Effect of Dietary Restriction on Gut Microbiota and Brain–Gut Short Neuropeptide F in Mud Crab, Scylla paramamosain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. High-Throughput Sequencing and Bioinformatic Analysis
2.3. RNA Extraction and cDNA Synthesis
2.4. Whole-Mount Immunohistochemistry
2.5. Expression Profile of sNPF in Different Feeding Conditions
2.6. Statistical Analyses
3. Result
3.1. Summary of Sequencing
3.2. Richness and Diversity Analysis
3.3. Microbiome Composition
3.4. Localization of sNPF in the Brain and Midgut
3.5. Effects of Different Feeding Conditions on sNPF Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Namrata, S.; Sanjay, N.; Pallavi, C. Effect of starvation on the biochemical composition of freshwater fish Channa punctatus. Recent Res. Sci. Technol. 2011, 3, 17–19. [Google Scholar]
- Davis, K.B.; Gaylord, T.G. Effect of fasting on body composition and responses to stress in sunshine bass. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 30–36. [Google Scholar] [PubMed]
- Okada, T.; Fukuda, S.; Hase, K.; Nishiumi, S.; Izumi, Y.; Yoshida, M.; Hagiwara, T.; Kawashima, R.; Yamazaki, M.; Oshio, T.; et al. Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nat. Commun. 2013, 4, 1654. [Google Scholar]
- Tran, N.T.; Xiong, F.; Hao, Y.T.; Zhang, J.; Wu, S.G.; Wang, G.T. Starvation influences the microbiota assembly and expression of immunity-related genes in the intestine of grass carp (Ctenopharyngodon idellus). Aquaculture 2018, 489, 121–129. [Google Scholar]
- Sakyi, M.E.; Cai, J.; Tang, J.F.; Xia, L.; Li, P.; Abarike, E.D.; Kuebutornye, F.K.; Jian, J. Short term starvation and re-feeding in Nile tilapia (Oreochromis niloticus, Linnaeus 1758): Growth measurements, and immune responses. Aqua. Rep. 2020, 16, 100261. [Google Scholar]
- Dai, W.F.; Zhang, J.J.; Qiu, Q.F.; Chen, J.; Yang, W.; Ni, S.; Xiong, J.B. Starvation stress affects the interplay among shrimp gut microbiota, digestion and immune activities. Fish Shellfish Immunol. 2018, 80, 191–199. [Google Scholar] [PubMed]
- Foysal, M.J.; Fotedar, R.; Tay, A.C.Y.; Gupta, S.K. Effects of long-term starvation on health indices, gut microbiota and innate immune response of fresh water crayfish, marron Cherax cainii, Austin 2002. Aquaculture 2020, 514, 734444. [Google Scholar]
- Feng, Q.; Chen, W.D.; Wang, Y.D. Gut microbiota: An integral moderator in health and disease. Front. Microbiol. 2018, 9, 151. [Google Scholar]
- Sherwin, E.; Rea, K.; Dinan, T.G.; Cryan, J.F. A gut microbiome feeling about the brain. Curr. Opin. Gastroenterol. 2016, 32, 96–102. [Google Scholar]
- Shen, R.; Wang, B.; Giribaldi, M.G.; Ayres, J.; Thomas, J.B.; Montminy, M. Neuronal energy-sensing pathway promotes energy balance by modulating disease tolerance. Proc. Natl. Acad. Sci. USA 2016, 113, E3307–E3314. [Google Scholar]
- Mikani, A.; Wang, Q.S.; Takeda, M. Brain-midgut short neuropeptide F mechanism that inhibits digestive activity of the American cockroach, Periplaneta americana, upon starvation. Peptides 2012, 34, 135–144. [Google Scholar]
- Zhang, P.; Wu, X.; Liang, S.; Shao, X.; Wang, Q.; Chen, R.; Zhu, W.; Shao, C.; Jin, F.; Jia, C. A dynamic mouse peptidome landscape reveals probiotic modulation of the gut-brain axis. Sci. Signal. 2020, 13, eabb0443. [Google Scholar]
- Xu, Z.; Wei, Y.; Guo, S.; Lin, D.; Ye, H. Short neuropeptide F enhances the immune response in the hepatopancreas of mud crab (Scylla paramamosain). Fish Shellfish Immunol. 2020, 101, 244–251. [Google Scholar] [PubMed]
- Bao, C.C.; Yang, Y.Y.; Huang, H.Y.; Ye, H.H. Inhibitory role of the mud crab short neuropeptide F in vitellogenesis and oocyte maturation via autocrine/paracrine signaling. Front. Endocrinol. 2018, 9, 390. [Google Scholar]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2014; pp. 195–219. [Google Scholar]
- Wei, H.; Wang, H.; Tang, L.; Mu, C.; Ye, C.; Chen, L.; Wang, C. High-throughput sequencing reveals the core gut microbiota of the mud crab Scylla paramamosain in different coastal regions of southern China. BMC Genom. 2019, 20, 829. [Google Scholar]
- Brown, M.R.; Crim, J.W.; Arata, R.C.; Cai, H.N.; Chun, C.; Shen, P. Identification of a Drosophila brain-gut peptide related to the neuropeptide Y family. Peptides 1999, 20, 1035–1042. [Google Scholar] [PubMed]
- Ren, G.R.; Hauser, F.; Rewitz, K.F.; Kondo, S.; Engelbrecht, A.F.; Didriksen, A.K.; Schjøtt, S.R.; Sembach, F.E.; Li, S.; Søgaard, K.C.; et al. CCHamide-2 is an orexigenic brain-gut peptide in Drosophila. PLoS ONE 2015, 10, e0133017. [Google Scholar]
- Wu, K.; Li, S.; Wang, J.; Ni, Y.; Huang, W.; Liu, Q.; Ling, E. Peptide hormones in the insect midgut. Front. Physiol. 2020, 11, 191. [Google Scholar]
- Wegener, C.; Veenstra, J.A. Chemical identity, function and regulation of enteroendocrine peptides in insects. Curr. Opin. Insect Sci. 2015, 11, 8–13. [Google Scholar]
- Root, C.M.; Ko, K.I.; Jafari, A.; Wang, J.W. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 2011, 145, 133–144. [Google Scholar]
- Jiang, H.B.; Gui, S.H.; Xu, L.; Pei, Y.X.; Smagghe, G.; Wang, J.J. The short neuropeptide F modulates olfactory sensitivity of Bactrocera dorsalis upon starvation. J. Insect Physiol. 2017, 99, 78–85. [Google Scholar] [PubMed]
- Reiher, W.; Shirras, C.; Kahnt, J.; Baumeister, S.; Isaac, R.E.; Wegener, C. Peptidomics and peptide hormone processing in the Drosophila midgut. J. Proteome Res. 2011, 10, 1881–1892. [Google Scholar]
- Ye, H.; Tao, Y.; Wang, G.; Lin, Q.; Chen, X.; Li, S. Experimental nursery culture of the mud crab Scylla paramamosain Estampador in China. Aquacult. Int. 2011, 19, 313–321. [Google Scholar]
- Li, S.J. Effects of starvation on survival and development of Zoea-1 larvae of the mud crab, Scylla serrata. J. Xiamen Univ. 2001, 40, 788–790. [Google Scholar]
- Liu, D.; Wang, F.; Yang, C.; Hu, N.; Sun, Y. Starvation and a conspecific competitor influence multiple predator effects in a swimming crab Portunus trituberculatus-Manila clam Ruditapes philippinarum foraging system. J. Exp. Mar. Biol. Ecol. 2017, 495, 35–42. [Google Scholar]
- Sun, Y.; Wang, F.; Dong, S. A comparative study of the effect of starvation regimes on the foraging behavior of Portunus trituberculatus and Charybdis japonica. Physiol. Behav. 2015, 151, 168–177. [Google Scholar] [PubMed]
- Herlemann, D.P.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatic 2014, 30, 2114–2120. [Google Scholar]
- Reyon, D.; Tsai, S.Q.; Khayter, C.; Foden, J.A.; Sander, J.D.; Joung, J.K. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 2012, 30, 460–465. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar]
- Johard, H.A.; Enell, L.E.; Gustafsson, E.; Trifilieff, P.; Veenstra, J.A.; Nässel, D.R. Intrinsic neurons of Drosophila mushroom bodies express short neuropeptide F: Relations to extrinsic neurons expressing different neurotransmitters. J. Comp. Neurol. 2008, 507, 1479–1496. [Google Scholar]
- Wang, M.; Ye, H.; Miao, L.; Li, X. Role of short neuropeptide F in regulating eyestalk neuroendocrine systems in the mud crab Scylla paramamosain. Aquaculture 2022, 560, 738493. [Google Scholar]
- Christie, A.E.; Stemmler, E.A.; Peguero, B.; Messinger, D.I.; Provencher, H.L.; Scheerlinck, P.; Hsu, Y.-W.A.; Guiney, M.E.; de la Iglesia, H.O.; Dickinson, P.S.; et al. Identification, physiological actions, and distribution of VYRKPPFNGSIFamide Val1-SIFamide in the stomatogastric nervous system of the American lobster Homarus americanus. J. Comp. Neurol. 2006, 496, 406–421. [Google Scholar]
- Liu, A.; Liu, F.; Shi, W.; Huang, H.; Wang, G.; Ye, H. C-Type allatostatin and its putative receptor from the mud crab serve an inhibitory role in ovarian development. J. Exp. Biol. 2019, 222, jeb207985. [Google Scholar]
- Hama, H.; Kurokawa, H.; Kawano, H.; Ando, R.; Shimogori, T.; Noda, H.; Fukami, K.; Sakaue-Sawano, A.; Miyawaki, A. Scale: A chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat. Neurosci. 2011, 14, 1481–1488. [Google Scholar]
- Benson, A.K.; Kelly, S.A.; Legge, R.; Ma, F.; Low, S.J.; Kim, J.; Zhang, M.; Oh, P.L.; Nehrenberg, D.; Hua, K.; et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18933–18938. [Google Scholar]
- Dehler, C.E.; Secombes, C.J.; Martin, S.A.M. Environmental and physiological factors shape the gut microbiota of Atlantic salmon parr (Salmo salar L.). Aquaculture 2017, 467, 149–157. [Google Scholar]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar]
- Simon, C.J.; Truong, H.H.; Noble, T.H.; Osborne, S.A.; Wynne, J.W.; Wade, N.M. Microbial biomass, marine invertebrate meals and feed restriction influence the biological and gut microbiota response of shrimp Penaeus monodon. Aquaculture 2020, 520, 734679. [Google Scholar]
- Peter, M.; Lv, H.; Jiang, X.; Liu, Y.; Hur, J.W.; Esbon, M.; Samwel, C.; Superius, E.; Khalfan, Z.; Gao, Y.; et al. Effects of starvation on enzyme activities and intestinal microflora composition in loach (Paramisgurnus dabryanus). Aquat. Rep. 2020, 18, 100467. [Google Scholar]
- Sun, S.; Su, Y.; Yu, H.; Ge, X.; Zhang, C. Starvation affects the intestinal microbiota structure and the expression of inflammatory-related genes of the juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2020, 517, 734764. [Google Scholar]
- Miao, F.; Zhao, Z.; Li, Q.; Song, J.; Wang, Y.; Hu, M. Impact of initial feeding and molting on Tachypleus tridentatus gut microbiota. Curr. Microbiol. 2020, 77, 2847–2858. [Google Scholar]
- Cornejo-Granados, F.; Lopez-Zavala, A.A.; Gallardo-Becerra, L.; Mendoza-Vargas, A.; Sánchez, F.; Vichido, R.; Brieba, L.G.; Viana, M.T.; Sotelo-Mundo, R.R.; Ochoa-Leyva, A. Microbiome of Pacific White leg shrimp reveals differential bacterial community composition between Wild, Aquacultured and AHPND/EMS outbreak conditions. Sci. Rep. 2017, 7, 11783. [Google Scholar]
- Zhao, J.; Wen, X.; Li, S.; Zhu, D.; Li, Y. Effects of dietary lipid levels on growth, feed utilization, body composition and antioxidants of juvenile mud crab Scylla paramamosain Estampador. Aquaculture 2015, 435, 200–206. [Google Scholar]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill–Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.-Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724. [Google Scholar]
- Xia, J.H.; Lin, G.; Fu, G.H.; Wan, Z.Y.; Lee, M.; Wang, L.; Liu, X.J.; Yue, G.H. The intestinal microbiome of fish under starvation. BMC Genom. 2014, 15, 266. [Google Scholar]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [PubMed]
- Gavriilidou, A.; Gutleben, J.; Versluis, D.; Forgiarini, F.; van Passel, M.W.J.; Ingham, C.J.; Smidt, H.; Sipkema, D. Comparative genomic analysis of Flavobacteriaceae: Insights into carbohydrate metabolism, gliding motility and secondary metabolite biosynthesis. BMC Genom. 2020, 21, 569. [Google Scholar]
- Li, S.H.; Kang, I.; Cho, J.C. Metabolic versatility of the family Halieaceae revealed by the genomics of novel cultured isolates. Microbiol. Spectr. 2023, 11, e03879-22. [Google Scholar]
- Kohl, K.D.; Amaya, J.; Passement, C.A.; Dearing, M.D.; Mccue, M.D. Unique and shared responses of the gut microbiota to prolonged fasting: A comparative study across five classes of vertebrate hosts. FEMS Microbiol. Ecol. 2014, 90, 883–894. [Google Scholar]
- Xiong, J.; Dai, W.; Li, C. Advances, challenges, and directions in shrimp disease control: The guidelines from an ecological perspective. Appl. Microbiol. Biotechnol. 2016, 100, 6947–6954. [Google Scholar]
- Liu, C.; Liu, M.; Wang, Y.; Shi, B.; Pan, D. Insights into the gut microbiota of the freshwater crab Sinopotamon planum across three seasons and its associations with the surrounding aquatic microbiota. Diversity 2023, 15, 519. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Wang, P.; Li, T. Insights into the microbiota of larval and postlarval Pacific white shrimp (Penaeus vannamei) along early developmental stages: A case in pond level. Mol. Genet. Genom. 2020, 295, 1517–1528. [Google Scholar]
- Michl, S.C.; Ratten, J.M.; Beyer, M.; Hasler, M.; LaRoche, J.; Schulz, C. The malleable gut microbiome of juvenile rainbow trout (Oncorhynchus mykiss): Diet-dependent shifts of bacterial community structures. PLoS ONE 2017, 12, e0177735. [Google Scholar]
- Ardissone, S.; Greub, G. The Chlamydia-related Waddlia chondrophila encodes functional type II toxin-antitoxin systems. Appl. Environ. Microbiol. 2024, 90, e00681-23. [Google Scholar]
- Deeg, C.M.; Zimmer, M.M.; George, E.E.; Husnik, F.; Keeling, P.J.; Suttle, C.A. Chromulinavorax destructans, a pathogen of microzooplankton that provides a window into the enigmatic candidate phylum dependentiae. PLoS Pathog. 2019, 15, e1007801. [Google Scholar]
- Wei, P.; Keller, C.; Li, L. Neuropeptides in gut-brain axis and their influence on host immunity and stress. Comput. Struct. Biotechnol. J. 2020, 18, 843–851. [Google Scholar]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [PubMed]
- El Karim, I.A.; Linden, G.J.; Orr, D.F.; Lundy, F.T. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J. Neuroimmunol. 2008, 200, 11–16. [Google Scholar] [PubMed]
- Kim, B.; Kanai, M.I.; Oh, Y.; Kyung, M.; Kim, E.K.; Jang, I.H. Response of the microbiome–gut–brain axis in Drosophila to amino acid deficit. Nature 2021, 593, 570–574. [Google Scholar]
- Nässel, D.R.; Wegener, C. A comparative review of short and long neuropeptide F signaling in invertebrates: Any similarities to vertebrate neuropeptide Y signaling? Peptides 2011, 32, 1335–1355. [Google Scholar] [PubMed]
- Nässel, D.R.; Enell, L.E.; Santos, J.G.; Wegener, C.; Johard, H.A. A large population of diverse neurons in the drosophila central nervous system expresses short neuropeptide F, suggesting multiple distributed peptide functions. BMC Neurosci. 2008, 9, 90. [Google Scholar]
- Harzsch, S.; Krieger, J. Crustacean olfactory systems: A comparative review and a crustacean perspective on olfaction in insects. Prog. Neurobiol. 2018, 161, 23–60. [Google Scholar]
- Sandeman, D.; Sandeman, R.; Derby, C.; Schmidt, M. Morphology of the brain of crayfish, crabs, and spiny lobsters: A common nomenclature for homologous structures. Biol. Bull. 1992, 183, 304–326. [Google Scholar] [PubMed]
- Medla, M.; Daubnerová, I.; Koči, J.; Roller, L.; Slovák, M.; Žitňan, D. Identification and expression of short neuropeptide F and its receptors in the tick Ixodes ricinus. J. Insect Physiol. 2023, 147, 104524. [Google Scholar]
- Li, M.M.; Yang, Q.; Chen, L.H.; Li, Y.Y.; Wu, J.X.; Xu, X.L. Effect of short neuropeptide F signaling on larval feeding in Mythimna separata. Insect Sci. 2023, 31, 417–434. [Google Scholar]

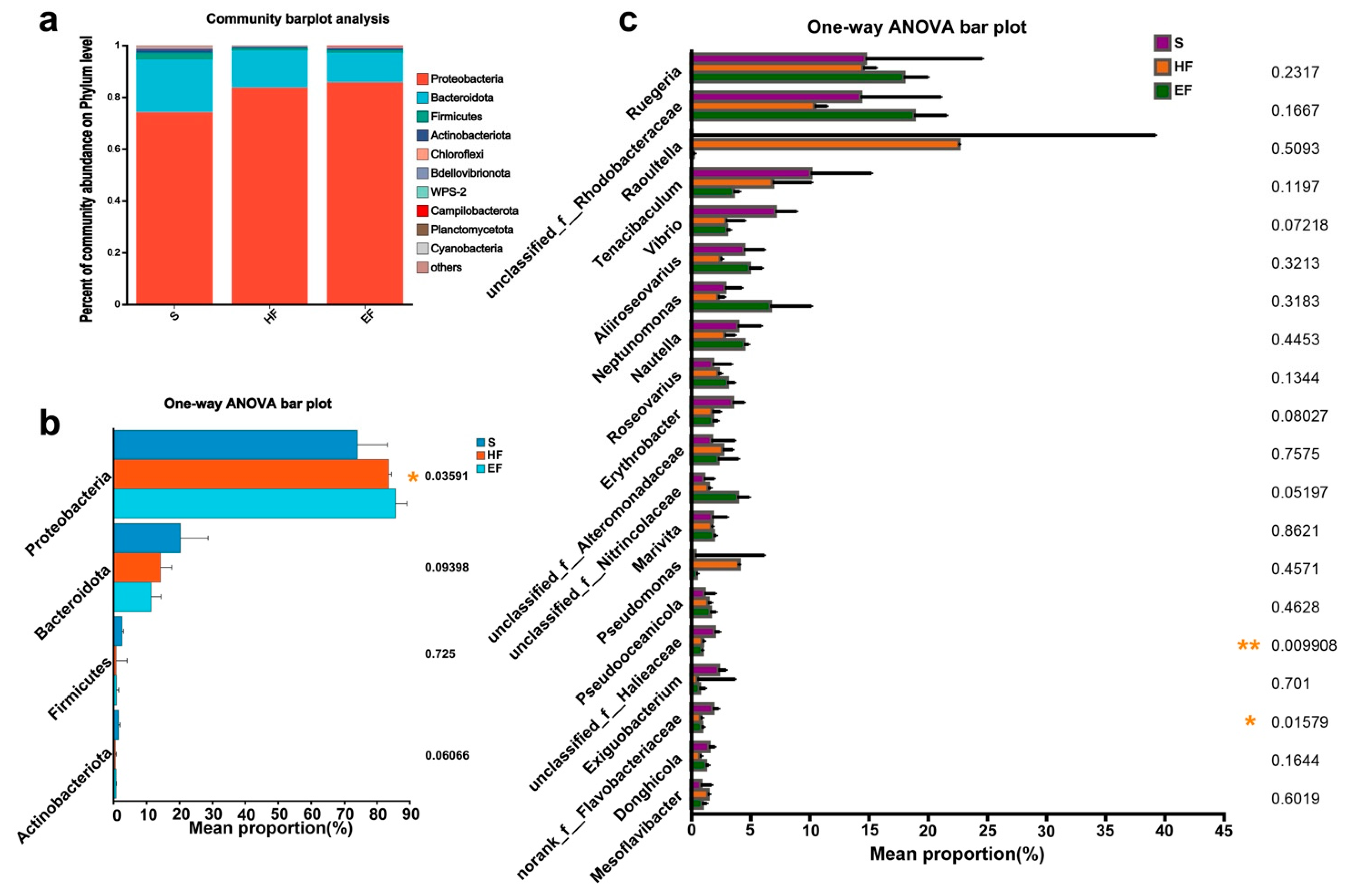

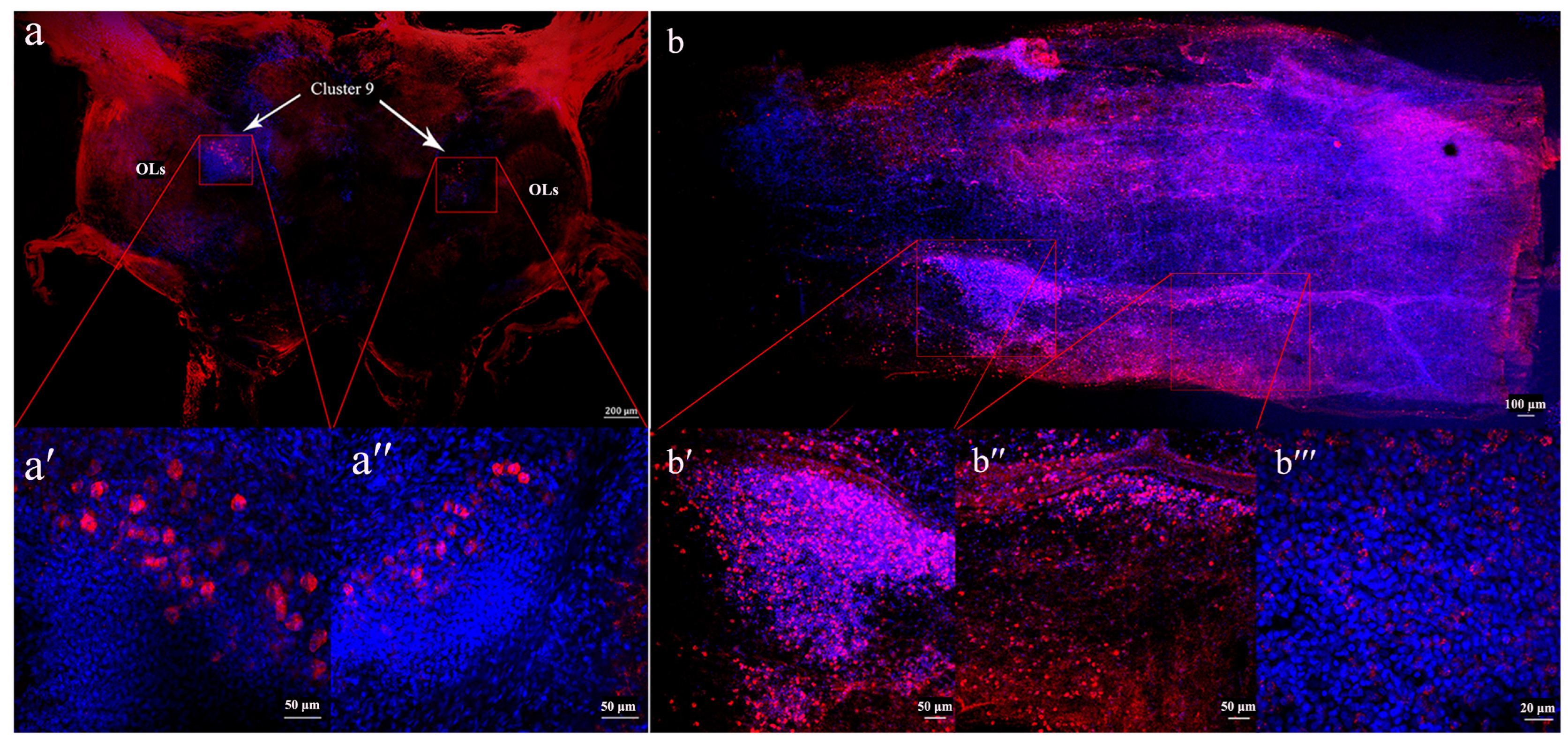
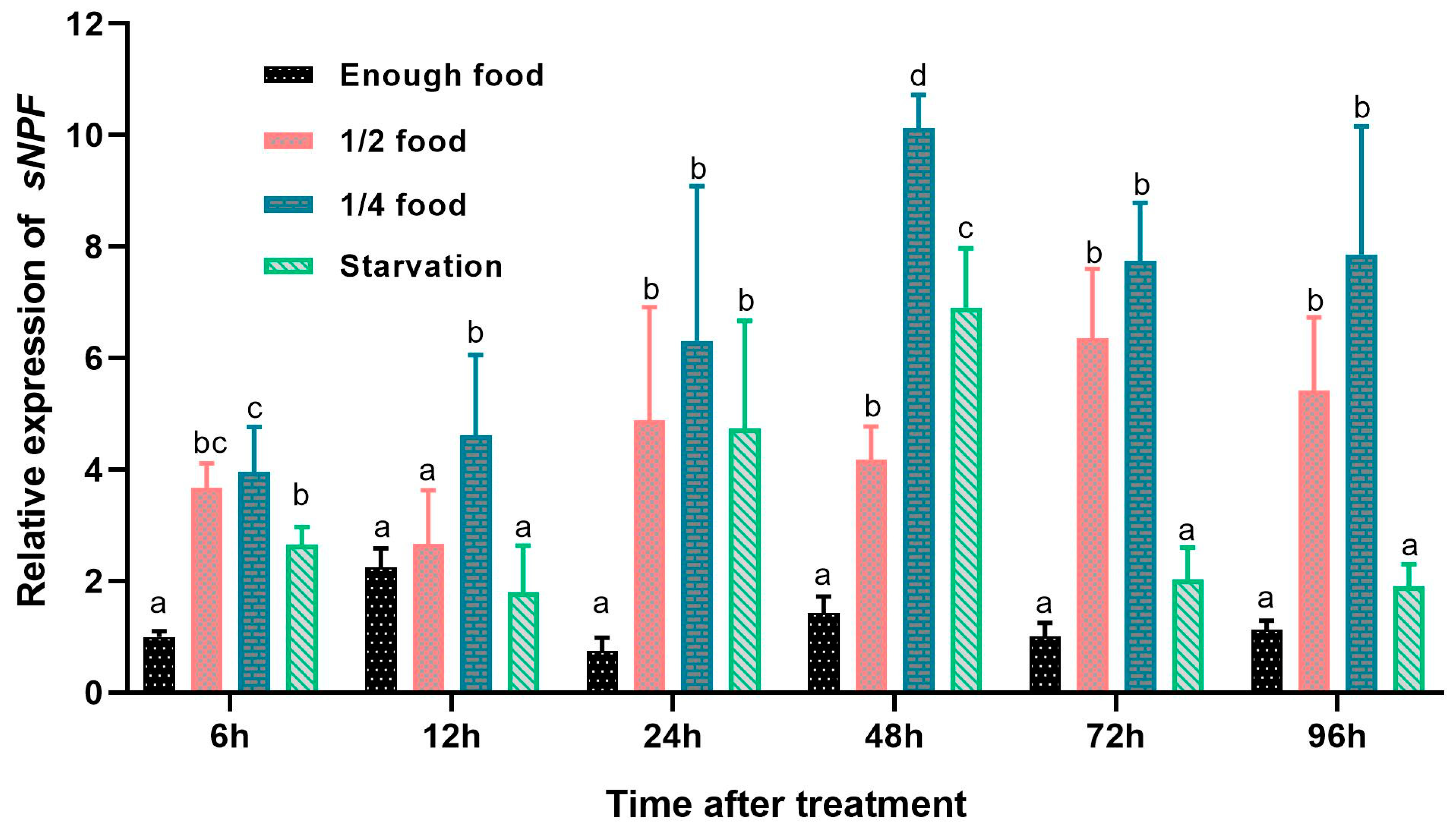
| Sample | Richness Estimators | Diversity Indices | ||
|---|---|---|---|---|
| Chao | ACE | Shannon | Simpson | |
| S | 21.33 ± 1.16 a | 21.59 ± 1.42 a | 0.78 ± 0.07 a | 0.59 ± 0.01 a |
| HF | 18.67 ± 23.51 a | 21.03 ± 1.48 a | 0.52 ± 0.22 a | 0.73 ± 0.13 a |
| EF | 16.67 ± 2.31 a | 16.64 ± 2.29 b | 0.53 ± 0.09 a | 0.75 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, C.; Yang, Y.; Ye, H. Effect of Dietary Restriction on Gut Microbiota and Brain–Gut Short Neuropeptide F in Mud Crab, Scylla paramamosain. Animals 2024, 14, 2415. https://doi.org/10.3390/ani14162415
Bao C, Yang Y, Ye H. Effect of Dietary Restriction on Gut Microbiota and Brain–Gut Short Neuropeptide F in Mud Crab, Scylla paramamosain. Animals. 2024; 14(16):2415. https://doi.org/10.3390/ani14162415
Chicago/Turabian StyleBao, Chenchang, Yanan Yang, and Haihui Ye. 2024. "Effect of Dietary Restriction on Gut Microbiota and Brain–Gut Short Neuropeptide F in Mud Crab, Scylla paramamosain" Animals 14, no. 16: 2415. https://doi.org/10.3390/ani14162415





