Effect of Different Dietary Iron Contents on Liver Transcriptome Characteristics in Wujin Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Feeding Conditions
2.2. Diet Preparation
2.3. Sample Collection
2.4. Growth Performance
2.5. Analysis of Serum Iron Metabolism Parameters
2.6. RNA Isolation and Sequencing and Sequence Data Processing
2.6.1. RNA Extraction
2.6.2. Quantitative and Qualitative RNA Analysis
2.6.3. Library Preparation of Transcriptome Sequencing
2.6.4. Clustering and Sorting
2.6.5. Quality Control
2.6.6. Contrast Ratio Analysis
2.6.7. Gene Function Annotation
2.7. Bioinformatics Analysis
2.7.1. Gene Expression Level Quantification, Differential Gene Screening, and Functional Enrichment and Cluster Analyses
2.7.2. Hypoxia Regulation and Screening of Key Signal Pathways of Iron Metabolism
2.8. Gene Expression
2.9. Statistical Analysis
3. Results
3.1. Effect of Different Dietary Iron Contents on the Growth Performance of Piglets
3.2. Effects of Different Dietary Iron Contents on Serum Iron Metabolism Parameters of Piglets
3.3. Sequencing Results and Quality Control
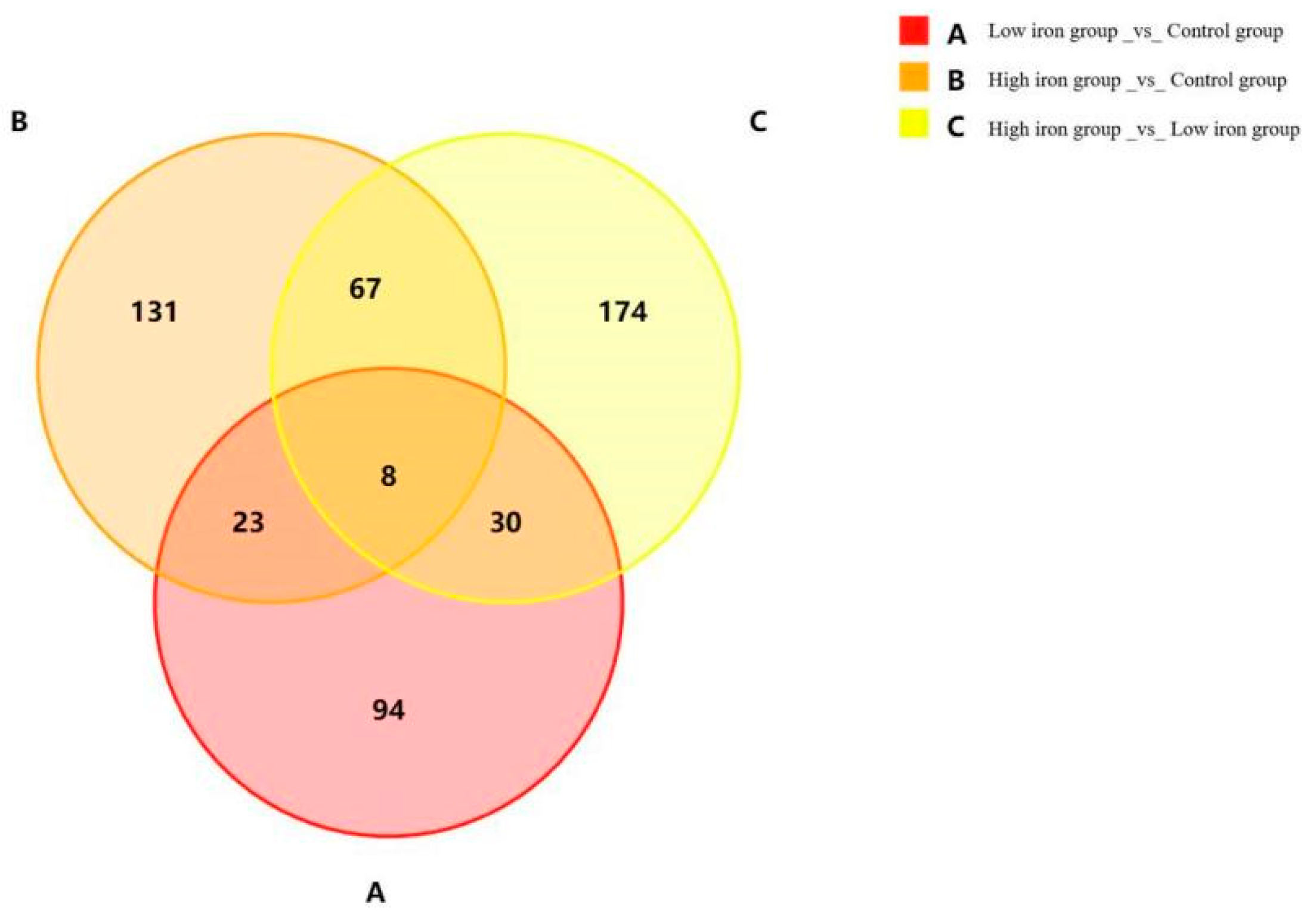
3.4. Screening of Differentially Expressed Genes
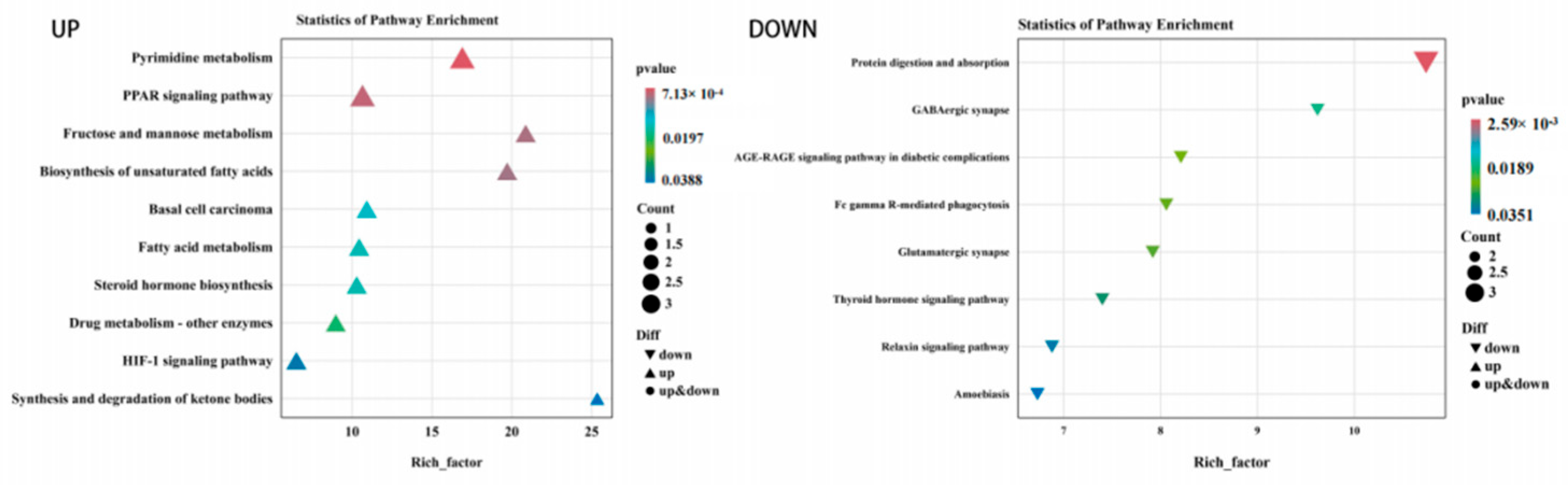
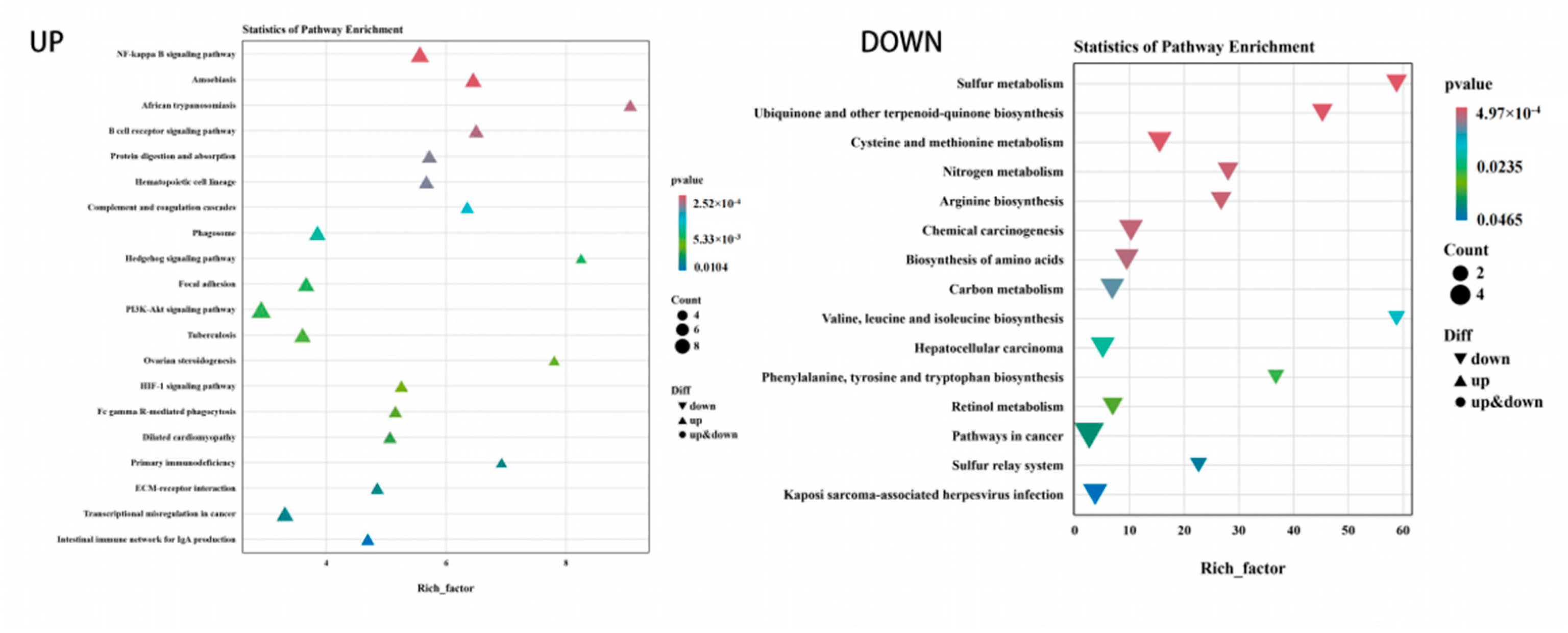
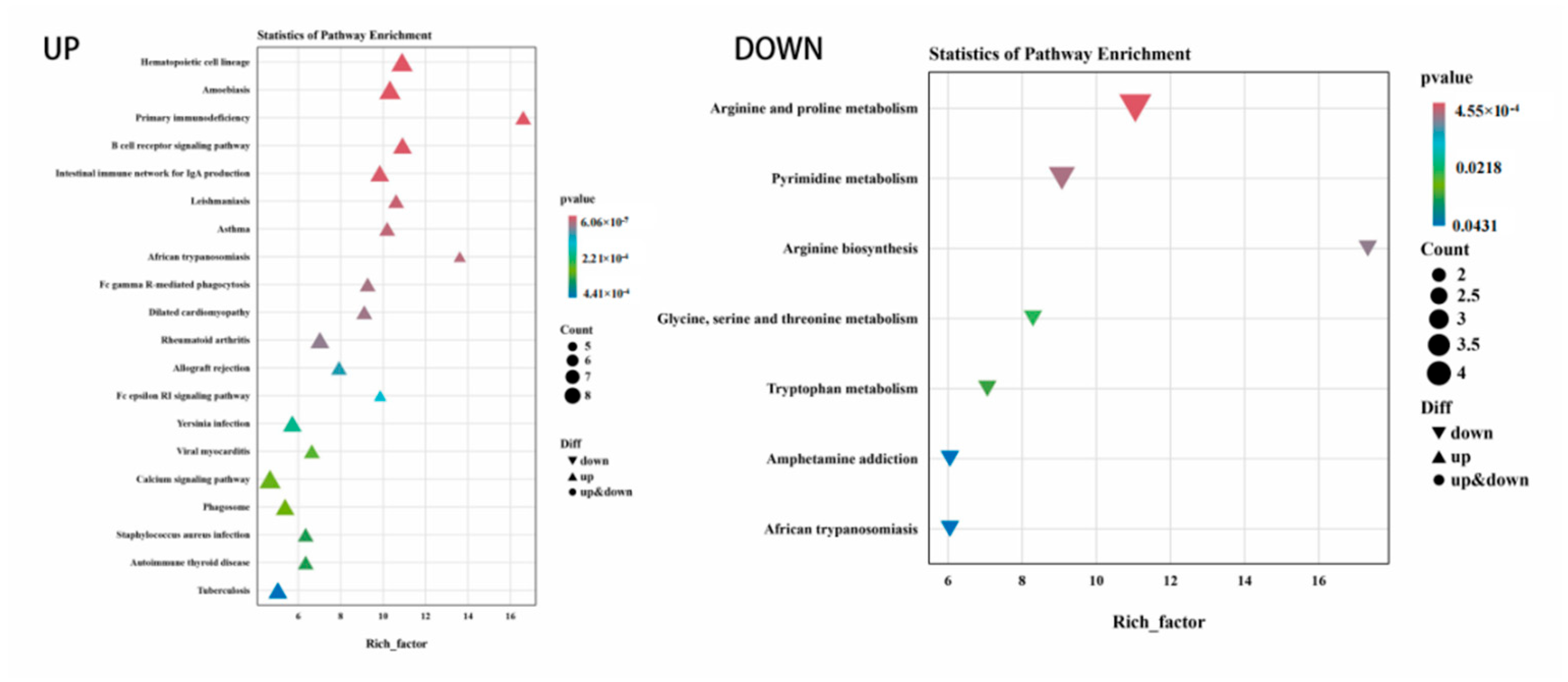
3.5. Functional Enrichment of DEGs
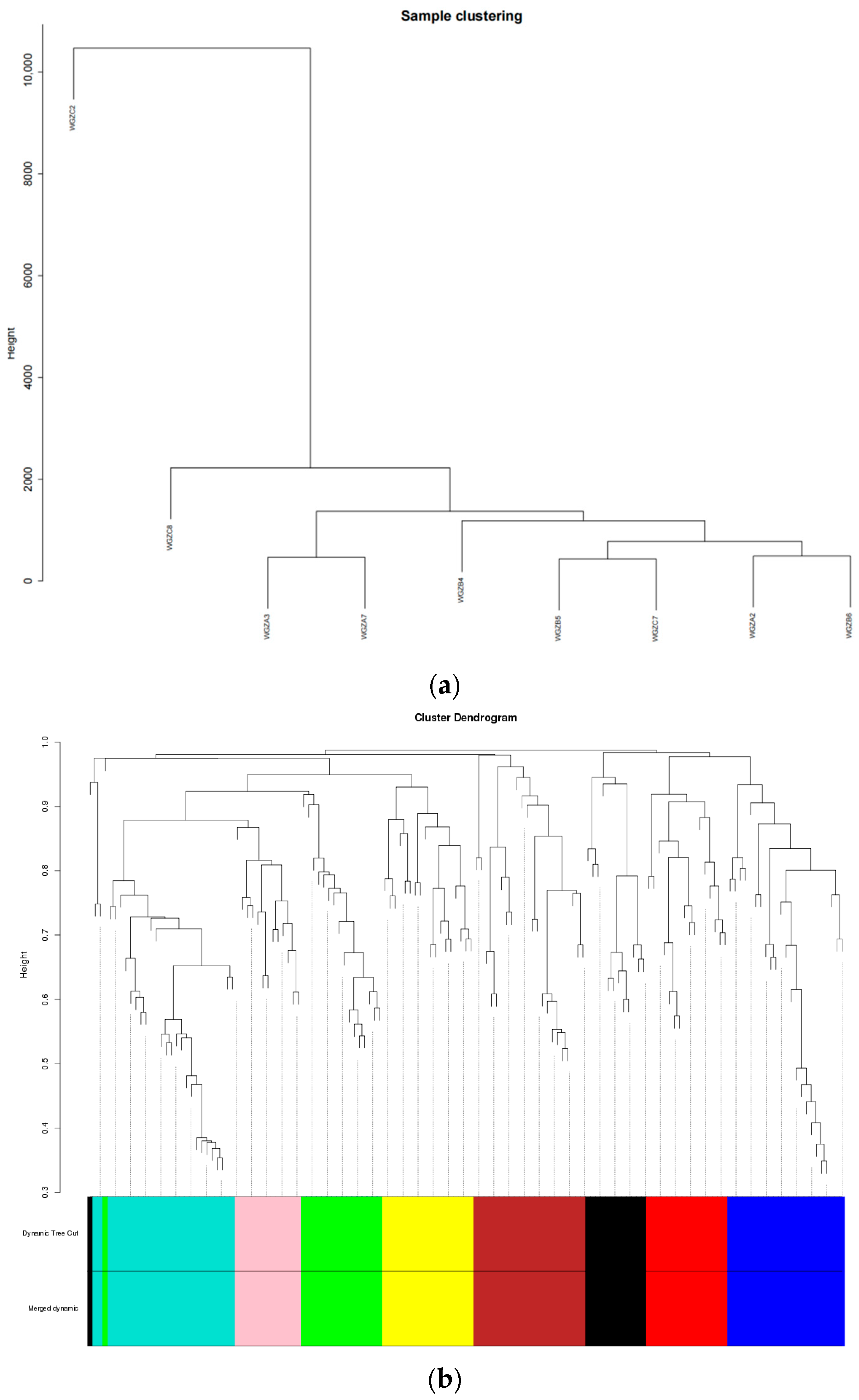
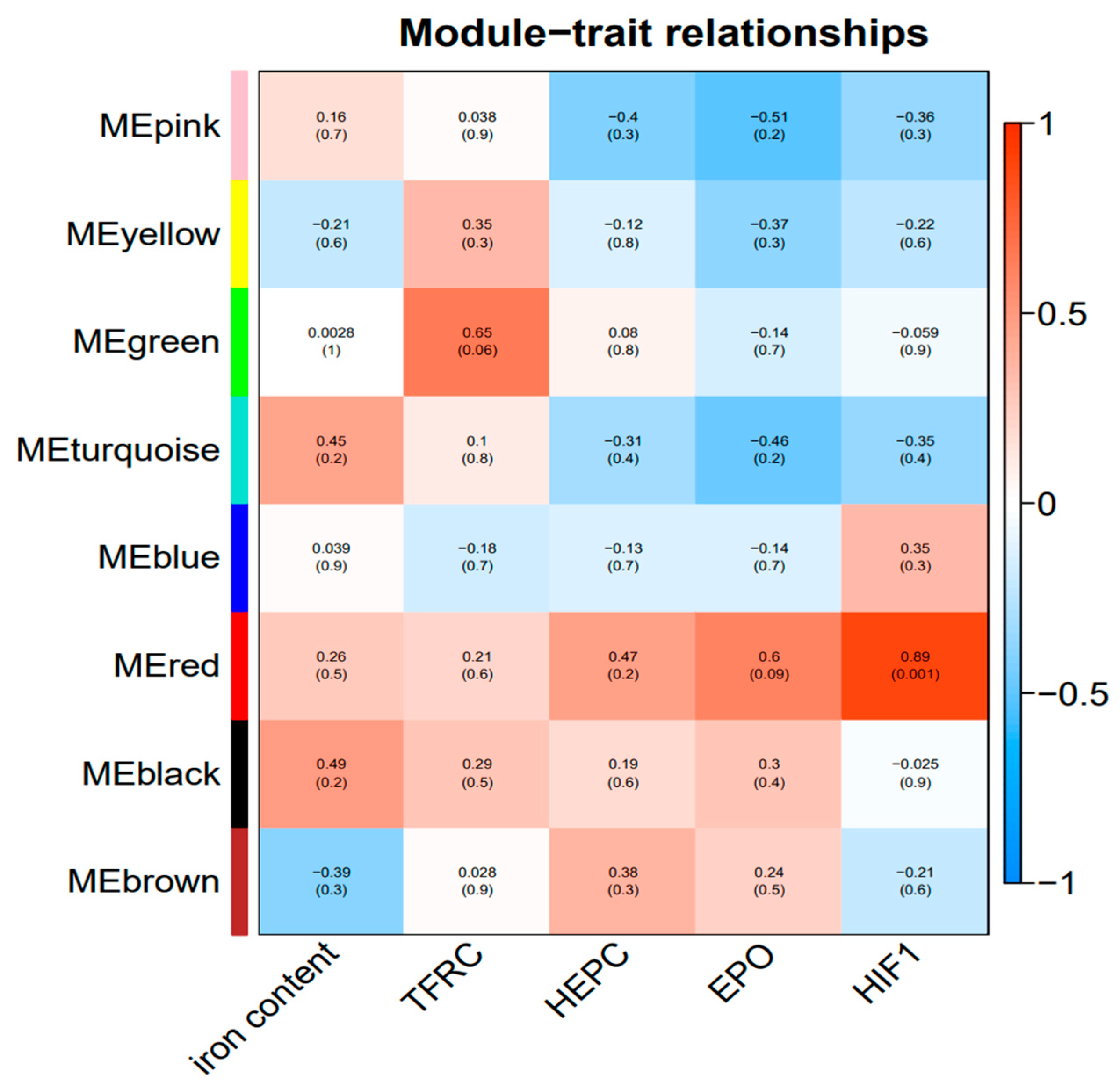
3.6. Screening of Hypoxic Regulation- and Iron Metabolism-Related Pathways Based on WGCNA and Pathway Enrichment Analyses
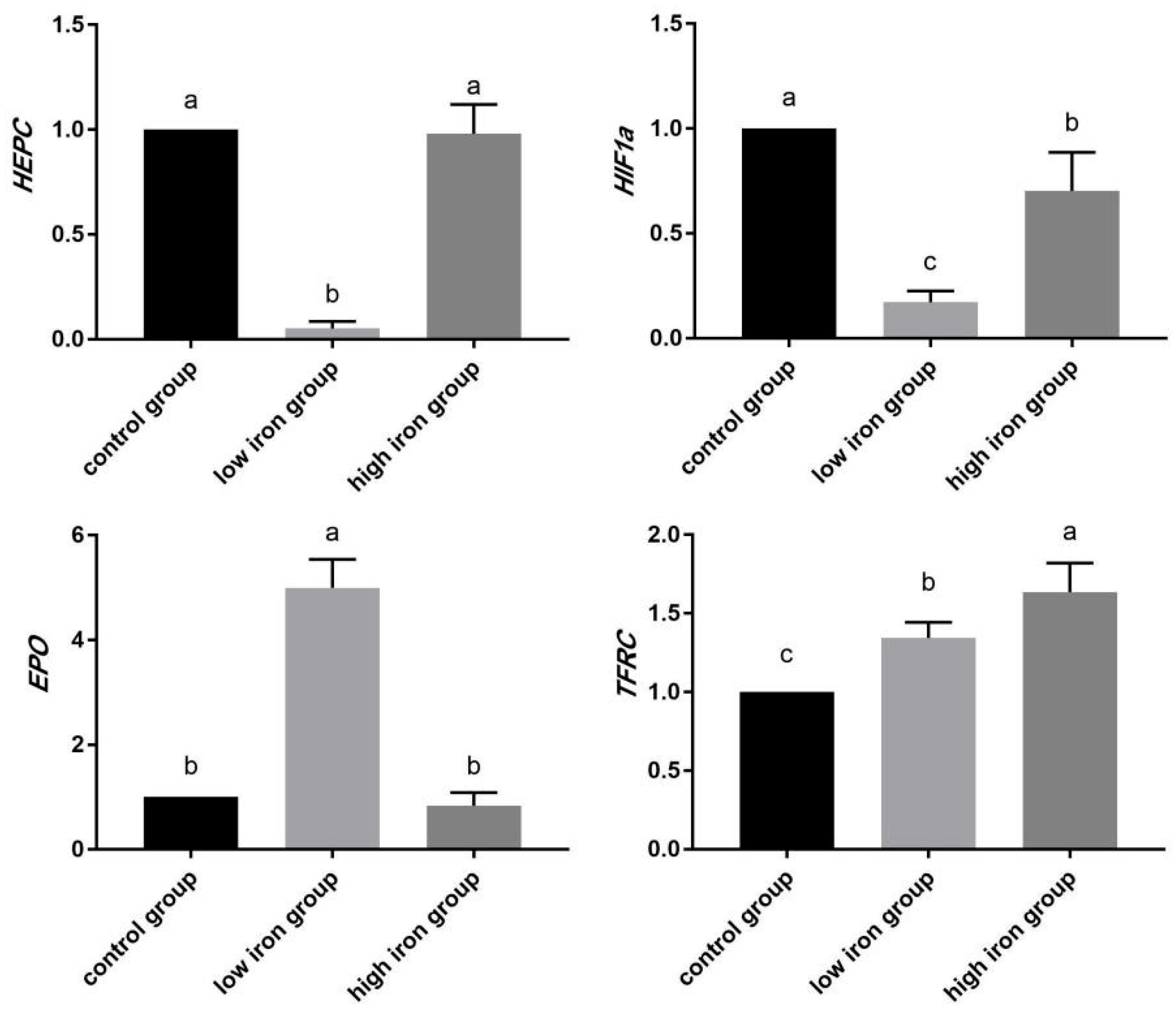
3.7. Relative Expression Analysis of Hypoxia Regulation and Iron Metabolism Gene mRNA in Piglets
4. Discussion
4.1. Growth Performance
4.2. Changes in Serum Iron Metabolism Parameters
4.3. Transcriptional Analysis
4.4. Screening Key Signaling Pathways for Hypoxia Regulation and Iron Metabolism Based on WGCNA and Transcriptome Analysis
4.5. Effects of Dietary Iron Level on HIF-1 Signaling Pathway and TGF-β Signaling Pathway in Wujin Piglets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Ma, X.; Yang, C.-W.; Jiang, S.; Huang, L.; Li, Y.; Zhang, F.; Jiao, N.; Yang, W. Low Level of Dietary Organic Trace Elements Improve the Eggshell Strength, Trace Element Utilization, and Intestinal Function in Late-Phase Laying Hens. Front. Vet. Sci. 2022, 9, 903615. [Google Scholar] [CrossRef] [PubMed]
- Ettle, T.; Schlegel, P.; Roth, F. Investigations on Iron Bioavailability of Different Sources and Supply Levels in Piglets. J. Anim. Physiol. Anim. Nutr. 2008, 92, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Study on the Change of Iron Content in Pig Plasma and Its Relationship with Weight Gain Rate. J. Jiangsu Agric. Coll. 1990, 3, 21–24. [Google Scholar]
- Aisen, P.; Listowsky, I. Iron Transport and Storage Proteins. Annual Review of Biochemistry. Annu. Rev. Biochem. 1980, 49, 357–393. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wan, D.; Yang, H.; Li, G.; Zhang, Y.; Zhou, X.; Wu, X.; Yin, Y. Effects of Iron Deficiency on Serum Metabolome, Hepatic Histology, and Function in Neonatal Piglets. Animals 2020, 10, 1353. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yu, X.; Feng, J. Iron Homeostasis Disorder in Piglet Intestine. Metallomics 2020, 12, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhao, Y.; Yu, X.; Chen, L.; Han, J.; Feng, J. Tolerable Upper Intake Level of Iron Damages the Liver of Weaned Piglets. J. Anim. Physiol. Anim. Nutr. 2021, 105, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, X.; Wang, X.; Shao, Y.; Tu, Q.; Yang, H.; Yin, J.; Yin, Y. Responses of Intestinal Microbiota and Immunity to Increasing Dietary Levels of Iron Using a Piglet Model. Front. Cell Dev. Biol. 2020, 8, 603392. [Google Scholar] [CrossRef]
- Huang, R.-Q.; Yang, X.-J.; Xie, G.-M.; Li, J.; Jian, Y.-H.; Yang, J.; Zhu, Y.-W. Effects of Dietary Iron Sources on Growth Performance, Iron Status, Fe-Containing Enzyme Activity and Gene Expression Related to Iron Homeostasis in Tissues of Weaned Pigs. Front. Vet. Sci. 2023, 10, 1111257. [Google Scholar] [CrossRef]
- Lee, S.H.; Shinde, P.; Choi, J.; Park, M.; Ohh, S.; Kwon, I.K.; Pak, S.I.; Chae, B.J. Effects of Dietary Iron Levels on Growth Performance, Hematological Status, Liver Mineral Concentration, Fecal Microflora, and Diarrhea Incidence in Weanling Pigs. Biol. Trace Elem. Res. 2008, 126, 57–68. [Google Scholar] [CrossRef]
- Li, Y.; Hansen, S.L.; Borst, L.B.; Spears, J.W.; Moeser, A.J. Dietary Iron Deficiency and Oversupplementation Increase Intestinal Permeability, Ion Transport, and Inflammation in Pigs123. J. Nutr. 2016, 146, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Ashwell, M.S.; Moeser, A.J.; Fry, R.S.; Knutson, M.D.; Spears, J.W. High Dietary Iron Reduces Transporters Involved in Iron and Manganese Metabolism and Increases Intestinal Permeability in Calves. J. Dairy Sci. 2010, 93, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Hrdlickova, R.; Toloue, M.; Tian, B. RNA-Seq Methods for Transcriptome Analysis. WIREs RNA 2017, 8, e1364. [Google Scholar] [CrossRef]
- Lee, J.; Jang, H.; Doo, M.; Kim, B.-H.; Ha, J.-H. High Iron Consumption Modifies the Hepatic Transcriptome Related to Cholesterol Metabolism. J. Med. Food 2024. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.; Kaur, G.; Joon, R.; Shukla, V.; Choudhary, P.; Roy, J.K.; Singh, B.; Pandey, A.K. Transcriptome and Biochemical Analysis in Hexaploid Wheat with Contrasting Tolerance to Iron Deficiency Pinpoints Multi-Layered Molecular Process. Plant Physiol. Biochem. 2024, 207, 108336. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, Y.; Liu, W.; Ou, X.; Zhang, W.; Wang, J.; Chang, Y.; Wang, F.; Gao, M.; Liu, S. Zonated Iron Deposition in the Periportal Zone of the Liver Is Associated with Selectively Enhanced Lipid Synthesis. Liver Int. 2024, 44, 589–602. [Google Scholar] [CrossRef]
- Wang, R.-L.; Biales, A.; Bencic, D.; Lattier, D.; Kostich, M.; Villeneuve, D.; Ankley, G.T.; Lazorchak, J.; Toth, G. DNA Microarray Application in Ecotoxicology: Experimental Design, Microarray Scanning, and Factors Affecting Transcriptional Profiles in a Small Fish Species. Environ. Toxicol. Chem. 2008, 27, 652–663. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R Package for Identifying Differentially Expressed Genes from RNA-Seq Data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Han, Y.; Gao, S.; Muegge, K.; Zhang, W.; Zhou, B. Advanced Applications of RNA Sequencing and Challenges. Bioinform. Biol. Insights 2015, 9 (Suppl. S1), 29–46. [Google Scholar] [CrossRef]
- Nookaew, I.; Papini, M.; Pornputtapong, N.; Scalcinati, G.; Fagerberg, L.; Uhlén, M.; Nielsen, J. A Comprehensive Comparison of RNA-Seq-Based Transcriptome Analysis from Reads to Differential Gene Expression and Cross-Comparison with Microarrays: A Case Study in Saccharomyces Cerevisiae. Nucleic Acids Res. 2012, 40, 10084–10097. [Google Scholar] [CrossRef]
- Adiconis, X.; Borges-Rivera, D.; Satija, R.; DeLuca, D.S.; Busby, M.A.; Berlin, A.M.; Sivachenko, A.; Thompson, D.A.; Wysoker, A.; Fennell, T.; et al. Comparative Analysis of RNA Sequencing Methods for Degraded or Low-Input Samples. Nat. Methods 2013, 10, 623–629. [Google Scholar] [CrossRef]
- Li, S.; Tighe, S.W.; Nicolet, C.M.; Grove, D.; Levy, S.; Farmerie, W.; Viale, A.; Wright, C.; Schweitzer, P.A.; Gao, Y.; et al. Multi-Platform Assessment of Transcriptome Profiling Using RNA-Seq in the ABRF next-Generation Sequencing Study. Nat. Biotechnol. 2014, 32, 915–925. [Google Scholar] [CrossRef]
- Van Dijk, E.L.; Jaszczyszyn, Y.; Thermes, C. Library Preparation Methods for Next-Generation Sequencing: Tone down the Bias. Exp. Cell Res. 2014, 322, 12–20. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.; An, Q.; Pan, H.; Chen, K.; Guo, R. The Study of Hypoxia Adaptive Differences of Yunnan Wujin and Yuedawu Pigs. Acta Vet. Zootech. Sin. 2015, 46, 965–973. [Google Scholar]
- Wang, Z.; Guo, F. Research Progress of Wujin Pig. Heilongjiang Anim. Husb. Vet. 2017, 19, 90–92. [Google Scholar]
- Li, X.; Niu, Y.; Long, G.; Li, J.; Zhang, X.; Li, L.; Qiu, Z.; Wang, W.; Long, Y. Study on Breeding of Dahe Black Pig II. Swine Prod. 2019, 1, 57–62. [Google Scholar]
- Che, L.; Li, C.; Xu, N.; Zhang, C.; Liu, S.; An, Q.; Guo, R. Effects of Lactoferrin on Reproductive Performance of Dahe Black Sows and Iron Nutrition, Blood Cell Parameters, Immune Indicators, and Porcine β—Defensin Gene Expression of Sows and Piglets. Chin. J. Anim. Nutr. 2020, 32, 3089–3098. [Google Scholar]
- De Lange, C.F.M. New NRC (2012) Nutrient Requirements of Swine. Adv. Pork Prod. 2013, 24, 17–28. [Google Scholar]
- GB/T 39235-2020; Nutrient Requirements of Swine. China Livestock Standardization Technical Committee: Beijing, China, 2020.
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript Assembly and Quantification by RNA-Seq Reveals Unannotated Transcripts and Isoform Switching during Cell Differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rincker, M.J.; Clarke, S.L.; Eisenstein, R.S.; Link, J.E.; Hill, G.M. Effects of Iron Supplementation on Binding Activity of Iron Regulatory Proteins and the Subsequent Effect on Growth Performance and Indices of Hematological and Mineral Status of Young Pigs1,2. J. Anim. Sci. 2005, 83, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Vaňhara, J.; Berlinská, J. Parenteral Iron Administration in Suckling Piglets—A Review. Acta Vet. Brno 2017, 86, 249–261. [Google Scholar] [CrossRef]
- Svoboda, M.; Mikulková, K. Oral Iron Administration in Suckling Piglets—A Review. Acta Vet. Brno 2018, 87, 77–83. [Google Scholar] [CrossRef]
- Szudzik, M.; Lipiński, P.; Jończy, A.; Mazgaj, R.; Pieszka, M.; Kamyczek, M.; Smuda, E.; Starzyński, R.R. Long-Term Effect of Split Iron Dextran/Hemoglobin Supplementation on Erythrocyte and Iron Status, Growth Performance, Carcass Parameters, and Meat Quality of Polish Large White and 990 Line Pigs. Biol. Trace Elem. Res. 2020, 196, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.W. Erythropoietin: Physiologic and Pharmacologic Aspects. Proc. Soc. Exp. Biol. Med. 1997, 216, 358–369. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J. Effects of Hypoxia on Expression of Erythropoietin and Hypoxia Induced-Factor 1α Gene in Rat Liver and Kidney. Chin. Crit. Care Emerg. Med. 2004, 16, 9–12. [Google Scholar]
- Westermann, A.J.; Vogel, J. Cross-Species RNA-Seq for Deciphering Host–Microbe Interactions. Nat. Rev. Genet. 2021, 22, 361–378. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Terpilowska, S. Chapter 11—Pro- and Antioxidant Activity of Chromium(III), Iron(III), Molybdenum(III), or Nickel(II). In Toxicology; Patel, V.B., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 99–106. ISBN 978-0-12-819092-0. [Google Scholar]
- Yiu, W.H.; Wong, D.W.L.; Wu, H.J.; Li, R.X.; Yam, I.; Chan, L.Y.Y.; Leung, J.C.K.; Lan, H.Y.; Lai, K.N.; Tang, S.C.W. Kallistatin Protects against Diabetic Nephropathy in Db/Db Mice by Suppressing AGE-RAGE-Induced Oxidative Stress. Kidney Int. 2016, 89, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, D.; Zheng, M.; Hao, Y.; Hou, L.; Zhang, S. Effect of Pioglitazone on Insulin Resistance in Fructose-Drinking Rats Correlates with AGEs/RAGE Inhibition and Block of NAPDH Oxidase and NF Kappa B Activation. Eur. J. Pharmacol. 2010, 629, 153–158. [Google Scholar] [CrossRef]
- Schleicher, E.; Friess, U. Oxidative Stress, AGE, and Atherosclerosis. Kidney Int. 2007, 72, S17–S26. [Google Scholar] [CrossRef]
- Zeng, B.; Liu, L.; Liao, X.; Zhang, C.; Ruan, H. Thyroid Hormone Protects Cardiomyocytes from H2O2-Induced Oxidative Stress via the PI3K-AKT Signaling Pathway. Exp. Cell Res. 2019, 380, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Zhi, T.; He, W.; Tang, J. Identification of Important Modules and Biomarkers in Breast Cancer Based on WGCNA. OncoTargets Ther. 2020, 13, 6805–6817. [Google Scholar]
- Lin, W.; Wang, Y.; Chen, Y.; Wang, Q.; Gu, Z.; Zhu, Y. Role of Calcium Signaling Pathway-Related Gene Regulatory Networks in Ischemic Stroke Based on Multiple WGCNA and Single-Cell Analysis. Oxidative Med. Cell. Longev. 2021, 2021, 8060477. [Google Scholar] [CrossRef]
- Han, Y.; Wu, J.; Gong, Z.; Zhou, Y.; Li, H.; Wang, B.; Qian, Q. Identification and Development of a Novel 5-Gene Diagnostic Model Based on Immune Infiltration Analysis of Osteoarthritis. J. Transl. Med. 2021, 19, 522. [Google Scholar] [CrossRef]
- Chen, X.; Ma, J. Weighted Gene Co-Expression Network Analysis (WGCNA) to Explore Genes Responsive to Streptococcus Oralis Biofilm and Immune Infiltration Analysis in Human Gingival Fibroblasts Cells. Bioengineered 2021, 12, 1054–1065. [Google Scholar] [CrossRef]
- Fanalli, S.L.; Gomes, J.D.; de Novais, F.J.; Gervásio, I.C.; Fukumasu, H.; Moreira, G.C.M.; Coutinho, L.L.; Koltes, J.; Amaral, A.J.; Cesar, A.S.M. Key Co-Expressed Genes Correlated with Blood Serum Parameters of Pigs Fed with Different Fatty Acid Profile Diets. Front. Genet. 2024, 15, 1394971. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Betancor, M.B.; McMillan, S.; Norambuena, F.; Tocher, D.R.; Douglas, A.; Martin, S.A.M. Modulation of Metabolic and Immunoregulatory Pathways in the Gut Transcriptome of Atlantic Salmon (Salmo salar L.) after Early Nutritional Programming during First Feeding with Plant-Based Diet. Front. Immunol. 2024, 15, 1412821. [Google Scholar] [CrossRef] [PubMed]
- Hyun Seung Ban, Y.U.; Nakamura, H. Hypoxia-Inducible Factor (HIF) Inhibitors: A Patent Survey (2016–2020). Expert Opin. Ther. Pat. 2021, 31, 387–397. [Google Scholar] [CrossRef]
- Deji, B.Z.; Shang, P.; Danzeng, W.J.; Zhang, H.; Qiangba, Y.Z. Expression and Hypoxia Adaptation Analysis of the EPO Gene in Different Tissues of Plateau Tibetan Pigs. Genet. Mol. Res. 2015, 14, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, C.; Fandrey, J. Hypoxia-induced erythropoietin production: A paradigm for oxygen-regulated gene expression. Clin. Exp. Pharmacol. Physiol. 2006, 33, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, H. Study on Erythropoietin Expression and Hypoxia Adaptation in Tibetan Pigs. Hubei Prov. Anim. Husb. Vet. 2016, 37, 8–9. [Google Scholar]
- Silva, M.; de Brito Magalhães, C.L.; de Paula Oliveira, R.; Silva, M.E.; Pedrosa, M.L. Differential Expression of Iron Metabolism Proteins in Diabetic and Diabetic Iron-Supplemented Rat Liver. J. Biochem. Mol. Toxicol. 2012, 26, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Qian, Z.M.; Gong, Q.; Zhu, Z.J.; Lu, L.; Ke, Y. The Iron Regulatory Hormone Hepcidin Inhibits Expression of Iron Release as Well as Iron Uptake Proteins in J774 Cells. J. Nutr. Biochem. 2012, 23, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver. J. Biol. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef]
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.; Barut, B.; Zapata, A.; et al. Positional Cloning of Zebrafish Ferroportin1 Identifies a Conserved Vertebrate Iron Exporter. Nature 2000, 403, 776–781. [Google Scholar] [CrossRef]
- Abboud, S.; Haile, D.J. A Novel Mammalian Iron-Regulated Protein Involved in Intracellular Iron Metabolism. J. Biol. Chem. 2000, 275, 19906–19912. [Google Scholar] [CrossRef] [PubMed]
- McKie, A.T.; Marciani, P.; Rolfs, A.; Brennan, K.; Wehr, K.; Barrow, D.; Miret, S.; Bomford, A.; Peters, T.J.; Farzaneh, F.; et al. A Novel Duodenal Iron-Regulated Transporter, IREG1, Implicated in the Basolateral Transfer of Iron to the Circulation. Mol. Cell 2000, 5, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Nemeth, E. Manipulation of the Hepcidin Pathway for Therapeutic Purposes. Haematologica 2013, 98, 1667–1676. [Google Scholar] [CrossRef]
- Xiao, J.J.; Krzyzanski, W.; Wang, Y.-M.; Li, H.; Rose, M.J.; Ma, M.; Wu, Y.; Hinkle, B.; Perez-Ruixo, J.J. Pharmacokinetics of Anti-Hepcidin Monoclonal Antibody Ab 12B9m and Hepcidin in Cynomolgus Monkeys. AAPS J. 2010, 12, 646–657. [Google Scholar] [CrossRef] [PubMed]




| Ingredient | Content (%) | Nutrient Level 2 | |
|---|---|---|---|
| Corn | 30.0 | CP (%) | 19.0 |
| Soybean meal | 15.0 | Ca (%) | 0.80 |
| Expanded soybean | 15.0 | Fe (mg/kg) | 76.8 |
| Whey powder | 12.0 | AP (%) | 0.42 |
| Rice | 10.0 | Lys (%) | 1.22 |
| Flour | 6.0 | Met (%) | 0.39 |
| Glucose | 4.0 | Methionine + cystine (%) | 0.67 |
| Peruvian fish meal | 3.0 | Thr (%) | 0.77 |
| Citric acid | 1.50 | Trp (%) | 0.23 |
| Vegetable oil | 1.00 | DE (MJ/kg) | 13.38 |
| Fine stone powder | 0.84 | ||
| Calcium hydrogen Phosphate | 0.81 | ||
| Lysine | 0.18 | ||
| Fungicide | 0.10 | ||
| DL-methionine | 0.08 | ||
| Premix 1 | 0.49 |
| Genes | Accession | Primer Sequence (5′-3′) | Amplification Length/bp |
|---|---|---|---|
| HEPC | NM_214117 | F: AGAGGCTAAGAAGAGACA | 76 |
| R: ATCCCACAGATTGCTTTG | |||
| EPO | NM_214134 | F: TGCAGCTTCAGTGAGAATATC | 75 |
| R: CTGGACCTCCATCCTCTT | |||
| HIF-1α | NM_001123124 | F: TACAACATCACCATACAG | 118 |
| R: GACAGATAACACATTAGGA | |||
| TFRC | NM_214001 | F: CACAAGGAGTAACCACAT | 117 |
| R: GCCAAGTAGCCAATCATA | |||
| ACTB | XM_003124280.5 | F: GCGGCATCCACGAAACTA | 75 |
| R: TGTTGGCGTAGAGGTCCTT |
| Item | Low-Iron Group | Control Group | High-Iron Group |
|---|---|---|---|
| IBW (kg) | 15.21 ± 1.81 | 15.1 ± 1.93 | 15.56 ± 1.70 |
| FBW (kg) | 27.43 ± 5.33 | 28.79 ± 4.57 | 28.56 ± 3.06 |
| ADG (kg/d) | 0.38 ± 0.11 | 0.43 ± 0.10 | 0.41 ± 0.08 |
| ADFI (kg/d) | 1.04 ± 0.24 | 1.04 ± 0.20 | 1.00 ± 0.17 |
| F/D | 2.81 ± 0.45 | 2.48 ± 0.36 | 2.50 ± 0.25 |
| Item | Low-Iron Group | Control Group | High-Iron Group |
|---|---|---|---|
| Serum iron content (mg/L) | 19.79 ± 2.35 | 18.51 ± 1.33 | 16.17 ± 1.88 |
| TIBC (µmol/L) | 135.96 ± 6.62 | 143.63 ± 6.80 | 142.19 ± 9.46 |
| TfR (nmol/L) | 16.01 ± 0.68 | 16.65 ± 0.27 | 17.15 ± 0.69 |
| HB (µg/mL) | 63.42 ± 6.71 | 61.41 ± 1.79 | 58.33 ± 4.44 |
| HEPC (ng/mL) | 1135.86 ± 54.75 | 1179.33 ± 22.76 | 1108.57 ± 30.38 |
| HIF-1 (pg/mL) | 447.67 ± 37.95 a | 394.5 ± 13.87 ab | 370.52 ± 16.31 b |
| EPO (mIU/mL) | 4.98 ± 0.29 | 4.89 ± 0.10 | 4.51 ± 0.19 |
| Samples | Clean Reads | Clean Bases | GC Content (%) | % ≥ Q30 (%) | Total Reads | Mapped Reads | Mapping (%) |
|---|---|---|---|---|---|---|---|
| WGZA2 | 19,088,882 | 5,715,504,106 | 49.33% | 94.05% | 38,177,764 | 36,841,761 | 96.50% |
| WGZA3 | 22,129,212 | 6,623,511,384 | 49.78% | 93.17% | 44,258,424 | 42,654,731 | 96.38% |
| WGZA7 | 19,728,590 | 5,898,720,736 | 49.43% | 94.35% | 39,457,180 | 38,023,783 | 96.37% |
| WGZB4 | 19,980,454 | 5,981,164,218 | 49.48% | 94.53% | 39,960,908 | 38,591,996 | 96.57% |
| WGZB5 | 20,097,375 | 6,016,690,334 | 48.62% | 93.81% | 40,194,750 | 38,823,158 | 96.59% |
| WGZB6 | 19,062,074 | 5,707,294,314 | 49.53% | 93.77% | 38,124,148 | 36,698,850 | 96.26% |
| WGZC2 | 26,959,352 | 8,070,051,376 | 49.73% | 93.78% | 53,918,704 | 52,099,313 | 96.63% |
| WGZC7 | 20,765,796 | 6,210,772,130 | 49.66% | 94.17% | 41,531,592 | 39,963,275 | 96.22% |
| WGZC8 | 23,796,379 | 7,123,213,814 | 49.34% | 95.17% | 47,592,758 | 46,181,559 | 97.03% |
| DEG Set | DEG Number | Upregulated | Downregulated |
|---|---|---|---|
| Low iron group _vs._ Control group | 155 | 78 | 77 |
| High iron group _vs._ Control group | 229 | 164 | 65 |
| High iron group _vs._ Low iron group | 279 | 165 | 114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Xing, X.; Guo, R.; Li, Q.; Xu, Y.; Pan, H.; Ji, P.; Wang, P.; Yu, C.; Li, J.; et al. Effect of Different Dietary Iron Contents on Liver Transcriptome Characteristics in Wujin Pigs. Animals 2024, 14, 2399. https://doi.org/10.3390/ani14162399
Gao L, Xing X, Guo R, Li Q, Xu Y, Pan H, Ji P, Wang P, Yu C, Li J, et al. Effect of Different Dietary Iron Contents on Liver Transcriptome Characteristics in Wujin Pigs. Animals. 2024; 14(16):2399. https://doi.org/10.3390/ani14162399
Chicago/Turabian StyleGao, Lin, Xiaokun Xing, Rongfu Guo, Qihua Li, Yan Xu, Hongbin Pan, Peng Ji, Ping Wang, Chuntang Yu, Jintao Li, and et al. 2024. "Effect of Different Dietary Iron Contents on Liver Transcriptome Characteristics in Wujin Pigs" Animals 14, no. 16: 2399. https://doi.org/10.3390/ani14162399





