansen, B.; Brun, E.; Brun-Hansen, H.; Westermann, S.; et al. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasites Vectors 2014, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.C.; Golovljova, I.; Jaenson, T.G.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, G.; Iatta, R.; Lia, R.P.; D’Alessio, N.; Manoj RR, S.; Veneziano, V.; Otranto, D. Spotted fever group rickettsiae in Dermacentor marginatus from wild boars in Italy. Transbound. Emerg. Dis. 2021, 68, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Noll, M.; Wall, R.; Makepeace, B.L.; Newbury, H.; Adaszek, L.; Bødker, R.; Estrada-Peña, A.; Guillot, J.; Pereira da Fonseca, I.; Probst, J.; et al. Predicting the distribution of Ixodes ricinus and Dermacent or reticulatus in Europe: A comparison of climate niche modelling approaches. Parasites Vectors 2023, 16, 384. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Ixodes Ricinus—Current Known Distribution. February 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/ixodes-ricinus-current-known-distribution-february-2023 (accessed on 9 June 2024).
- Gandy, S.L.; Hansford, K.M.; Medlock, J.M. Possible expansion of Ixodes ricinus in the United Kingdom identified through the Tick Surveillance Scheme between 2013 and 2020. Med. Vet. Entomol. 2023, 37, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Tagliapietra, V.; Rosà, R.; Arnoldi, D.; Cagnacci, F.; Capelli, G.; Montarsi, F.; Hauffe, H.C.; Rizzoli, A. Saturation deficit and deer density affect questing activity and local abundance of Ixodes ricinus (Acari, Ixodidae) in Italy. Vet. Parasitol. 2011, 183, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalská, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Hansford, K.M.; Wheeler, B.W.; Tschirren, B.; Medlock, J.M. Questing Ixodes ricinus ticks and Borrelia spp. in urban green space across Europe: A review. Zoonoses Public Health 2022, 69, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, L.A.; Pintore, M.D.; Tomassone, L.; Pautasso, A.; Bisanzio, D.; Mignone, W.; Casalone, C.; Mannelli, A. Habitat and occurrence of ixodid ticks in the Liguria region, northwest Italy. Exp. Appl. Acarol. 2014, 64, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, A.; Quesada, M.; López-Claessens, S.; Castellà, J.; Sanfeliu, I.; Antón, E.; Segura-Porta, F. The role of wild boar (Sus scrofa) in the eco-epidemiology of R. slovaca in northeastern Spain. Vector-Borne Zoonotic Dis. 2007, 7, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Grech-Angelini, S.; Stachurski, F.; Lancelot, R.; Boissier, J.; Allienne, J.F.; Marco, S.; Maestrini, O.; Uilenberg, G. Ticks (Acari: Ixodidae) infesting cattle and some other domestic and wild hosts on the French Mediterranean island of Corsica. Parasit Vectors 2016, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors 2010, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Dantas-Torres, F.; Estrada-Peña, A.; Levin, M. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013, 4, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Curioni, V.; Cerquetella, S.; Scuppa, P.; Pasqualini, L.; Beninati, T.; Favia, G. Lyme disease and babesiosis: Preliminary findings on the transmission risk in highly frequented areas of the Monti Sibillini National Park (Central Italy). Vector-Borne Zoonotic Dis. 2004, 4, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Marco, I.; Lopez-Olvera, J.R.; Rosell, R.; Vidal, E.; Hurtado, A.; Juste, R.; Pumarola, M.; Lavin, S. Severe outbreak of disease in the southern chamois (Rupicapra pyrenaica) associated with border disease virus infection. Vet. Microbiol. 2007, 120, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Habib, J.; Zenner, L.; Garel, M.; Mercier, A.; Poirel, M.T.; Itty, C.; Appolinaire, J.; Amblard, T.; Benedetti, P.; Sanchis, F.; et al. Prevalence of tick-borne pathogens in ticks collected from the wild mountain ungulates mouflon and chamois in 4 regions of France. Parasite 2024, 31, 21. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Bertelloni, F.; Turchi, B.; Filogari, D.; Cerri, D. Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy. Asian Pacific J. Trop. Med. 2015, 8, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Pascucci, I.; Di Domenico, M.; Curini, V.; Cocco, A.; Averaimo, D.; D’Alterio, N.; Cammà, C. Diversity of Rickettsia in Ticks Collected in Abruzzi and Molise Regions (Central Italy). Microorganisms 2019, 7, 696. [Google Scholar] [CrossRef] [PubMed]
- Chisu, V.; Foxi, C.; Masala, G. First molecular detection of the human pathogen Rickettsia raoultii and other spotted fever group rickettsiae in Ixodid ticks from wild and domestic mammals. Parasitol. Res. 2018, 117, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Remesar, S.; Cano-Terriza, D.; Morrondo, P.; Jiménez-Ruiz, S.; López, C.M.; Jiménez-Martín, D.; Díaz, P.; Paniagua, J.; García-Bocanegra, I. Molecular detection of Rickettsia spp. in wild ungulates and their ticks in Mediterranean areas of southwestern Spain. Zoonoses Public Health 2023, 70, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Michalski, M.M.; Kubiak, K.; Szczotko, M.; Dmitryjuk, M. Tick-Borne Pathogens in Ticks Collected from Wild Ungulates in North-Eastern Poland. Pathogens 2021, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Ciccozzi, M.; Lo Presti, A.; Cella, E.; Giovanetti, M.; Di Luca, M.; Toma, L.; Bianchi, R.; Khoury, C.; Rezza, G.; et al. Characterization of spotted fever group Rickettsiae in ticks from a city park of Rome, Italy. Ann. Ist. Super Sanita 2015, 51, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Barroso, D.; Vescio, M.F.; Bella, A.; Ciervo, A.; Busani, L.; Rizzo, C.; Rezza, G.; Pezzotti, P. Mediterranean spotted fever rickettsiosis in Italy, 2001–2015: Spatio-temporal distribution based on hospitalization records. Ticks Tick-Borne Dis. 2019, 10, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Menandro, M.L.; Cassini, R.; Mondin, A.; Pasotto, D.; Grillini, M.; Rocca, G.; Drigo, M. High Prevalence of tick-borne zoonotic Rickettsia slovaca in ticks from wild boars, northeastern Italy. Animals 2022, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Barbiero, A.; Manciulli, T.; Spinicci, M.; Vellere, I.; Colao, M.G.; Rossolini, G.M.; Bartoloni, A.; Raoult, D.; Zammarchi, L. Scalp eschar and neck lymph adenopathy after a tick bite (SENLAT) in Tuscany, Italy (2015–2022). Infection 2023, 51, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Guccione, C.; Rubino, R.; Colomba, C.; Anastasia, A.; Caputo, V.; Iaria, C.; Cascio, A. Rickettsiosis with Pleural Effusion: A Systematic Review with a Focus on Rickettsiosis in Italy. Trop. Med. Infect. Dis. 2022, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- ECDC—Epidemiological Situation of Rickettsioses in EU/EFTA Countries. Available online: https://op.europa.eu/en/publication-detail/-/publication/afe46de4-e6fb-4c38-9661-d2ce0d3b05b0/language-en (accessed on 9 June 2024).
- Zhang, Y.Y.; Hay, S.I.; Fang, L.Q.; Sun, Y.; Chen, J.; Teng, A.; Wang, T.; Li, H.; Hay, S.I.; Fang, L.; et al. Mapping the global distribution of spotted fever group rickettsiae: A systematic review with modelling analysis. Lancet Digit. Health 2023, 5, e5–e15. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Drigo, M.; Cassini, R.; Mondin, A.; Pasotto, D.; Sinigaglia, R.; Rocca, G.; Menandro, M.L. High Prevalence of Rickettsia Slovaca in Dermacentor Marginatus in Euganean Hills Regional Park. Int. J. Infect. Dis. 2022, 116, S120. [Google Scholar] [CrossRef]
- Boldis, V.; Spitalská, E. Dermacentor marginatus and Ixodes ricinus ticks versus L929 and Vero cell lines in Rickettsia slovaca life cycle evaluated by quantitative real time PCR. Exp. Appl. Acarol. 2010, 50, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Raele, D.A.; Galante, D.; Pugliese, N.; Salandra, G.; Cafiero, M.A. Spotted fever group rickettsiae associated with ixodid ticks in wild environment in Southern Italy. Microbiol. Open 2018, 7, e00527. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vozmediano, A.; Giglio, G.; Ramassa, E.; Nobili, F.; Rossi, L.; Tomassone, L. Dermacentor marginatus and Dermacentor reticulatus, and their infection by SFG Rickettsiae and Francisella-like endosymbionts, in mountain and periurban habitats of Northwestern Italy. Vet. Sci. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Blanda, V.; Torina, A.; la Russa, F.; D’Agostino, R.; Randazzo, K.; Scimeca, S.; Giudice, E.; Caracappa, S.; Cascio, A.; de la Fuente, J. A Retrospective Study of the Characterization of Rickettsia Species in Ticks Collected from Humans. Ticks Tick-Borne Dis. 2017, 8, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, G.; Biernat, B.; Stańczak, J.; Szewczyk, T.; Werszko, J. The role of particular tick developmental stages in the circulation of tick-borne pathogens affecting humans in Central Europe. 3. Rickettsiae. Ann. Parasitol. 2016, 62, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, G.; Mancini, F.; Caddeo, A.; Ciervo, A.; Babudieri, S.; Maida, I.; Fiori, M.L.; Rezza, G.; Mura, M.S. Rickettsia monacensis as cause of Mediterranean spotted fever-like illness, Italy. Emerg. Infect. Dis. 2012, 18, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Maioli, G.; Pistone, D.; Bonilauri, P.; Pajoro, M.; Barbieri, I.; Mulatti, P.; Vicari, N.; Dottori, M. Etiological agents of rickettsiosis and anaplasmosis in ticks collected in Emilia-Romagna region (Italy) during 2008 and 2009. Exp. Appl. Acarol. 2012, 57, 199–208. [Google Scholar] [CrossRef]
- Scarpulla, M.; Barlozzari, G.; Marcario, A.; Salvato, L.; Blanda, V.; De Liberato, C.; D’Agostini, C.; Torina, A.; Macrì, G. Molecular detection and characterization of spotted fever group rickettsiae in ticks from Central Italy. Ticks Tick Borne Dis. 2016, 7, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Torina, A.; Valenzise, M.; Blanda, V.; Camarda, N.; Bombaci, S.; Iaria, C.; De Luca, F.; Wasniewska, M. Scalp eschar and neck lymphadenopathy caused by Rickettsia massiliae. Emerg. Infect. Dis. 2013, 19, 836. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Matsumoto, K.; Samoylenko, I.; Drancourt, M.; Roux, V.; Rydkina, E.; Davoust, B.; Tarasevich, I.; Brouqui, P.; Fournier, P.E. Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int. J. Syst. Evolut. Microbiol. 2008, 58, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Chochlakis, D.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Human anaplasmosis and Anaplasma ovis variant. Emerg. Infect. Dis. 2010, 16, 1031. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, Q.; Qin, X.; Lyu, Y.; Teng, Z.; Li, K.; Yu, L.; Jin, X.; Chang, H.; Wang, W.; et al. Anaplasma bovis infection in fever and thrombocytopenia patients—Anhui Province, China, 2021. China CDC Wkly. 2022, 4, 249. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, F.; Liao, Y.; Shen, J.J.; Xu, J.M.; Chen, Y.Z.; Li, J.H.; Holmes, E.C.; Zhang, Y.Z. Epidemiology and diversity of rickettsiales bacteria in humans and animals in Jiangsu and Jiangxi provinces, China. Sci. Rep. 2019, 9, 13176. [Google Scholar] [CrossRef] [PubMed]
- Da Rold, G.; Obber, F.; Monne, I.; Milani, A.; Ravagnan, S.; Toniolo, F.; Sgubin, S.; Zamperin, G.; Foiani, G.; Vascellari, M.; et al. Clinical tick-borne encephalitis in a roe deer (Capreolus capreolus L.). Viruses 2022, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Pacilly, F.C.A.; Benning, M.E.; Jacobs, F.; Leidekker, J.; Sprong, H.; Van Wieren, S.E.; Takken, W. Blood feeding on large grazers affects the transmission of Borrelia burgdorferi sensu lato by Ixodes ricinus. Ticks Tick-Borne Dis. 2014, 5, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Pascucci, I.; Curini, V.; Cocco, A.; Dall’Acqua, F.; Pompilii, C.; Cammà, C. Detection of Anaplasma phagocytophilum genotypes that are potentially virulent for human in wild ruminants and Ixodes ricinus in central Italy. Ticks Tick-Borne Dis. 2016, 7, 782–787. [Google Scholar] [CrossRef] [PubMed]
| Pathogen | Primers | Target Gene | Reference |
|---|---|---|---|
| Anaplasma spp. | 16SANA-F 5′-CAGAGTTTGATCCTGGCTCAGAACG-3′ 16SANA-R 5′-GAGTTTGCCGGGACTTCTTCT GTA-3′ | 16S rRNA | Stuen et al. [36] |
| Borrelia burgdorferi s.l. | FLA1 5′-AGAGCAACTTACAGACGAAATTAAT-3′ FLA2 5′-CAAGTCTATTTTGGAAAGCACCTAA-3′ | FLA | Skotarczak et al. [37] |
| Coxiella burnetii | Trans1 5′-TATGTATCCACCGTAGCCAG C-3′ Trans2 5′-CCCAACAACACCTCCTTATTC-3′ | IS1111 | Berri et al. [38] |
| Rickettsia spp. | RpCS.877p 5′-GGGGGCCTGCTCACGGCGG-3′ RpCS.1258n 5′-ATTGCAAAAAGTACAGTGAACA-3′ | citrate synthase | Regnery et al. [39] |
| Tick-borne encephalitis (TBE) virus | F-TBE 5′-GGGCGGTTCTTGTTCTCC-3′ R-TBE 5′-ACACATCACCTCCTTGTCAGACT-3′ TBE-Probe-WTTGAGCCACCATCACCCAGACACA | 3′ non-coding region | Schwaiger et al. [35] |
| Pathogen | Primers | Target Gene | Reference |
|---|---|---|---|
| Rickettsia spp. | Rr190.70p 5′-ATGGCGAATATTTCTCCAAAA-3′ Rr190.701n 5′-GTTCCGTTAATGGCAGCATCT-3′ Rr190.602n 5′-AGTGCAGCATTCGCTCCCCCT-3′ | OmpA | Oteo et al. [40] |
| rompB OF 5′-GTAACCGGAAGTAATCGTTTCGTAA-3′ rompB OR 5′-GCTTTATAACCAGCTAAACCACC-3′ rompB SFG IF 5′-GTTTAATACGTGCTGCTAACCAA-3′ rompB SFG IR 5′-GGTTTGGCCCATATACCATAAG-3′ | OmpB | Choi et al. [41] | |
| RpCS.877p 5′-GGGGGCCTGCTCACGGCGG-3′ RpCS.1258n 5′-ATTGCAAAAAGTACAGTGAACA-3′ | Citrate synthase | Regnery et al. [39] | |
| Anaplasma spp. | EE1 5′-TCCTGGCTCAGAACGAACGCTGGCGGC-3′ EE2 5′-AGTCACTGACCCAACCTTAAATGGCTG-3′ EE3 5′-GTCGAACGGATTATTCTTTATAGCTTGC-3′ EE4 5′-CCCTTCCGTTAAGAAGGATCTAATCTCC-3′ | 16S-rRNA | Richter et al. [42] |
| Coxiella burnetii | sIS1pri F 5′-CGGGTTAAGCGTGCTCAGTAT-3′ sIS1pri R 5′-TCCACACGCTTCCATCACCAC-3′ Tqpro sIS1 (5′-FAM/3′-BHQ1) 5′-AGCCCACCTTAAGACTGGCTACGGTGGAT-3′ | IS1111 | Schets et al. [43] |
| Borrelia burgdorferi s.l. | Bor_OspA_F 5′-AATATTTATTGGGAATAGGTCTAA-3′ Bor_OspA_R 5′-CACCAGGCAAATCTACTGA-3′ Bor_OspA_TM (5′-FAM/3′-BHQ1) 5′-TTAATAGCATGYAAGCAAAATGTTAGCA-3′ | OspA | Briciu et al. [44] |
| Identified Tick Species | Roe Deer (n Host = 105) | Fallow Deer (n Host = 49) | Chamois (n Host = 2) | Wild Boar (n Host = 61) | Overall |
|---|---|---|---|---|---|
| Ixodes ricinus | 276 (91.1%) *** | 168 (74.7%) ** | 0 *** | 12 (8.2%) *** | 456 (66.8%) |
| Dermacentor marginatus | 0 *** | 0 *** | 0 ns | 108 (73.5%) *** | 108 (15.8%) |
| Rhipicephalus sanguineus s.s. | 24 (7.9%) *** | 53 (23.6%) *** | 3 (37.5%) ns | 27 (18.4%) ns | 107 (15.7%) |
| Rhipicephalus spp. | 3 (1.0%) ns | 3 (1.3%) ns | 0 ns | 0 ns | 6 (0.9%) |
| Haemaphysalis punctata | 0 * | 1 (0.4%) ns | 5 (62.5%) *** | 0 ns | 6 (0.9%) |
|
Overall number of collected ticks | 303 | 225 | 8 | 147 | 683 |
| Identified Pathogen | Tick Species | Host Species | PCR and Sequencing (CRABART): N of Confirmed Positive Pools |
|---|---|---|---|
| Rickettsia slovaca | Ixodes ricinus | Roe deer | 20 |
| Fallow deer | 5 | ||
| Dermacentor marginatus | Wild boar | 6 | |
| Rhipicephalus sanguineus | Fallow deer | 2 | |
| Roe deer | 1 | ||
| Rickettsia monacensis | Ixodes ricinus | Roe deer | 13 |
| Fallow deer | 4 | ||
| Dermacentor marginatus | Wild boar | 1 | |
| Rickettsia helvetica | Ixodes ricinus | Roe deer | 3 |
| Fallow deer | 1 | ||
| Rickettsia massiliae | Ixodes ricinus | Roe deer | 1 |
| Rickettsia raoultii | Dermacentor marginatus | Wild boar | 1 |
| Anaplasma phagocytophilum | Ixodes ricinus | Fallow deer | 3 |
| B. burgdorferi s.l. | Ixodes ricinus | Roe deer | 1 |
| Coxiella burnetii | Ixodes ricinus | Roe deer | 0 |
| Host | Tick Species | N Ticks | Tested Pools | Rickettsia slovaca % [95% CI] | Rickettsia monacensis % [95% CI] | Rickettsia helvetica % [95% CI] | Rickettsia massiliae % [95% CI] | Rickettsia raoultii % [95% CI] | Anaplasma sp. % [95% CI] | Borrellia sp. [95% CI] |
|---|---|---|---|---|---|---|---|---|---|---|
| Chamois | Haemaphysalis punctata | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chamois | Rhipicephalus sanguineus s.s. | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Roe deer | Ixodes ricinus | 263 | 103 | 19.42 [12.28–28.38] | 11.65 [6.17–19.47] | 2.91 [0.60–8.28] | 0.97 [0.02–5.29] | 0 | 0 | 0.97 [0.02–5.29] |
| Roe deer | Rhipicephalus sanguineus s.s | 24 | 3 | 33.33 [0.84–90.57] | 0 | 0 | 0 | 0 | 0 | 0 |
| Roe deer | Rhipicephalus spp. | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wild boar | Dermacentor marginatus | 106 | 50 | 12.00 [4.53–24.31] | 2.00 [0.05–13.65] | 0 | 0 | 2.00 [0.05–13.65] | 0 | 0 |
| Wild boar | Ixodes ricinus | 12 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wild boar | Rhipicephalus sanguineus s.s. | 28 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fallow deer | Haemaphysalis punctata | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fallow deer | Ixodes ricinus | 167 | 36 | 13.89 [4.67–29.50] | 8.33 [1.75–22.47] | 2.78 [0.07–14.53] | 0 | 0 | 8.33 [1.75–22.47] | 0 |
| Fallow deer | Rhipicephalus sanguineus s.s. | 51 | 12 | 8.33 [0.21–38.48] | 0 | 0 | 0 | 0 | 0 | 0 |
| Fallow deer | Rhipicephalus spp. | 5 | 3 | 33.33 [0.84–90.57] | 0 | 0 | 0 | 0 | 0 | 0 |
| Factors | Category | n° Pool | Rickettsia spp. Prevalence | Chi Square | p Value |
|---|---|---|---|---|---|
| Host | Chamois | 2 | 0.00% | 10.83 | 0.01 |
| Roe deer | 107 | 34.58% | |||
| Wild boar | 61 | 13.11% | |||
| Fallow deer | 52 | 21.15% | |||
| Tick species | Dermacentor marginatus | 50 | 12.73% | 6.28 | 0.04 |
| Ixodex ricinus | 147 | 37.42% | |||
| Rhipicephalus spp. | 23 | 5.85% | |||
| Season | Autumn | 37 | 0.00% | 25.67 | <0.0001 |
| Spring | 30 | 7.69% | |||
| Winter | 155 | 34.84% | |||
| Area | East | 12 | 0.00% | 4.28 | 0.04 |
| West | 210 | 26.67% |
| Parameter | Baseline | OR | 95% CI OR | Wald χ2 | p-Value χ2 |
|---|---|---|---|---|---|
| host | Roe deer | 3.2 | 1.4–7.4 | 10.1384 | 0.0015 |
| season | winter | 12.9 | 2.9–57.0 | 11.9671 | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guardone, L.; Nogarol, C.; Accorsi, A.; Vitale, N.; Listorti, V.; Scala, S.; Brusadore, S.; Miceli, I.N.; Wolfsgruber, L.; Guercio, A.; et al. Ticks and Tick-Borne Pathogens: Occurrence and Host Associations over Four Years of Wildlife Surveillance in the Liguria Region (Northwest Italy). Animals 2024, 14, 2377. https://doi.org/10.3390/ani14162377
Guardone L, Nogarol C, Accorsi A, Vitale N, Listorti V, Scala S, Brusadore S, Miceli IN, Wolfsgruber L, Guercio A, et al. Ticks and Tick-Borne Pathogens: Occurrence and Host Associations over Four Years of Wildlife Surveillance in the Liguria Region (Northwest Italy). Animals. 2024; 14(16):2377. https://doi.org/10.3390/ani14162377
Chicago/Turabian StyleGuardone, Lisa, Chiara Nogarol, Annalisa Accorsi, Nicoletta Vitale, Valeria Listorti, Sonia Scala, Sonia Brusadore, Ilaria Nina Miceli, Lara Wolfsgruber, Annalisa Guercio, and et al. 2024. "Ticks and Tick-Borne Pathogens: Occurrence and Host Associations over Four Years of Wildlife Surveillance in the Liguria Region (Northwest Italy)" Animals 14, no. 16: 2377. https://doi.org/10.3390/ani14162377






