Stallion Sperm Freezing with Different Extenders: Role of Antioxidant Activity and Nitric Oxide Production
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Collection and Breeding Care
2.3. Sperm Collection, Dilution, and Shipping
2.4. Sperm Concentration and Kinetics
2.5. Sperm Mitochondrial Membrane Potential (MMP) and Intracellular Hydrogen Peroxide (ROS) Content
2.6. Sperm Freezing
2.7. Sperm Packaging, Freezing, and Thawing
2.8. pH and Osmolarity Evaluation
2.9. Biochemical Analyses
2.10. Statistical Analysis
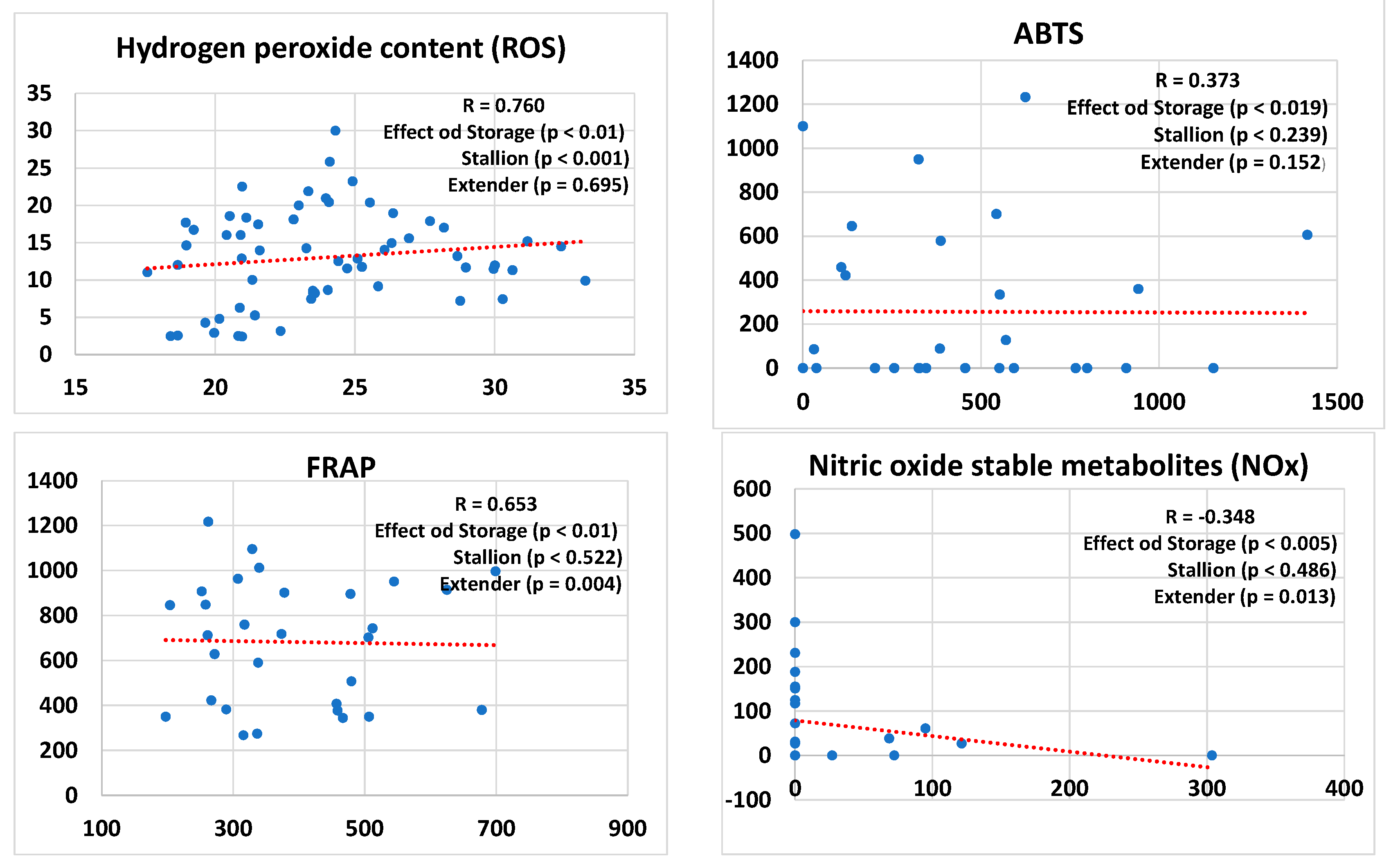
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazur, P. Principles of Cryobiology. In Life in the Frozen State; Fuller, B.J., Lane, N., Benson, E.E., Eds.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Pegg, D.E.; Diaper, M.P. The “unfrozen fraction” hypothesis of freezing injury to human erythrocytes: A critical examination of the evidence. Cryobiology 1989, 26, 30–43. [Google Scholar] [CrossRef]
- Edashige, K. The movement of water and cryoprotectants across the plasma membrane of mammalian oocytes and embryos and its relevance to vitrification. J. Reprod. Dev. 2016, 62, 317–321. [Google Scholar] [CrossRef]
- Benson, J.D.; Woods, E.J.; Walters, E.M.; Critser, J.K. The cryobiology of spermatozoa. Theriogenology 2012, 78, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Neild, D.M.; Brouwers, J.F.; Colenbrander, B.; Agüero, A.; Gadella, B.M. Lipid peroxide formation in relation to membrane stability of fresh and frozen thawed stallion spermatozoa. Mol. Reprod. Dev. 2005, 72, 230–238. [Google Scholar] [CrossRef]
- Fahy, G.M.; Lilley, T.H.; Linsdell, H.; Douglas, M.S.; Meryman, H.T. Cryoprotectant toxicity and cryoprotectant toxicity reduction: In search of molecular mechanisms. Cryobiology 1990, 27, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Bryant, S.J.; Wilkinson, B.L.; Bryant, G. The need for novel cryoprotectants and cryopreservation protocols: Insights into the importance of biophysical investigation and cell permeability. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129749. [Google Scholar] [CrossRef]
- Polge, C.; Smith, A.U.; Parkes, A.S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949, 164, 666. [Google Scholar] [CrossRef]
- Modun, D.; Music, I.; Katalinic, V.; Dujic, Z.; Boban, M. Glycerol and Ethanol in Red Wine Are Responsible for Urate-Related Increases in Plasma Antioxidant Capacity. Clin. Chem. 2006, 52, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, J.E.; Bishop, M.W. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature 1959, 183, 1394–1395. [Google Scholar] [CrossRef]
- Sanmartín-Suárez, C.; Soto-Otero, R.; Sánchez-Sellero, I.; Méndez-Álvarez, E. Antioxidant properties of dimethyl sulfoxide and its viability as a solvent in the evaluation of neuroprotective antioxidants. J. Pharmacol. Toxicol. Methods 2011, 63, 209–215. [Google Scholar] [CrossRef]
- Alvarenga, M.A.; Papa, F.O.; Landim-Alvarenga, F.C.; Medeiros, A.S. Amides as cryoprotectants for freezing stallion semen: A review. Anim. Reprod. Sci. 2005, 89, 105–113. [Google Scholar] [CrossRef]
- Mendoza-Betancourt, J.A.; Kross, R.D.; Moro, M.A.; Lizasoain, I.; Pérez-Astudillo, L.H.; Alva-Félix-Díaz, A.; Villanueva, C. Dimethylformamide Reduces Cerebral Ischaemia in Diabetic Rats Hours after Its Occurrence; A New Horizon. In Free Radicals, Antioxidants and Diseases; IntechOpen: London, UK, 2018. [Google Scholar]
- Kim, T.H.; Kim, Y.W.; Shin, S.M.; Kim, C.W.; Yu, I.J.; Kim, S.G. Synergistic hepatotoxicity of N,N-dimethylformamide with carbon tetrachloride in association with endoplasmic reticulum stress. Chem. Biol. Interact. 2010, 184, 492–501. [Google Scholar] [CrossRef]
- Aitken, R.J. Impact of oxidative stress on male and female germ cells: Implications for fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Burdon, R. Free radical-lipid interactions and their pathological consequences. Prog. Lipid Res. 1993, 32, 71–110. [Google Scholar] [CrossRef] [PubMed]
- Christova, Y.; James, P.S.; Jones, R. Lipid diffusion in sperm plasma membranes exposed to peroxidative injury from oxygen free radicals. Mol. Reprod. Dev. 2004, 68, 365–372. [Google Scholar] [CrossRef]
- Aitken, R.J.; Buckingham, D.W.; Carreras, A.; Irvine, D.S. Superoxide dismutase in human sperm suspensions: Relationship with cellular composition, oxidative stress, and sperm function. Free Radic. Biol. Med. 1996, 21, 495–504. [Google Scholar] [CrossRef]
- Gallo, A.; Esposito, M.C.; Tosti, E.; Boni, R. Sperm Motility, Oxidative Status, and Mitochondrial Activity: Exploring Correlation in Different Species. Antioxidants 2021, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- Akbarinejad, V.; Fathi, R.; Shahverdi, A.; Esmaeili, V.; Rezagholizadeh, A.; Ghaleno, L.R. The Relationship of Mitochondrial Membrane Potential, Reactive Oxygen Species, Adenosine Triphosphate Content, Sperm Plasma Membrane Integrity, and Kinematic Properties in Warmblood Stallions. J. Equine Vet. Sci. 2020, 94, 103267. [Google Scholar] [CrossRef] [PubMed]
- Gibb, Z.; Lambourne, S.R.; Aitken, R.J. The paradoxical relationship between stallion fertility and oxidative stress. Biol. Reprod. 2014, 91, 77. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Tischner, M. Evaluation of deep-frozen semen in stallions. J. Reprod. Fertil. Suppl. 1979, 53–59. [Google Scholar]
- Morrell, J.M. Stallion Sperm Selection: Past, Present, and Future Trends. J. Equine Vet. Sci. 2012, 32, 436–440. [Google Scholar] [CrossRef]
- Cecchini Gualandi, S.; Giangaspero, B.; Di Palma, T.; Macchia, G.; Carluccio, A.; Boni, R. Oxidative profile and protease regulator potential to predict sperm functionality in donkey (Equus asinus). Sci. Rep. 2021, 11, 20551. [Google Scholar] [CrossRef]
- Pagl, R.; Aurich, J.E.; Müller-Schlösser, F.; Kankofer, M.; Aurich, C. Comparison of an extender containing defined milk protein fractions with a skim milk-based extender for storage of equine semen at 5 °C. Theriogenology 2006, 66, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.R.; da Silva, Y.F.R.S.; Resende, H.L.; Guasti, P.N.; Monteiro, G.A.; Papa, P.M.; Júnior, J.A.D.A.; Puoli Filho, J.N.P.; Alvarenga, M.A.; Papa, F.O. Control methods and evaluation of bacterial growth on fresh and cooled stallion semen. J. Equine Vet. Sci. 2015, 35, 277–282. [Google Scholar] [CrossRef]
- Pillet, E.; Labbe, C.; Batellier, F.; Duchamp, G.; Beaumal, V.; Anton, M.; Desherces, S.; Schmitt, E.; Magistrini, M. Liposomes as an alternative to egg yolk in stallion freezing extender. Theriogenology 2012, 77, 268–279. [Google Scholar] [CrossRef]
- Boni, R.; Gallo, A.; Cecchini, S. Kinetic activity, membrane mitochondrial potential, lipid peroxidation, intracellular pH and calcium of frozen/thawed bovine spermatozoa treated with metabolic enhancers. Andrology 2017, 5, 133–145. [Google Scholar] [CrossRef]
- Di Palma, T.; Cecchini, S.; Macchia, G.; Pasolini, M.P.; Cocchia, N.; Boni, R. Kinematic, bioenergetic and oxidative evaluations of donkey sperm preserved at +4 °C. Zygote 2020, 28, 300–307. [Google Scholar] [CrossRef]
- Hernández-Avilés, C.; Ramírez-Agámez, L.; Varner, D.D.; Love, C.C. Effects of egg yolk level, penetrating cryoprotectant, and pre-freeze cooling rate, on the post-thaw quality of stallion sperm. Anim. Reprod. Sci. 2023, 248, 107162. [Google Scholar] [CrossRef]
- Pillet, E.; Duchamp, G.; Batellier, F.; Beaumal, V.; Anton, M.; Desherces, S.; Schmitt, E.; Magistrini, M. Egg yolk plasma can replace egg yolk in stallion freezing extenders. Theriogenology 2011, 75, 105–114. [Google Scholar] [CrossRef]
- Batellier, F.; Magistrini, M.; Fauquant, J.; Palmer, E. Effect of milk fractions on survival of equine spermatozoa. Theriogenology 1997, 48, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Cochran, J.D.; Amann, R.P.; Froman, D.P.; Pickett, B.W. Effects of centrifugation, glycerol level, cooling to 5 °C, freezing rate and thawing rate on the post-thaw motility of equine sperm. Theriogenology 1984, 22, 25–38. [Google Scholar] [CrossRef]
- Nishikawa, Y. Studies on the preservation of raw and frozen horse semen. J. Reprod. Fertil. Suppl. 1975, 23, 99–104. [Google Scholar]
- Kavak, A.; Johannisson, A.; Lundeheim, N.; Rodriguez-Martinez, H.; Aidnik, M.; Einarsson, S. Evaluation of cryopreserved stallion semen from Tori and Estonian breeds using CASA and flow cytometry. Anim. Reprod. Sci. 2003, 76, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Loomis, P.R.; Graham, J.K. Commercial semen freezing: Individual male variation in cryosurvival and the response of stallion sperm to customized freezing protocols. Anim. Reprod. Sci. 2008, 105, 119–128. [Google Scholar] [CrossRef]
- Contri, A.; De Amicis, I.; Molinari, A.; Faustini, M.; Gramenzi, A.; Robbe, D.; Carluccio, A. Effect of dietary antioxidant supplementation on fresh semen quality in stallion. Theriogenology 2011, 75, 1319–1326. [Google Scholar] [CrossRef]
- Zeitoun, M.M.; Ateah, M.A.; Almaiman, A.T.; Mansour, M.M. Spirulina Supplementation to the Semen Extender Influences the Quality and Antioxidant Parameters of Chilled or Cryopreserved Arabian Stallion Spermatozoa. J. Equine Vet. Sci. 2022, 118, 104108. [Google Scholar] [CrossRef]
- Catalán, J.; Yánez-Ortiz, I.; Tvarijonaviciute, A.; González-Aróstegui, L.G.; Rubio, C.P.; Barranco, I.; Yeste, M.; Miró, J. Seminal Plasma Antioxidants Are Related to Sperm Cryotolerance in the Horse. Antioxidants 2022, 11, 1279. [Google Scholar] [CrossRef]
- Rosselli, M.; Keller, P.J.; Dubey, R.K. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum. Reprod. Update 1998, 4, 3–24. [Google Scholar] [CrossRef]
- Staicu, F.D.; Lopez-Úbeda, R.; Romero-Aguirregomezcorta, J.; Martínez-Soto, J.C.; Matás Parra, C. Regulation of boar sperm functionality by the nitric oxide synthase/nitric oxide system. J. Assist. Reprod. Genet. 2019, 36, 1721–1736. [Google Scholar] [CrossRef] [PubMed]
- Rosselli, M.; Dubey, R.K.; Imthurn, B.; Macas, E.; Keller, P.J. Effects of nitric oxide on human spermatozoa: Evidence that nitric oxide decreases sperm motility and induces sperm toxicity. Hum. Reprod. 1995, 10, 1786–1790. [Google Scholar] [CrossRef]
- Clulow, J.; Gibb, Z. Liquid storage of stallion spermatozoa—Past, present and future. Anim. Reprod. Sci. 2022, 247, 107088. [Google Scholar] [CrossRef]
- Varner, D.D.; Blanchard, T.L.; Love, C.L.; Garcia, M.C.; Kenney, R.M. Effects of cooling rate and storage temperature on equine spermatozoal motility parameters. Theriogenology 1988, 29, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Ramires Neto, C.; Monteiro, G.A.; Sancler-Silva, Y.F.R.; Papa, P.; Guasti, P.N.; Resende, H.L.; Papa, F.O.; Dellaqua, J.A.; Alvarenga, M.A. Comparison of different freezing extenders for semen cryopreservation from stallions with poor and good semen freezability. J. Equine Vet. Sci. 2014, 34, 58–60. [Google Scholar] [CrossRef]
- Gutiérrez-Cepeda, L.; Crespo, F.; Blazquez, J.C.; Serres, C. Optimization of the Equine-Sperm Freeze Test in Purebred Spanish Horses by Incorporating Colloidal Centrifugation. Animals 2023, 13, 382. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, S.; Bollwein, H.; Siuda, M.; Handler, J. Comparison of the Effects of Five Semen Extenders on the Quality of Frozen-Thawed Equine Epididymal Sperm. J. Equine Vet. Sci. 2019, 79, 1–8. [Google Scholar] [CrossRef]
- Alamaary, M.S.; Haron, A.W.; Ali, M.; Hiew, M.W.H.; Adamu, L.; Peter, I.D. Effects of four extenders on the quality of frozen semen in Arabian stallions. Vet. World 2019, 12, 34–40. [Google Scholar] [CrossRef]
- Papa, F.O.; Felício, G.B.; Melo-Oña, C.M.; Alvarenga, M.A.; De Vita, B.; Trinque, C.; Puoli-Filho, J.N.P.; Dell’Aqua, J.A. Replacing egg yolk with soybean lecithin in the cryopreservation of stallion semen. Anim. Reprod. Sci. 2011, 129, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Lagares, M.A.; Martins, H.S.; Carvalho, I.A.; Oliveira, C.A., Jr.; Souza, M.R.; Penna, C.F.; Cruz, B.C.; Stahlberg, R.; Henry, M.R. Caseinate protects stallion sperm during semen cooling and freezing. Cryo Lett. 2012, 33, 214–219. [Google Scholar]

| Shipping/Centrifugation Extenders (SCEs) | Osmolarity | pH | ABTS | FRAP | NOx |
|---|---|---|---|---|---|
| mOsm L−1 | µM | µM | µM | ||
| Equiplus | 324 ± 3 A | 6.85 ± 0.05 | 61.1 ± 2.5 A | 163 ± 7 A | n.d. |
| BotuSemen | 390 ± 5 B | 6.78 ± 0.03 a | 212.3 ± 4.9 BC | 187 ± 10 A | n.d. |
| INRA96 | 317 ± 1 A | 6.91 ± 0.07 b | 32.0 ± 7.6 BD | 236 ± 7 B | n.d. |
| Freezing Extenders | |||||
| Spectrum Duo Red | 985 ± 5 A | 6.66 ± 0.11 A | n.d. | 1234 ± 10 A | 52.7 ± 2.1 A |
| BotuCrio | 1321 ± 7 BC | 6.73 ± 0.13 B | 79.1 ± 4.0 | 1213 ± 15 A | 52.3 ± 3.2 A |
| INRAFreeze | 734 ± 3 BDE | 6.85 ± 0.07 BC | n.d. | 379 ± 5 B | 65.3 ± 3.2 B |
| HF-20 | 809 ± 3 BDF | 7.02 ± 0.05 BD | n.d. | 360 ± 5 B | n.d. |
| ABTS | FRAP | NOx | |
|---|---|---|---|
| Stallion | µM | µM | µM |
| No. 1 | 129 ± 4 | 393 ± 7 | n.d. |
| No. 2 | 245 ± 6 | 550 ± 6 | n.d. |
| No. 3 | 2202 ± 11 | 405 ± 6 | n.d. |
| No. 4 | 28 ± 4 | 556 ± 15 | 64 ± 4 |
| No. 5 | 910 ± 11 | 934 ± 18 | 64 ± 6 |
| No. 6 | 780 ± 4 | 538 ± 10 | 106 ± 6 |
| No. 7 | 305 ± 8 | 259 ± 6 | n.d. |
| No. 8 | 673 ± 11 | 489 ± 8 | 350 ± 13 |
| No. 9 | 1611 ± 29 | 972 ± 20 | 529 ± 11 |
| No. 10 | 708 ± 10 | 301 ± 6 | 54 ± 5 |
| Conditioned Shipping/ | ABTS | FRAP | NOx |
|---|---|---|---|
| Centrifugation Extenders (cSCEs) | µM | µM | µM |
| Equiplus | 353 ± 301 | 356 ± 134 | 20 ± 40 |
| BotuSemen | 432 ± 344 | 347 ± 167 | 9 ± 29 |
| INRA 96 | 443 ± 428 | 415 ± 126 | 36 ± 91 |
| Conditioned Freezing Extenders | |||
| Spectrum Duo Red | 22 ± 46 A | 930 ± 152 A | 96 ± 154 A |
| BotuCrio | 88 ± 167 A | 728 ± 215 BC | 112 ± 99 A |
| INRA Freeze | 659 ± 365 B | 388 ± 98 BD | 4 ± 12 B |
| HF-20 | n.d. | 704 ± 111 BC | n.d. |
| Shipping/Centrifugation Extenders (SCEs) | Freezing Extenders | ||||||
|---|---|---|---|---|---|---|---|
| Equiplus | BotuSemen | INRA 96 | Spectrum Duo Red | BotuCrio | INRA Freeze | HF-20 | |
| TotMot (%) | 78.2 ± 14.4 | 80.0 ± 16.3 | 84.3 ± 16.2 | 28.4 ± 14.8 | 25.7 ± 13.7 | 30.5 ± 13.1 | 25.6 ± 14.9 |
| Prog (%) | 23.1 ± 7.0 | 19.6 ± 7.8 | 24.5 ± 5.5 | 11.7 ± 6.6 | 9.6 ± 6.2 | 8.6 ± 5.5 | 7.5 ± 5.1 |
| VCL (µm/s) | 95.7 ± 23.3 | 93.1 ± 20.1 | 94.1 ± 18.3 | 65.5 ± 10.9 | 62.8 ± 10.6 | 58.5 ± 7.9 | 59.4 ± 9.5 |
| VSL (µm/s) | 35.8 ± 10.2 | 34.9 ± 9.3 | 36.1 ± 8.1 | 34.4 ± 12.1 | 32.2 ± 11.9 | 26.4 ± 7.4 | 27.1 ± 7.7 |
| VAP (µm/s) | 59.3 ± 14.8 | 60.3 ±13.7 | 59.8 ±12.3 | 44.7 ± 12.3 a | 41.0 ± 11.3 | 36.1 ± 7.3 b | 36.8 ± 8.1 |
| MMP (F0B/F0A) | 34.9 ± 13.6 | 25.6 ± 20.2 | 24.8 ± 21.3 | 8.9 ± 6.8 | 10.1 ± 7.0 | 9.5 ± 6.4 | 9.2 ± 5.6 |
| ROS (a.u.) | 24.3 ± 4.3 | 24.1 ± 4.3 | 23.3 ± 2.8 | 12.7 ± 5.6 | 13.3 ± 6.9 | 13.1 ± 6.6 | 13.6 ± 8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boni, R.; Ruggiero, R.; Di Palma, T.; Ferrara, M.A.; Preziosi, G.; Cecchini Gualandi, S. Stallion Sperm Freezing with Different Extenders: Role of Antioxidant Activity and Nitric Oxide Production. Animals 2024, 14, 2465. https://doi.org/10.3390/ani14172465
Boni R, Ruggiero R, Di Palma T, Ferrara MA, Preziosi G, Cecchini Gualandi S. Stallion Sperm Freezing with Different Extenders: Role of Antioxidant Activity and Nitric Oxide Production. Animals. 2024; 14(17):2465. https://doi.org/10.3390/ani14172465
Chicago/Turabian StyleBoni, Raffaele, Raffaella Ruggiero, Tommaso Di Palma, Maria Antonietta Ferrara, Graziano Preziosi, and Stefano Cecchini Gualandi. 2024. "Stallion Sperm Freezing with Different Extenders: Role of Antioxidant Activity and Nitric Oxide Production" Animals 14, no. 17: 2465. https://doi.org/10.3390/ani14172465
APA StyleBoni, R., Ruggiero, R., Di Palma, T., Ferrara, M. A., Preziosi, G., & Cecchini Gualandi, S. (2024). Stallion Sperm Freezing with Different Extenders: Role of Antioxidant Activity and Nitric Oxide Production. Animals, 14(17), 2465. https://doi.org/10.3390/ani14172465







