Inhibitor of FTO, Rhein, Restrains the Differentiation of Myoblasts and Delays Skeletal Muscle Regeneration
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture and Cell Treatment
2.3. qRT-PCR
2.4. Immunofluorescence
2.5. Western Blotting
2.6. Hematoxylin and Eosin Staining and Tissue Immunofluorescence Staining
2.7. Detect m6A Levels
2.8. Statistical Analysis
3. Results
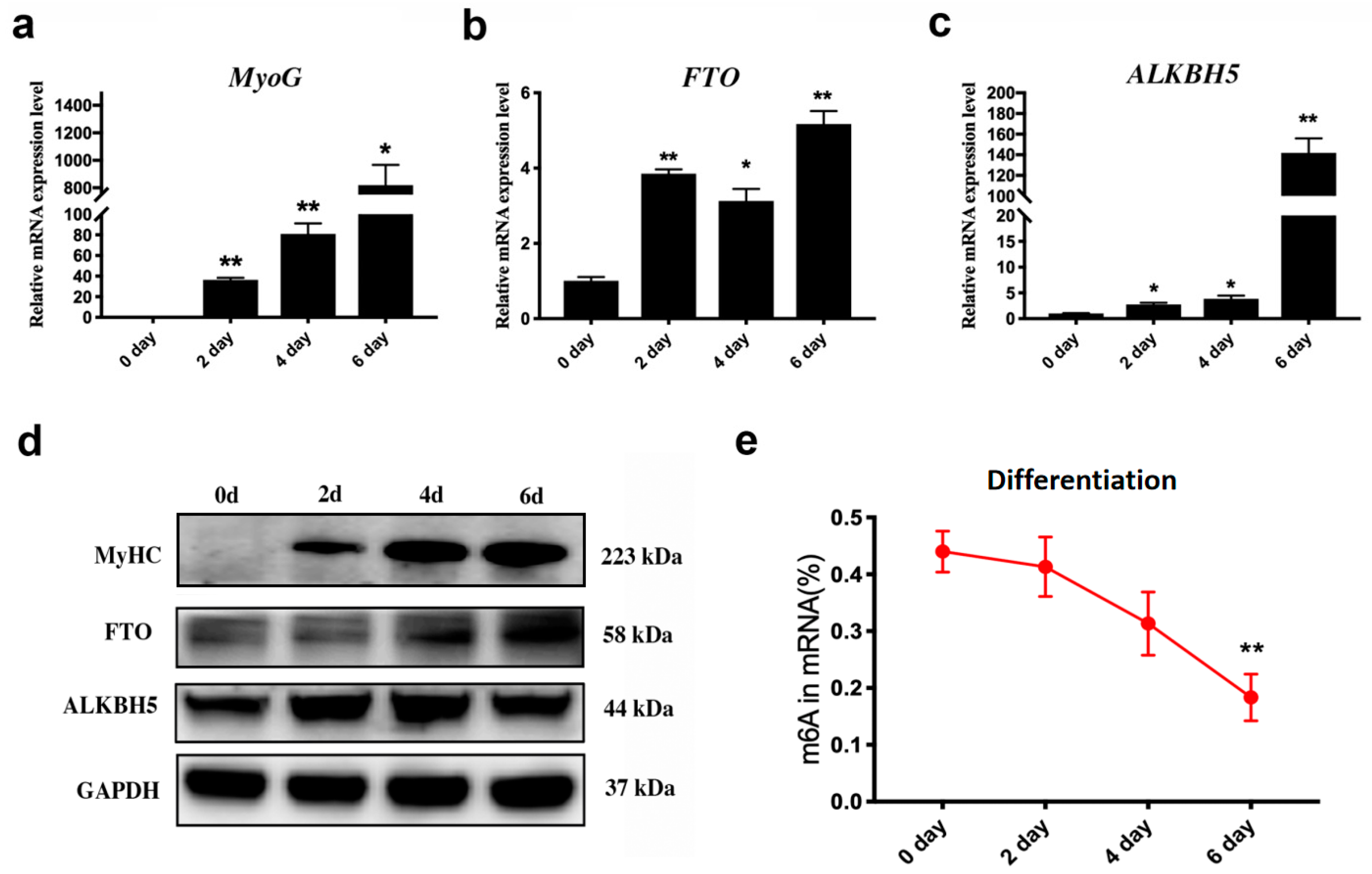
3.1. Dynamic Changes in m6A Erasers and m6A Levels during C2C12 Differentiation
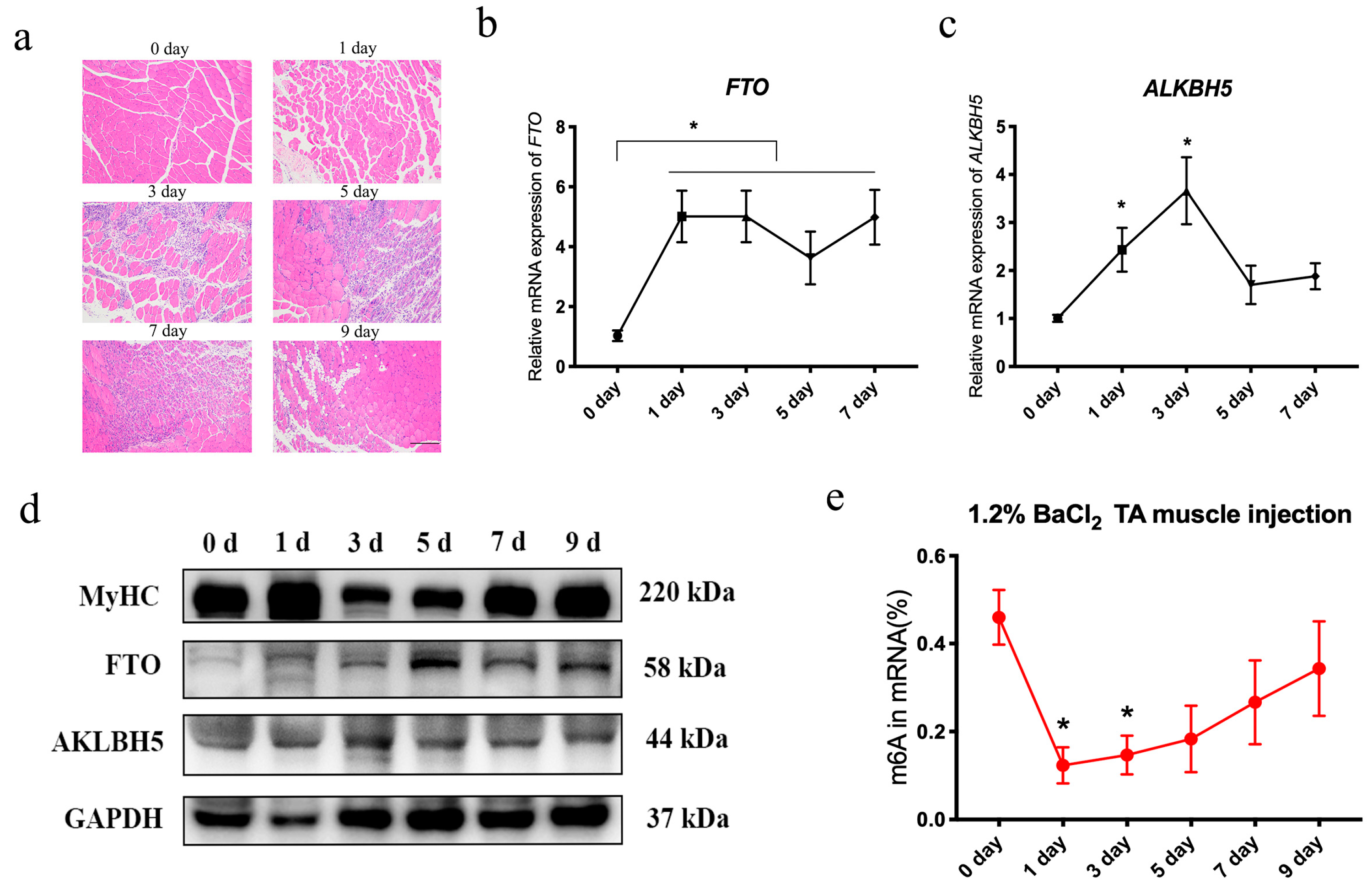
3.2. Dynamic Changes of m6A Erasers, Writers and Readers, and m6A Levels during Skeletal Muscle Injury
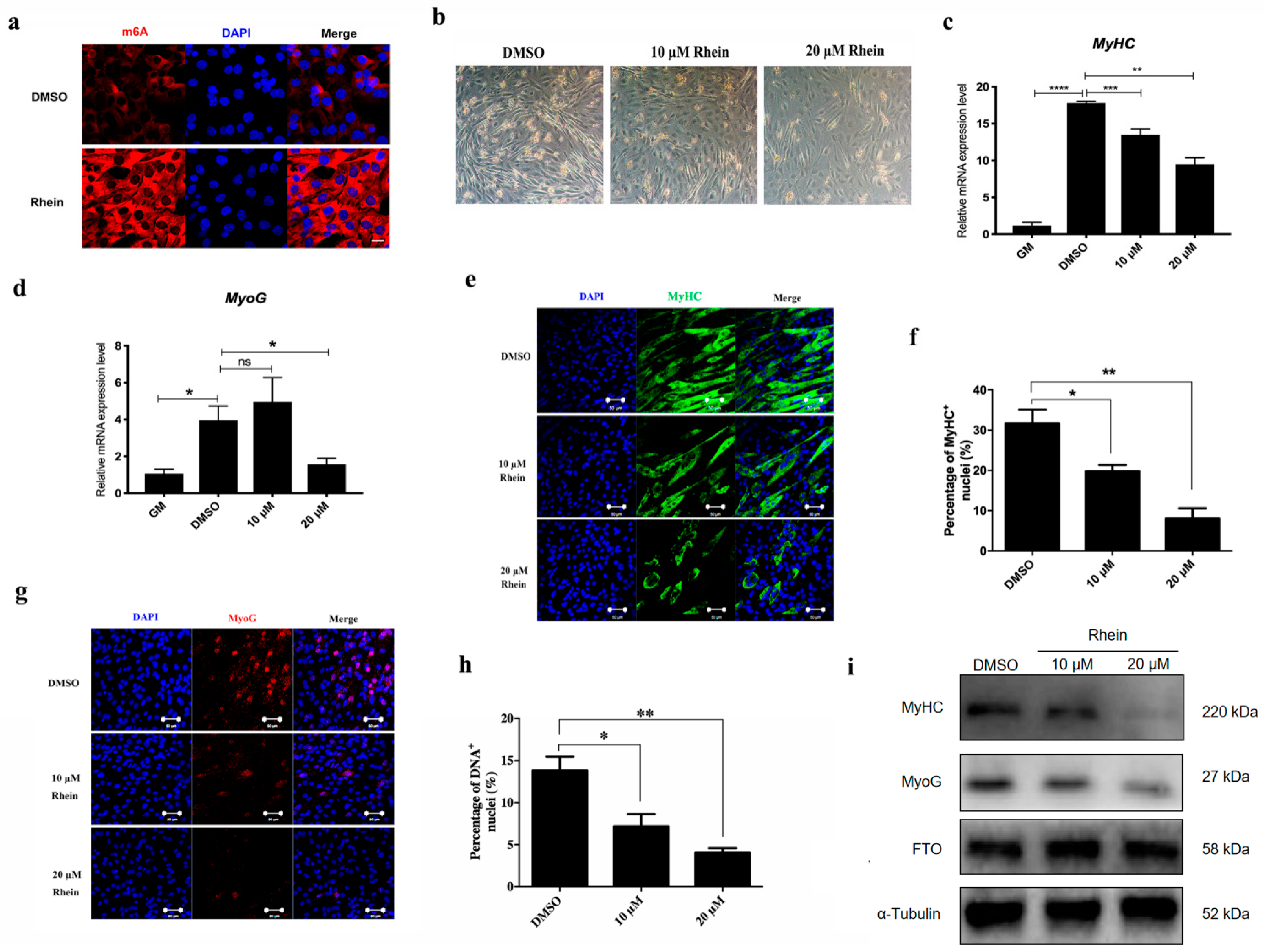
3.3. Increase m6A Level by Inhibiting FTO Inhibit C2C12 Differentiation
3.4. Increase of m6A Level by Inhibiting FTO Delays the Regeneration of Skeletal Muscle after Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, X.; Li, G.; Chen, Y.; Xiao, Q.; Yu, X.; Yu, X.; Lu, X.; Xiang, Z. Pharmacokinetics and Pharmacodynamics of the Combination of Rhein and Curcumin in the Treatment of Chronic Kidney Disease in Rats. Front. Pharmacol. 2020, 11, 573118. [Google Scholar]
- Wu, J.W.; Wei, Z.H.; Cheng, P.; Qian, C.; Xu, F.M.; Yang, Y.; Wang, A.Y.; Chen, W.X.; Sun, Z.G.; Lu, Y. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics 2020, 10, 10665–10679. [Google Scholar] [PubMed]
- Nguyen, A.T.; Kim, K.Y. Rhein inhibits the growth of Propionibacterium acnes by blocking NADH dehydrogenase-2 activity. J. Med. Microbiol. 2020, 69, 689–696. [Google Scholar] [PubMed]
- Barbosa, D.M.; Fahlbusch, P.; Herzfeld de Wiza, D.; Jacob, S.; Kettel, U.; Al-Hasani, H.; Kruger, M.; Ouwens, D.M.; Hartwig, S.; Lehr, S.; et al. Rhein a novel Histone Deacetylase (HDAC) inhibitor with antifibrotic potency in human myocardial fibrosis. Sci. Rep. 2020, 10, 4888. [Google Scholar]
- Carosio, S.; Berardinelli, M.G.; Aucello, M.; Musaro, A. Impact of ageing on muscle cell regeneration. Ageing Res. Rev. 2011, 10, 35–42. [Google Scholar]
- Tonkin, J.; Temmerman, L.; Sampson, R.D.; Gallego-Colon, E.; Barberi, L.; Bilbao, D.; Schneider, M.D.; Musaro, A.; Rosenthal, N. Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization. Mol. Ther. 2015, 23, 1189–1200. [Google Scholar] [PubMed]
- Baht, G.S.; Bareja, A.; Lee, D.E.; Rao, R.R.; Huang, R.; Huebner, J.L.; Bartlett, D.B.; Hart, C.R.; Gibson, J.R.; Lanza, I.R.; et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat. Metab. 2020, 2, 278–289. [Google Scholar]
- Liu, Z.; Zhang, X.; Lei, H.; Lam, N.; Carter, S.; Yockey, O.; Xu, M.; Mendoza, A.; Hernandez, E.R.; Wei, J.S.; et al. CASZ1 induces skeletal muscle and rhabdomyosarcoma differentiation through a feed-forward loop with MYOD and MYOG. Nat. Commun. 2020, 11, 911. [Google Scholar]
- Zhang, X.; Yao, Y.; Han, J.; Yang, Y.; Chen, Y.; Tang, Z.; Gao, F. Longitudinal epitranscriptome profiling reveals the crucial role of N(6)-methyladenosine methylation in porcine prenatal skeletal muscle development. J. Genet. Genom. 2020, 47, 466–476. [Google Scholar]
- Gheller, B.J.; Blum, J.E.; Fong, E.H.H.; Malysheva, O.V.; Cosgrove, B.D.; Thalacker-Mercer, A.E. A defined N6-methyladenosine (m(6)A) profile conferred by METTL3 regulates muscle stem cell/myoblast state transitions. Cell Death Discov. 2020, 6, 95. [Google Scholar]
- Li, X.; Yang, J.; Zhu, Y.; Liu, Y.; Shi, X.; Yang, G. Mouse Maternal High-Fat Intake Dynamically Programmed mRNA m(6)A Modifications in Adipose and Skeletal Muscle Tissues in Offspring. Int. J. Mol. Sci. 2016, 17, 1336. [Google Scholar] [CrossRef]
- Yang, Y.; Hsu, P.J.; Chen, Y.S.; Yang, Y.G. Dynamic transcriptomic m(6)A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [PubMed]
- Mo, B.W.; Li, X.M.; Li, S.M.; Xiao, B.; Yang, J.; Li, H.M. m6A echoes with DNA methylation: Coordinated DNA methylation and gene expression data analysis identified critical m6A genes associated with asthma. Gene 2022, 828, 146457. [Google Scholar]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [PubMed]
- Wang, X.; Huang, N.; Yang, M.; Wei, D.; Tai, H.; Han, X.; Gong, H.; Zhou, J.; Qin, J.; Wei, X.; et al. FTO is required for myogenesis by positively regulating mTOR-PGC-1alpha pathway-mediated mitochondria biogenesis. Cell Death Dis. 2017, 8, e2702. [Google Scholar]
- Wang, X.; Wu, R.; Liu, Y.; Zhao, Y.; Bi, Z.; Yao, Y.; Liu, Q.; Shi, H.; Wang, F.; Wang, Y. m(6)A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy 2020, 16, 1221–1235. [Google Scholar]
- Chen, B.; Ye, F.; Yu, L.; Jia, G.; Huang, X.; Zhang, X.; Peng, S.; Chen, K.; Wang, M.; Gong, S.; et al. Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J. Am. Chem. Soc. 2012, 134, 17963–17971. [Google Scholar]
- Fleming, J.W.; Capel, A.J.; Rimington, R.P.; Wheeler, P.; Leonard, A.N.; Bishop, N.C.; Davies, O.G.; Lewis, M.P. Bioengineered human skeletal muscle capable of functional regeneration. BMC Biol. 2020, 18, 145. [Google Scholar]
- Wang, C.; Yue, F.; Kuang, S. Muscle Histology Characterization Using H&E Staining and Muscle Fiber Type Classification Using Immunofluorescence Staining. Bio Protoc. 2017, 7, e2279. [Google Scholar]
- Ghazi, T.; Nagiah, S.; Chuturgoon, A.A. Fusaric acid induces hepatic global m6A RNA methylation and differential expression of m6A regulatory genes in vivo-a pilot study. Epigenetics 2022, 17, 695–703. [Google Scholar]
- Xiong, X.; Hou, L.; Park, Y.P.; Molinie, B.; Consortium, G.T.; Gregory, R.I.; Kellis, M. Genetic drivers of m(6)A methylation in human brain, lung, heart and muscle. Nat. Genet. 2021, 53, 1156–1165. [Google Scholar] [PubMed]
- Li, J.; Pei, Y.; Zhou, R.; Tang, Z.; Yang, Y. Regulation of RNA N(6)-methyladenosine modification and its emerging roles in skeletal muscle development. Int. J. Biol. Sci. 2021, 17, 1682–1692. [Google Scholar] [PubMed]
- Kudou, K.; Komatsu, T.; Nogami, J.; Maehara, K.; Harada, A.; Saeki, H.; Oki, E.; Maehara, Y.; Ohkawa, Y. The requirement of METTL3-promoted MyoD mRNA maintenance in proliferative myoblasts for skeletal muscle differentiation. Open Biol. 2017, 7, 170119. [Google Scholar]
- Deng, K.P.; Fan, Y.X.; Liang, Y.X.; Cai, Y.; Zhang, G.M.; Deng, M.T.; Wang, Z.B.; Lu, J.W.; Shi, J.F.; Wang, F.; et al. FTO-mediated demethylation of GADD45B promotes myogenesis through the activation of p38 MAPK pathway. Mol. Ther.-Nucleic Acids 2021, 26, 34–48. [Google Scholar] [PubMed]
- Sin, J.; Andres, A.M.; Taylor, D.J.R.; Weston, T.; Hiraumi, Y.; Stotland, A.; Kim, B.J.; Huang, C.Q.; Doran, K.S.; Gottlieb, R.A. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 2016, 12, 369–380. [Google Scholar] [PubMed]
- Liu, Y.T.; Zhou, T.J.; Wang, Q.H.; Fu, R.H.; Zhang, Z.F.; Chen, N.D.; Li, Z.Z.; Gao, G.Y.; Peng, S.L.; Yang, D.Z. A demethylase ALKBH5 drives denervation-induced muscle atrophy by targeting HDAC4 to activate FoxO3 signalling. J. Cachexia Sarcopenia Muscle 2022, 13, 1210–1223. [Google Scholar] [PubMed]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar]
- Heo, S.K.; Noh, E.K.; Kim, J.Y.; Jegal, S.; Jeong, Y.; Cheon, J.; Koh, S.; Baek, J.H.; Min, Y.J.; Choi, Y.; et al. Rhein augments ATRA-induced differentiation of acute promyelocytic leukemia cells. Phytomedicine 2018, 49, 66–74. [Google Scholar]


| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| MyoG | GAGACATCCCCCTATTTCTACCA | GCTCAGTCCGCTCATAGCC |
| FTO | CCGAGTCGTCCGGACTTTAC | GGAAACCACGTCTGTGAGGT |
| ALKBH5 | GACAACTACAAGGCGGGCA | CCAGAGGCATACAGGGAC |
| METTL3 | GGGCACTTGGATTTAAGGAACC | CTTAGGGCCGCTAGAGGTAGG |
| METTL14 | GAGCTGAGAGTGCGGATAGC | GCAGATGTATCATAGGAAGCCC |
| WTAP | ATGGCACGGGATGAGTTAATTC | TTCCCTTAAACCAGTCACATCG |
| YTHDF1 | ACAGTTACCCCTCGATGAGTG | GGTAGTGAGATACGGGATGGGA |
| YTHDF2 | GAGCAGAGACCAAAAGGTCAAG | CTGTGGGCTCAAGTAAGGTTC |
| YTHDF3 | GATCAGCCTATGCCATATCTGAC | CCCCTGGTTGACTAAAAACACC |
| GAPDH | ATCACTGCCACCCAGAAGACT | CATGCCAGTGAGCTTCCCGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Cao, Y.; Wu, W.; Liu, H.; Xu, S. Inhibitor of FTO, Rhein, Restrains the Differentiation of Myoblasts and Delays Skeletal Muscle Regeneration. Animals 2024, 14, 2434. https://doi.org/10.3390/ani14162434
Li R, Cao Y, Wu W, Liu H, Xu S. Inhibitor of FTO, Rhein, Restrains the Differentiation of Myoblasts and Delays Skeletal Muscle Regeneration. Animals. 2024; 14(16):2434. https://doi.org/10.3390/ani14162434
Chicago/Turabian StyleLi, Rongyang, Yan Cao, Wangjun Wu, Honglin Liu, and Shiyong Xu. 2024. "Inhibitor of FTO, Rhein, Restrains the Differentiation of Myoblasts and Delays Skeletal Muscle Regeneration" Animals 14, no. 16: 2434. https://doi.org/10.3390/ani14162434




