Effects of DNA Methylation of HPA-Axis Genes of F1 Juvenile Induced by Maternal Density Stress on Behavior and Immune Traits in Root Voles (Microtus oeconomus)—A Field Experiment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Root Voles in the Study Area
2.2. Experimental Facility
2.3. Establishment of Parental Populations and Live-Trapping
2.4. Behavioral Tests
2.5. Fecal and Blood Samples Preparation
2.6. Hypothalamic and Hippocampal Samples Preparation
2.7. Hematology Assays
2.8. FCM Assays
2.9. Cellular Immunity Assays
2.10. Humoral Immunity and Cytokines Assays
2.11. Coccidian Infection Assays
2.12. DNA Methylation Analysis
2.13. RNA Extraction, cDNA Synthesis, and RT-qPCR
2.14. Western Blotting
2.15. Statistical Analysis
3. Results
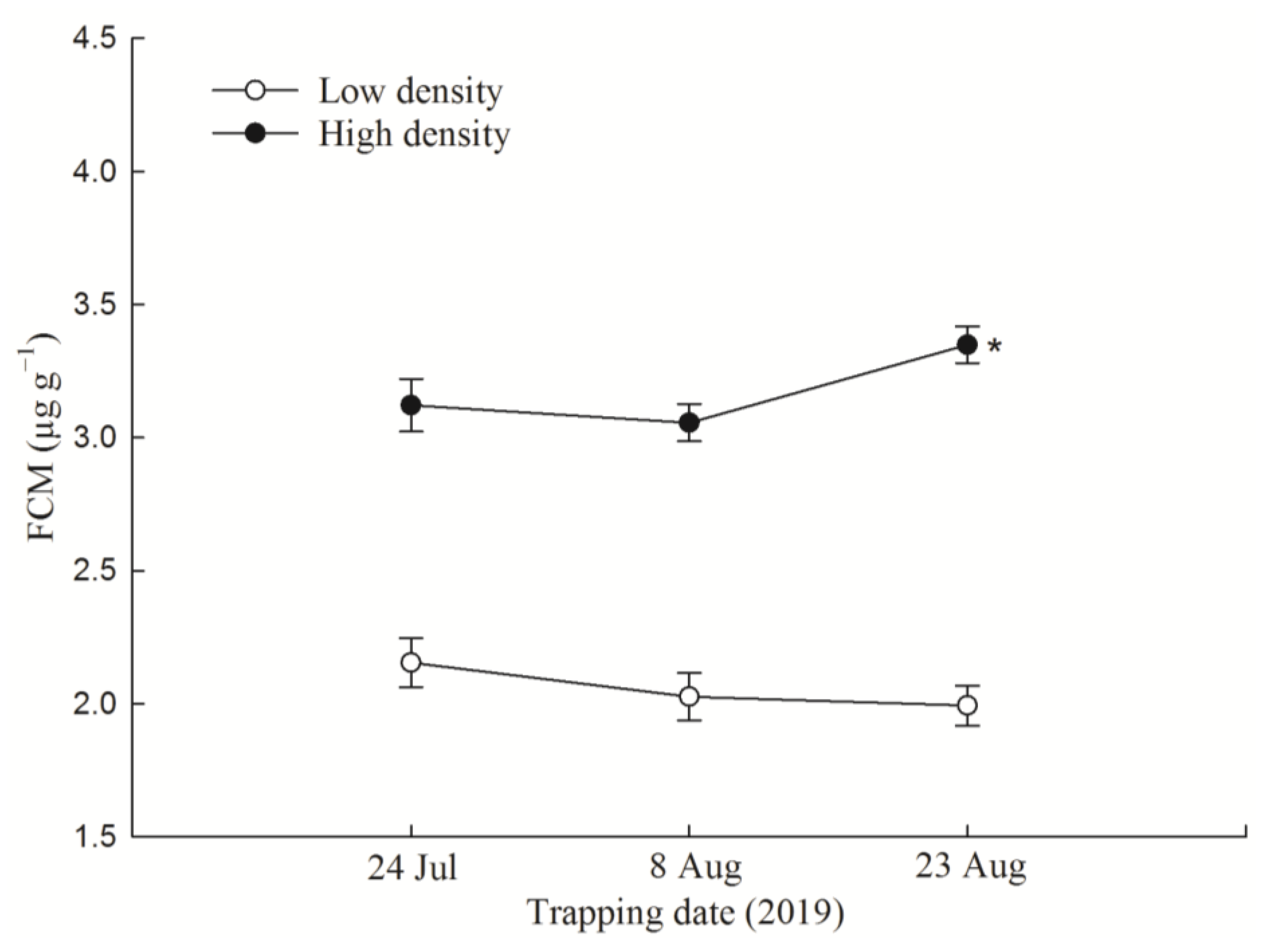
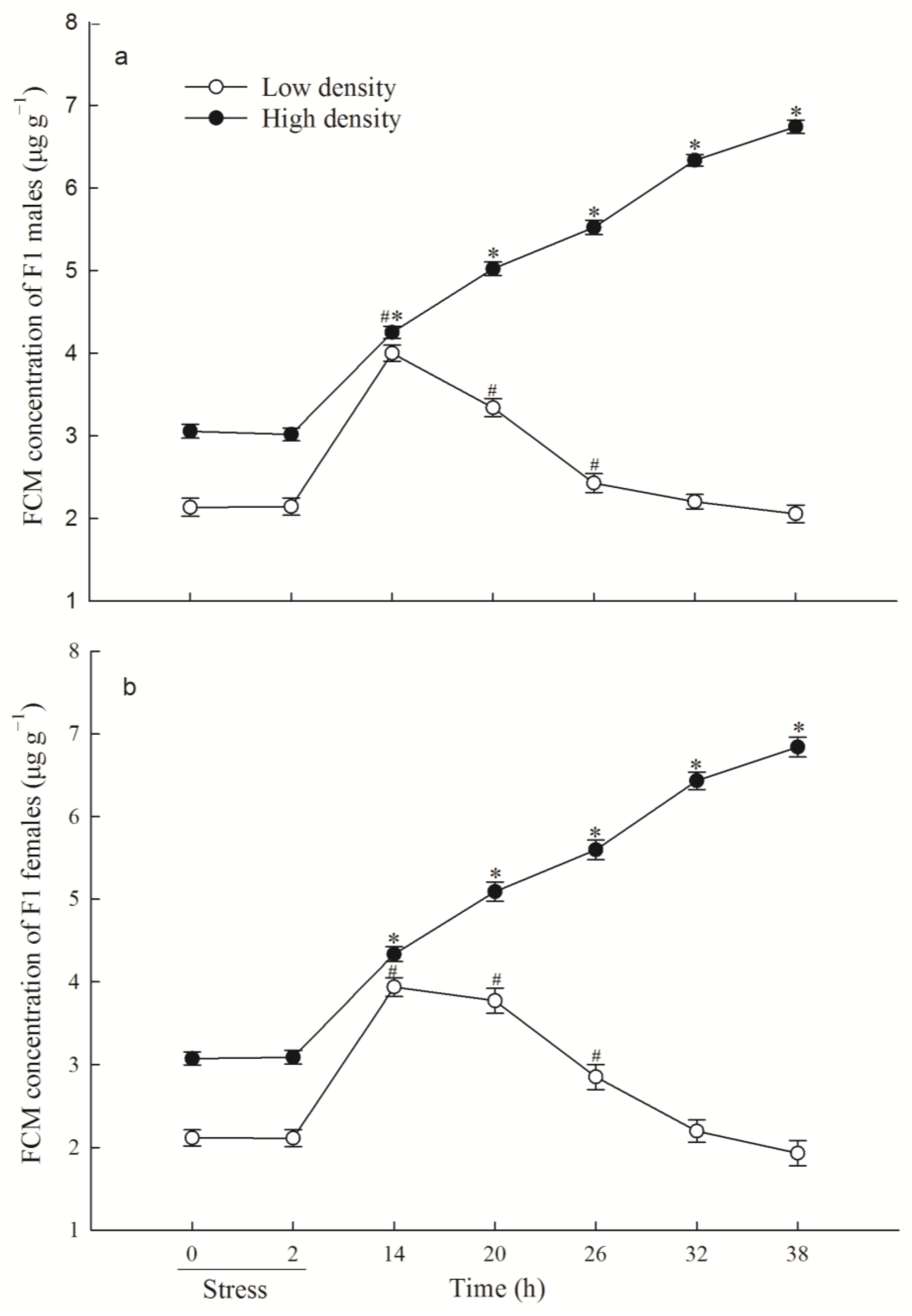
3.1. F1 FCM Levels and FCM Response to Acute Immobilization Stress
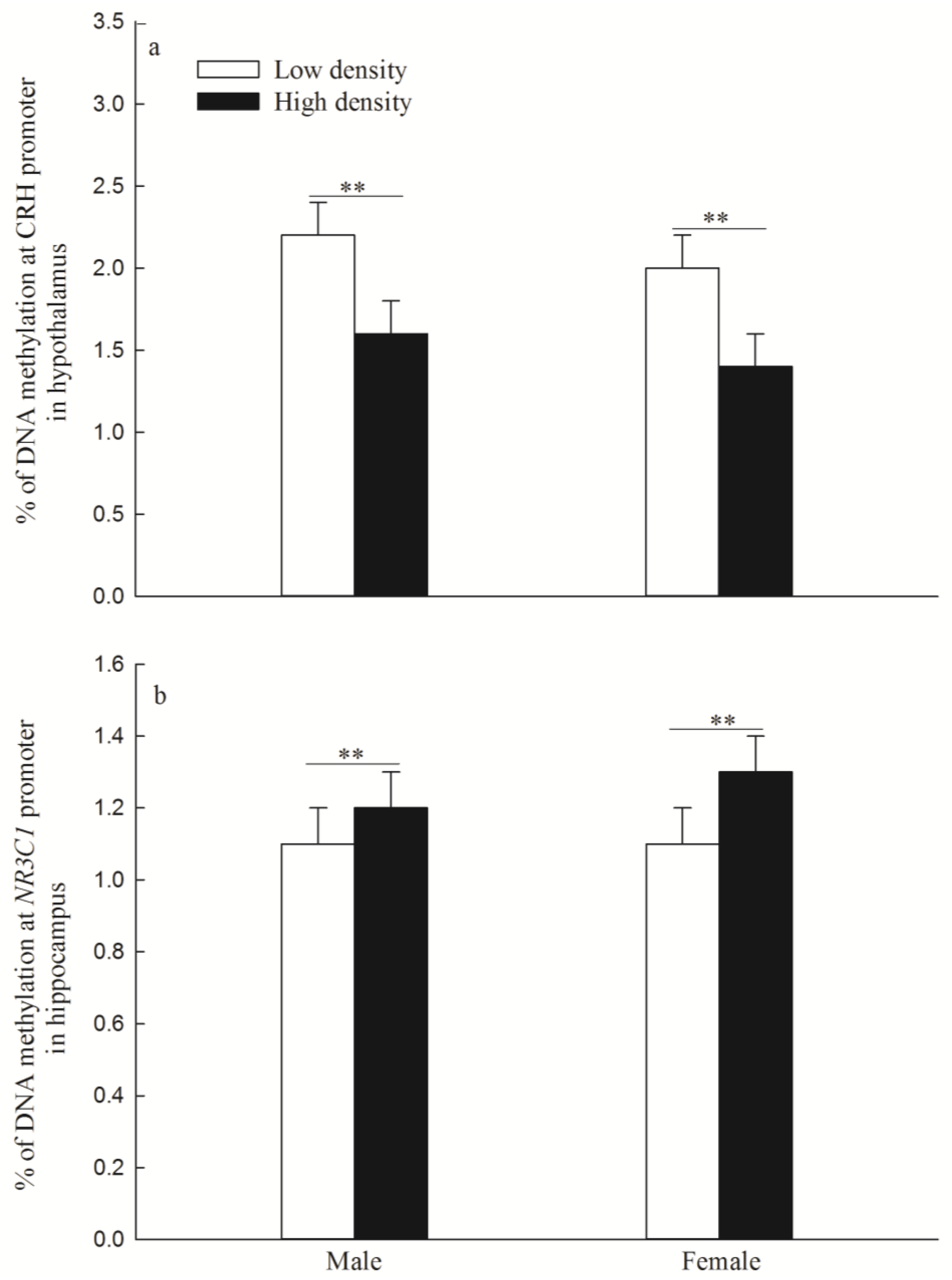
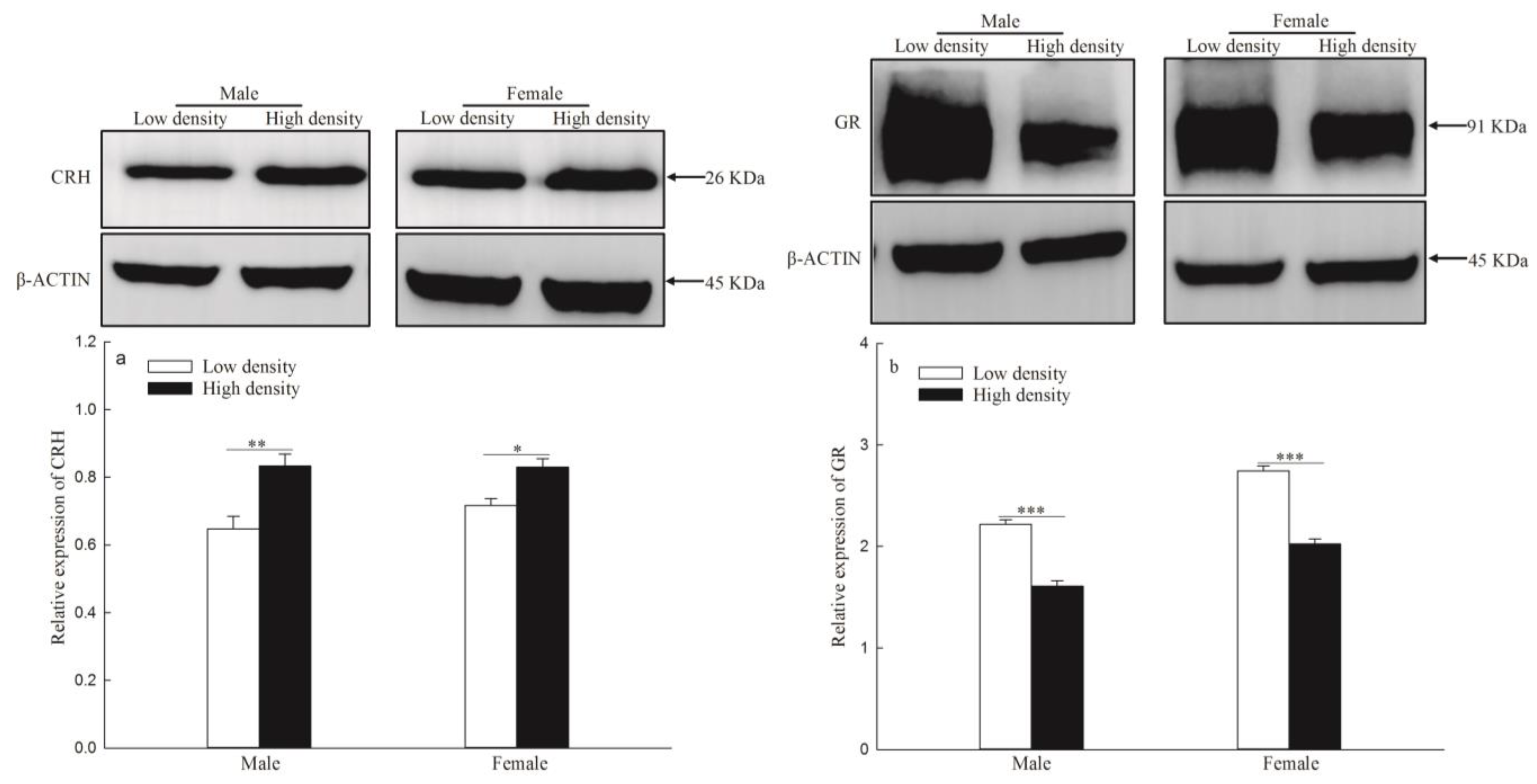
3.2. DNA Methylation Levels of CRH Gene in the Hypothalamus and NR3C1 in the Hippocampus
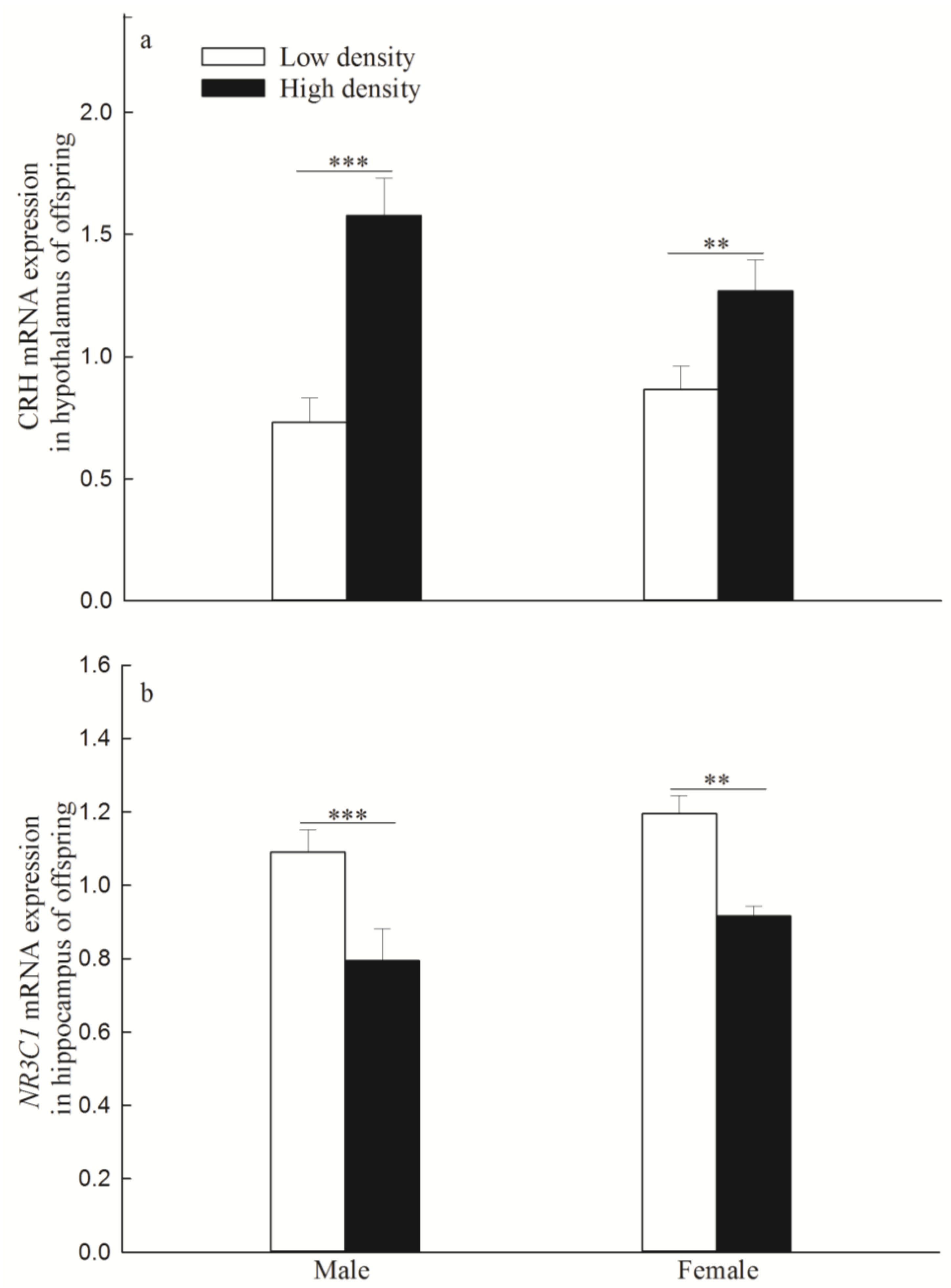
3.3. mRNA and Protein Expression of CRH Gene in the Hypothalamus and NR3C1 in the Hippocampus
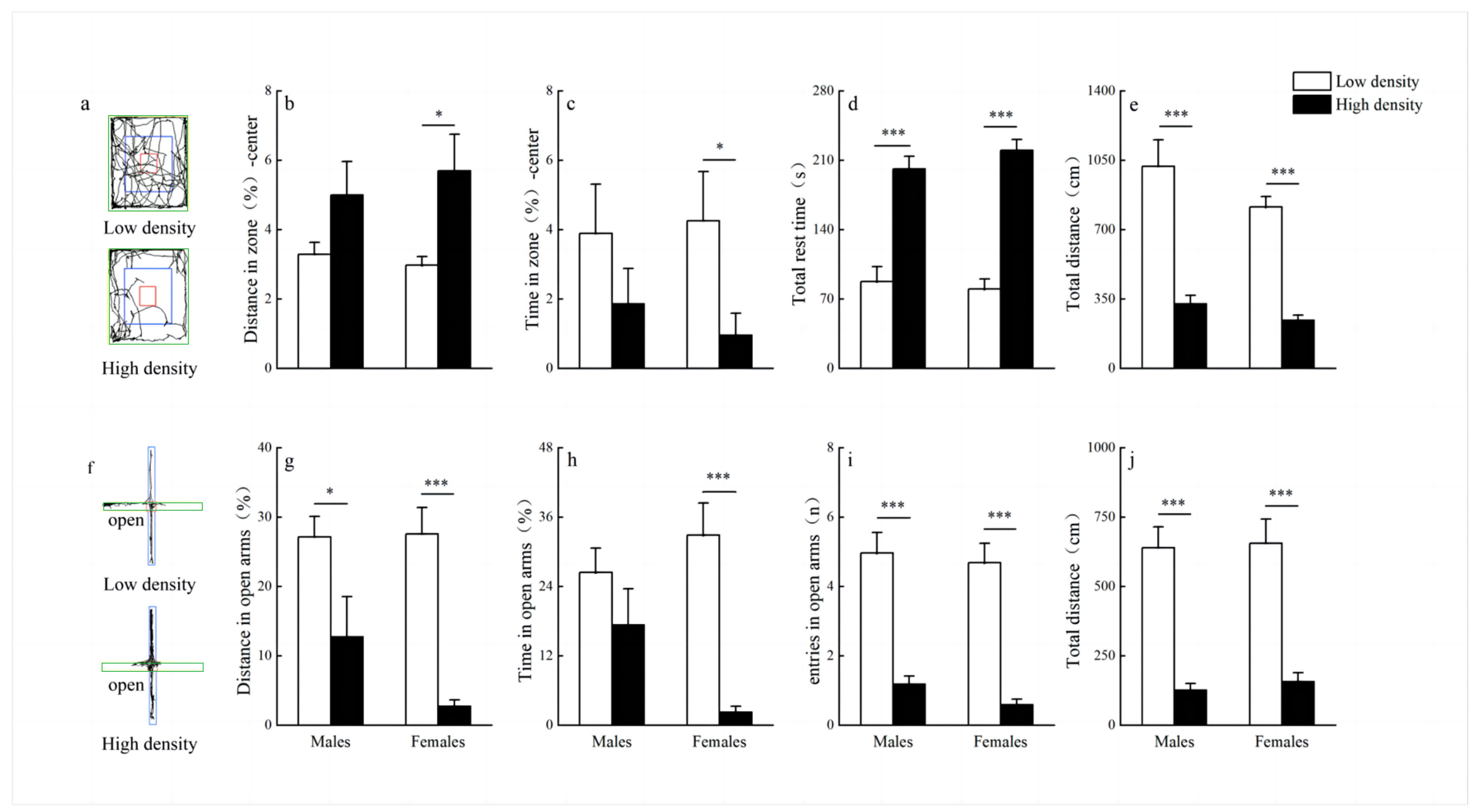
3.4. Behavioral Tests
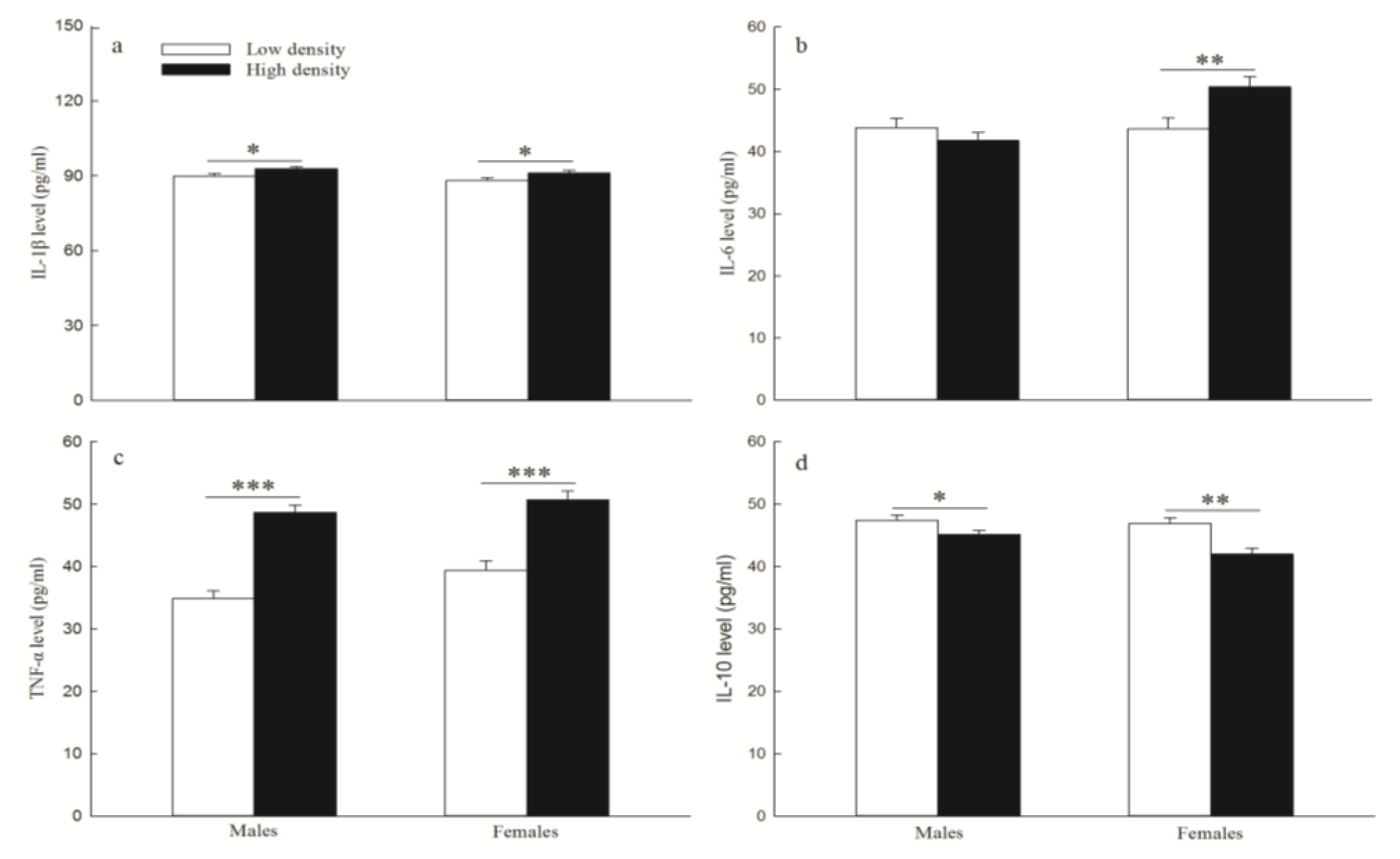
3.5. Inflammatory Cytokines Levels
3.6. Immune Traits
3.7. Coccidial Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Pech, R.; Davis, S.; Shi, D.; Wan, X.; Zhong, W. Extrinsic and intrinsic factors determine the eruptive dynamics of Brandt’s voles Microtus brandti in Inner Mongolia, China. Oikos 2003, 100, 299–310. [Google Scholar] [CrossRef]
- Van den Bergh, B.R.; Mulder, E.J.; Mennes, M.; Glover, V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neurosci. Biobehav. Rev. 2005, 29, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Wang, W.; Ding, X.; Yang, S.; Wang, A.; Yin, B.; Wei, W. Effects of maternal stress induced by predator odors during gestation on behavioral and physiological responses of F1 in Brandt’s vole (Lasiopodomys brandtii). Integr. Zool. 2018, 13, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Metzger, D.C.; Schulte, P.M. Maternal stress has divergent effects on gene expression patterns in the brains of male and female threespine stickleback. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161734. [Google Scholar] [CrossRef]
- Bian, J.H.; Du, S.Y.; Wu, Y.; Cao, Y.F.; Nie, X.H.; He, H.; You, Z.B. Maternal effects and population regulation: Maternal density-induced reproduction suppression impairs F1 capacity in response to immediate environment in root voles Microtus oeconomus. J. Anim. Ecol. 2015, 84, 326–336. [Google Scholar] [CrossRef]
- Hinds, J.A.; Sanchez, E.R. The role of the hypothalamus-pituitary-adrenal (HPA) axis in test-induced anxiety: Assessments, physiological responses, and molecular details. Stresses 2022, 2, 146–155. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Yin, S.; Lu, C.; Liu, D.; Jiang, H.; Pan, F. Long-term effects of early adolescent stress: Dysregulation of hypothalamic-pituitary-adrenal axis and central corticotropin releasing factor receptor 1 expression in adult male rats. Behav. Brain Res. 2015, 288, 39–49. [Google Scholar] [CrossRef]
- Agrawal, A.A. Phenotypic plasticity in the interactions and evolution of species. Science 2001, 294, 321–326. [Google Scholar] [CrossRef]
- Frésard, L.; Morisson, M.; Brun, J.M.; Collin, A.; Pain, B.; Minvielle, F.; Pitel, F. Epigenetics and phenotypic variability: Some interesting insights from birds. Genet. Sel. Evol. 2013, 45, 16. [Google Scholar] [CrossRef]
- Cao-Lei, L.; De Rooij, S.R.; King, S.; Matthews, S.G.; Metz, G.A.S.; Roseboom, T.J.; Szyf, M. Prenatal stress and epigenetics. Neurosci. Biobehav. Rev. 2020, 117, 198–210. [Google Scholar] [CrossRef]
- Bockmühl, Y.; Patchev, A.V.; Madejska, A.; Hoffmann, A.; Sousa, J.C.; Sousa, N.; Holsboer, F.; Almeida, O.F.X.; Spengler, D. Methylation at the CpG island shore region upregulates Nr3c1 promoter activity after early-life stress. Epigenetics 2015, 10, 247–257. [Google Scholar] [CrossRef]
- Mills, S.C.; Grapputo, A.; Jokinen, I.; Koskela, E.; Mappes, T.; Poikonen, T. Fitness trade-offs mediated by immunosuppression costs in a small mammal. Evolution 2010, 64, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Owens, I.P.; Wilson, K. Immunocompetence: A neglected life history trait or conspicuous red herring? Trends Ecol. Evol. 1999, 14, 170–172. [Google Scholar] [CrossRef]
- French, S.S.; Moore, M.C.; Demas, G.E. Ecological immunology: The organism in context. Integr. Comp. Biol. 2009, 49, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Du, S.Y.; Cao, Y.F.; Nie, X.H.; Wu, Y.; Bian, J.H. The synergistic effect of density stress during the maternal period and adulthood on immune traits of root vole (Microtus oeconomus) individuals-a field experiment. Oecologia 2016, 181, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, T.; Liu, L.; Han, Q.; Zhang, H.; Sun, Y.; Hao, R.; Ma, S. Seasonal photoperiodic influence of pineal melatonin on hypothalamic-pituitary-adrenal axis-hippocampal-receptor in male rats. J. Tradit. Chin. Med. Sci. 2022, 9, 143–152. [Google Scholar] [CrossRef]
- Kim, J.; Lei, Y.; Lu, X.Y.; Kim, C.S. Glucocorticoid-glucocorticoid receptor-HCN1 channels reduce neuronal excitability in dorsal hippocampal CA1 neurons. Mol. Psychiatr. 2022, 27, 4035–4049. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, S.; Wang, Z.; Zhen, Y.; Cui, R. Productivity investi-gation of the root vole (Microtus oeconomus) population in the Haibei alpine bushland (Potentilia fruticosa). I. Population dynamics. Acta Theirol. Sin. 1991, 11, 270–278, (In Chinese English Abstract). [Google Scholar]
- Sun, P.; Zhao, X.Q.; Xu, S.X.; Zhao, T.B.; Zhao, W. Changes after snow of the population characteristic of root vole (Microtus Oeconomus) in Haibei Alpine meadow. Acta. Theirol. Sin. 2002, 22, 318–320, (In Chinese English Abstract). [Google Scholar]
- Yang, Y.B.; Shang, G.Z.; Du, S.Y.; Zhang, X.; Wu, Y.; Bian, J.H. Maternal density stress and coccidian parasitism: Synergistic effects on overwinter survival in root voles. Funct. Ecol. 2018, 32, 2181–2193. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Zhang, J.; Zhou, Q.; Liu, X.; Zhou, G. Medicarpin improves depressive-like behaviors in a chronic unpredictable mild stress-induced mouse model of depression by upregulating liver X receptor β expression in the amygdala. Neurotox. Res. 2022, 40, 1937–1947. [Google Scholar] [CrossRef]
- He, H.; Cao, Y.; Chen, L.; Du, S.; Nie, X.; Bian, J. The utility of detecting corticosterone levels in feces of root vole (Microtus oeconomus). Acta Theriol. Sin. 2013, 33, 164–171, (In Chinese English abstract). [Google Scholar]
- Sheriff, M.J.; Krebs, C.J.; Boonstra, R. Assessing stress in animal populations: Do fecal and plasma glucocorticoids tell the same story? Gen. Comp. Endocr. 2010, 166, 614–619. [Google Scholar] [CrossRef]
- Chen, F.; Du, S.; Bian, J.; You, Z.B.; Wu, Y. Chronic hypoxia exposure during pregnancy is associated with a decreased active nursing activity in mother and an abnormal birth weight and postnatal growth in F1 of rats. Horm. Behav. 2012, 61, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, X.; Wang, D. Impairment of cellular and humoral immunity in overweight Mongolian gerbils (Meriones unguiculatus). Integr. Zool. 2011, 6, 352–365. [Google Scholar] [CrossRef]
- Wang, C.; Pu, W.; Zhao, D.; Zhou, Y.; Lu, T.; Chen, S.; He, Z.; Feng, X.; Wang, Y.; Li, C.; et al. Identification of hyper-methylated tumor suppressor genes-based diagnostic panel for esophageal squamous cell carcinoma (ESCC) in a Chinese Han population. Front. Genet. 2018, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, K.H.; Ünal, G.Ö.; Doğuç, D.K.; Toğay, V.A.; Koşar, P.A.; Sezik, M. Hypothalamic NR3C1 DNA methylation in rats exposed to prenatal stress. Mol. Biol. Rep. 2022, 49, 7921–7928. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Kamilaris, T.C.; Chrousos, G.P.; Gold, P.W. Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neurosci. Biobehav. Rev. 1992, 16, 115–130. [Google Scholar] [CrossRef]
- Hill, V.K.; Ricketts, C.; Bieche, I.; Vacher, S.; Gentle, D.; Lewis, C.; Maher, E.R.; Latif, F. Genome-wide DNA methylation profiling of CpG islands in breast cancer identifies novel genes associated with tumorigenicity. Cancer Res. 2011, 71, 2988–2999. [Google Scholar] [CrossRef]
- Hoffmann, A.; Spengler, D. DNA memories of early social life. Neuroscience 2014, 264, 64–75. [Google Scholar] [CrossRef]
- Mychasiuk, R.; Ilnytskyy, S.; Kovalchuk, O.; Kolb, B.; Gibb, R. Intensity matters: Brain; behaviour and the epigenome of prenatally stressed rats. Neuroscience 2011, 180, 105–110. [Google Scholar] [CrossRef]
- Morrison, K.E.; Rodgers, A.B.; Morgan, C.P.; Bale, T.L. Epigenetic mechanisms in pubertal brain maturation. Neuroscience 2014, 264, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Van Bodegom, M.; Homberg, J.R.; Henckens, M.J. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front. Cell. Neurosci. 2017, 11, 87. [Google Scholar]
- Torres-Berrío, A.; Issler, O.; Parise, E.M.; Nestler, E.J. Unraveling the epigenetic landscape of depression: Focus on early life stress. Dialogues. Clin. Neuro 2019, 21, 341–357. [Google Scholar] [CrossRef]
- Burns, S.B.; Szyszkowicz, J.K.; Luheshi, G.N.; Lutz, P.E.; Turecki, G. Plasticity of the epigenome during early-life stress. Semin. Cell. Dev. Biol. 2018, 77, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Huang, S.; Liu, J.; Batsuren, E.; Li, G.; Wan, X.; Zhao, J.; Wang, Z.; Han, W.; Zhang, Z. Effects of Group Size on Behavior, Reproduction, and mRNA Expression in Brains of Brandt’s Voles. Brain Sci. 2023, 13, 311. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Themann, A.; Garcia-Carachure, I.; Lira, O.; Robison, A.J.; Cushing, B.S.; Iñiguez, S.D. Chronic social defeat stress induces a depression-relevant outcome in male prairie voles. BioRxiv 2023, 16, 562541. [Google Scholar]
- Sheline, Y.I.; Liston, C.; McEwen, B.S. Parsing the hippocampus in depression: Chronic stress; hippocampal volume; and major depressive disorder. Biol. Psychiat. 2019, 85, 436–438. [Google Scholar] [CrossRef]
- Enayati, M.; Mosaferi, B.; Homberg, J.R.; Diniz, D.M.; Salari, A.A. Prenatal maternal stress alters depression-related symptoms in a strain-and sex-dependent manner in rodent offspring. Life Sci. 2020, 251, 117597. [Google Scholar] [CrossRef]
- Wei, Q.; Shi, H.; Ma, X.; Shi, Y.; Zhang, Y.; Wang, L. The impact of maternal stress on F1 birth weight and the mediating effect of dietary patterns: The Shanghai maternal-child pairs cohort study. J. Affect. Disord. 2021, 278, 643–649. [Google Scholar] [CrossRef]
- Wintermann, G.B.; Kirschbaum, C.; Petrowski, K. Predisposition or side effect of the duration: The reactivity of the HPA-axis under psychosocial stress in panic disorder. Int. J. Psychophysiol. 2016, 107, 9–15. [Google Scholar] [CrossRef]
- Walker, E.; Mittal, V.; Tessner, K. Stress and the hypothalamic-pituitary-adrenal axis in the developmental course of schizophrenia. Annu. Rev. Clin. Psychol. 2008, 4, 189–216. [Google Scholar] [CrossRef] [PubMed]
- McNeal, N.; Scotti, M.A.L.; Wardwell, J.; Chandler, D.L.; Bates, S.L.; LaRocca, M.; Trahanas, D.M.; Grippo, A.J. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton. Neurosci. 2014, 180, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.; Liu, Y.; Wang, Z. The Socially Monogamous Prairie Vole: A Rodent Model for Behavioral Neuroendocrine Research. In Model Animals in Neuroendocrinol: From Worm to Mouse to Man; John Wiley & Sons Ltd.: Tallahassee, FL, USA, 2018; pp. 181–205. [Google Scholar]
- Diz-Chaves, Y.; Pernía, O.; Carrero, P.; Garcia-Segura, L.M. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J. Neuroinflamm. 2012, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Hisada, C.; Kajimoto, K.; Tsugane, H.; Mitsuo, I.; Azuma, K.; Kubo, K.Y. Maternal chewing alleviates prenatal stress-related neuroinflammation mediated by microglia in the hippocampus of the mouse offspring. J. Prosthodont. Res. 2023, 67, 588–594. [Google Scholar] [CrossRef]
- Zhu, C.; Li, S. Role of CRH in colitis and colitis-associated cancer: A combinative result of central and peripheral effects? Front. Endocrinol. 2024, 15, 1363748. [Google Scholar] [CrossRef]
- Hu, P.; Lu, Y.; Pan, B.X.; Zhang, W.H. New insights into the pivotal role of the amygdala in inflammation-related depression and anxiety disorder. Int. J. Mol. Sci. 2022, 23, 11076. [Google Scholar] [CrossRef]
- Kohman, R.A.; Tarr, A.J.; Sparkman, N.L.; Bogale, T.M.; Boehm, G.W. Neonatal endotoxin exposure impairs avoidance learning and attenuates endotoxin-induced sickness behavior and central IL-1β gene transcription in adulthood. Behav. Brain Res. 2008, 194, 25–31. [Google Scholar] [CrossRef]
- Park, K.M.; Bowers, W.J. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell. Signal. 2010, 22, 977–983. [Google Scholar] [CrossRef]
- Kubera, M.; Obuchowicz, E.; Goehler, L.; Brzeszcz, J.; Maes, M. In animal models; psychosocial stress-induced (neuro) inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuro-Psychoph. 2011, 35, 744–759. [Google Scholar] [CrossRef]
- Pincus-Knackstedt, M.K.; Joachim, R.A.; Blois, S.M.; Douglas, A.J.; Orsal, A.S.; Klapp, B.F.; Wahn, U.; Hamelmann, E.; Arck, P.C. Prenatal stress enhances susceptibility of murine adult F1 toward airway inflammation. J. Immunol. 2006, 177, 8484–8492. [Google Scholar] [CrossRef]
- Viney, M.; Riley, E.M. The immunology of wild rodents: Current status and future prospects. Front. Immunol. 2017, 8, 1481. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.J.; Fine, J.B.; Demas, G.E.; Moffatt, C.A. Photoperiod and population density interact to affect reproductive and immune function in male prairie voles. Am. J. Physiol.-Reg. I. 1996, 270, R571–R577. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Li, G.; Zhao, J.; Lu, W.; Zhang, Z. High housing density increases stress hormone-or disease-associated fecal microbiota in male Brandt’s voles (Lasiopodomys brandtii). Horm. Behav. 2020, 126, 104838. [Google Scholar] [CrossRef] [PubMed]
- Saino, N.; Canova, L.; Fasola, M.; Martinelli, R. Reproduction and population density affect humoral immunity in bank voles under field experimental conditions. Oecologia 2000, 124, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bian, J.; Cao, Y. Maternal social stress affects immunocompetence of F1 in root voles (Microtus oeconomus): A field experiment. Acta Theirol. Sin. 2008, 28, 250–259, (In Chinese English Abstract). [Google Scholar]
- Demas, G.E.; Zysling, D.A.; Beechler, B.R.; Muehlenbein, M.P.; French, S.S. Beyond phytohaemagglutinin: Assessing vertebrate immune function across ecological contexts. J. Anim. Ecol. 2011, 80, 710–730. [Google Scholar] [CrossRef]
- Boonstra, R.; Hik, D.; Singleton, G.R.; Tinnikov, A. The impact of predator-induced stress on the snowshoe hare cycle. Ecol. Monogr. 1998, 68, 371–394. [Google Scholar] [CrossRef]
- Daugschies, A.; Imarom, S.; Bollwahn, W. Differentiation of porcine Eimeria spp. by morphologic algorithms. Vet. Parasitol. 1999, 81, 201–210. [Google Scholar] [CrossRef]
- Fekete, S.G.; Kellems, R.O. Interrelationship of feeding with immunity and parasitic infection: A review. Vet. Med.-Czech. 2007, 52, 131. [Google Scholar] [CrossRef]
- Hõrak, P.; Saks, L.; Karu, U.; Ots, I.; Surai, P.F.; McGraw, K.J. How coccidian parasites affect health and appearance of greenfinches. J. Anim. Ecol. 2004, 73, 935–947. [Google Scholar] [CrossRef]
- Devevey, G.; Niculita-Hirzel, H.; Biollaz, F.; Yvon, C.; Chapuisat, M.; Christe, P. Developmental, metabolic and immunological costs of flea infestation in the common vole. Funct. Ecol. 2008, 22, 1091–1098. [Google Scholar] [CrossRef]
- Shang, G.; Yang, Y.; Zhu, Y.; Wu, X.; Cao, Y.; Wu, Y.; Bian, J. A complex regulating pattern induced by the effects of predation and parasites on root vole (Microtus oeconomus) populations during the breeding season. J. Mammal. 2020, 101, 1345–1355. [Google Scholar] [CrossRef]


| Parameters | Low Density | High Density | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| White blood cell (×109) | 5.43 ± 0.59 (32) * | 5.59 ± 0.61 (19) # | 3.89 ± 0.42 (35) | 3.91 ± 0.42 (23) |
| Lymphocytes (×109) | 3.20 ± 0.22 (32) * | 3.22 ± 0.22 (19) # | 2.22 ± 0.15 (35) | 2.25 ± 0.16 (23) |
| Monocytes (×109) | 0.62 ± 0.04 (32) | 0.59 ± 0.04 (19) | 0.57 ± 0.04 (35) | 0.62 ± 0.04 (23) |
| Granulocytes (×109) | 0.18 ± 0.37 (32) | 0.18 ± 0.37 (19) | 0.16 ± 0.33 (35) | 0.17 ± 0.34 (23) |
| Plasma proteins (mg mL−1) | 10.32 ± 1.22 (32) * | 9.26 ± 1.33 (19) # | 3.87 ± 1.20 (35) | 3.77 ± 1.27(23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Shang, G.; Tian, X.; Liu, Z.; Yang, Y.; Niu, H.; Bian, J.; Wu, Y.; Ma, J. Effects of DNA Methylation of HPA-Axis Genes of F1 Juvenile Induced by Maternal Density Stress on Behavior and Immune Traits in Root Voles (Microtus oeconomus)—A Field Experiment. Animals 2024, 14, 2467. https://doi.org/10.3390/ani14172467
Du S, Shang G, Tian X, Liu Z, Yang Y, Niu H, Bian J, Wu Y, Ma J. Effects of DNA Methylation of HPA-Axis Genes of F1 Juvenile Induced by Maternal Density Stress on Behavior and Immune Traits in Root Voles (Microtus oeconomus)—A Field Experiment. Animals. 2024; 14(17):2467. https://doi.org/10.3390/ani14172467
Chicago/Turabian StyleDu, Shouyang, Guozhen Shang, Xin Tian, Zihan Liu, Yanbin Yang, Hongxing Niu, Jianghui Bian, Yan Wu, and Jinyou Ma. 2024. "Effects of DNA Methylation of HPA-Axis Genes of F1 Juvenile Induced by Maternal Density Stress on Behavior and Immune Traits in Root Voles (Microtus oeconomus)—A Field Experiment" Animals 14, no. 17: 2467. https://doi.org/10.3390/ani14172467





