Novel Single-Nucleotide Polymorphisms (SNPs) and Genetic Studies of the Shadow of Prion Protein (SPRN) in Quails
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Genomic DNA
2.3. Genetic Analysis of the Quail SPRN Gene
2.4. Genetic Polymorphisms of the SPRN Gene among Several Species
2.5. The Impact of Amino Acid Substitution by In Silico Evaluation
2.6. The 3D Structure Analysis
2.7. Statistical Analysis
3. Results
3.1. SPRN Gene Sequence in Quails
3.2. Identification of Novel Polymorphisms of the Quail SPRN Gene
3.3. Comparison of the Number of SNPs in the SPRN Gene among Prion Disease-Related Species
3.4. Polymorphism Distributions among Avian Species
3.5. Prediction of the 3D Structure of Quail Sho
3.6. In Silico Analysis of the Effects of Polymorphisms in the Quail SPRN Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prusiner, S.B. The prion diseases. Brain Pathol. 1998, 8, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef]
- Zayed, M.; Kook, S.H.; Jeong, B.H. Potential Therapeutic Use of Stem Cells for Prion Diseases. Cells 2023, 12, 2413. [Google Scholar] [CrossRef] [PubMed]
- Cohen, F.E.; Prusiner, S.B. Pathologic conformations of prion proteins. Annu. Rev. Biochem. 1998, 67, 793–819. [Google Scholar] [CrossRef]
- Büeler, H.; Fischer, M.; Lang, Y.; Bluethmann, H.; Lipp, H.P.; DeArmond, S.J.; Prusiner, S.B.; Aguet, M.; Weissmann, C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 1992, 356, 577–582. [Google Scholar] [CrossRef]
- Büeler, H.; Aguzzi, A.; Sailer, A.; Greiner, R.A.; Autenried, P.; Aguet, M.; Weissmann, C. Mice devoid of PrP are resistant to scrapie. Cell 1993, 73, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Lampo, E.; Van Poucke, M.; Hugot, K.; Hayes, H.; Van Zeveren, A.; Peelman, L.J. Characterization of the genomic region containing the Shadow of Prion Protein (SPRN) gene in sheep. BMC Genom. 2007, 8, 138. [Google Scholar] [CrossRef]
- Watts, J.C.; Drisaldi, B.; Ng, V.; Yang, J.; Strome, B.; Horne, P.; Sy, M.S.; Yoong, L.; Young, R.; Mastrangelo, P.; et al. The CNS glycoprotein Shadoo has PrP(C)-like protective properties and displays reduced levels in prion infections. EMBO J. 2007, 26, 4038–4050. [Google Scholar] [CrossRef]
- Onodera, T.; Nishimura, T.; Sugiura, K.; Sakudo, A. Function of Prion Protein and the Family Member, Shadoo. Curr. Issues Mol. Biol. 2020, 36, 67–88. [Google Scholar] [CrossRef]
- Premzl, M.; Sangiorgio, L.; Strumbo, B.; Marshall Graves, J.A.; Simonic, T.; Gready, J.E. Shadoo, a new protein highly conserved from fish to mammals and with similarity to prion protein. Genes 2003, 314, 89–102. [Google Scholar] [CrossRef]
- Premzl, M.; Gready, J.E.; Jermiin, L.S.; Simonic, T.; Marshall Graves, J.A. Evolution of vertebrate genes related to prion and Shadoo proteins--clues from comparative genomic analysis. Mol. Biol. Evol. 2004, 21, 2210–2231. [Google Scholar] [CrossRef] [PubMed]
- Ciric, D.; Richard, C.A.; Moudjou, M.; Chapuis, J.; Sibille, P.; Daude, N.; Westaway, D.; Adrover, M.; Béringue, V.; Martin, D.; et al. Interaction between Shadoo and PrP Affects the PrP-Folding Pathway. J. Virol. 2015, 89, 6287–6293. [Google Scholar] [CrossRef]
- Watts, J.C.; Westaway, D. The prion protein family: Diversity, rivalry, and dysfunction. Biochim. Biophys. Acta 2007, 1772, 654–672. [Google Scholar] [CrossRef]
- Beck, J.A.; Campbell, T.A.; Adamson, G.; Poulter, M.; Uphill, J.B.; Molou, E.; Collinge, J.; Mead, S. Association of a null allele of SPRN with variant Creutzfeldt-Jakob disease. J. Med. Genet. 2008, 45, 813–817. [Google Scholar] [CrossRef]
- Gabriel, J.M.; Oesch, B.; Kretzschmar, H.; Scott, M.; Prusiner, S.B. Molecular cloning of a candidate chicken prion protein. Proc. Natl. Acad. Sci. USA 1992, 89, 9097–9101. [Google Scholar] [CrossRef] [PubMed]
- Wopfner, F.; Weidenhöfer, G.; Schneider, R.; von Brunn, A.; Gilch, S.; Schwarz, T.F.; Werner, T.; Schätzl, H.M. Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein. J. Mol. Biol. 1999, 289, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Simonic, T.; Duga, S.; Strumbo, B.; Asselta, R.; Ceciliani, F.; Ronchi, S. cDNA cloning of turtle prion protein. FEBS Lett. 2000, 469, 33–38. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Kim, S.-K.; Jeong, B.-H. Scrapie susceptibility-associated indel polymorphism of shadow of prion protein gene (SPRN) in Korean native black goats. Sci. Rep. 2019, 9, 15261. [Google Scholar] [CrossRef] [PubMed]
- Won, S.Y.; Kim, Y.C.; Kim, S.K.; Jeong, B.H. The First Report of Genetic and Structural Diversities in the SPRN Gene in the Horse, an Animal Resistant to Prion Disease. Genes 2019, 11, 39. [Google Scholar] [CrossRef]
- Won, S.Y.; Kim, Y.C.; Do, K.; Jeong, B.H. The First Report of Genetic Polymorphisms of the Equine SPRN Gene in Outbred Horses, Jeju and Halla Horses. Animals 2021, 11, 2574. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, H.H.; Kim, A.D.; Jeong, B.H. Novel insertion/deletion polymorphisms and genetic features of the shadow of prion protein gene (SPRN) in dogs, a prion-resistant animal. Front. Vet. Sci. 2022, 9, 942289. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, H.H.; Jeong, B.H. The First Report of Polymorphisms and Genetic Characteristics of the Shadow of Prion Protein (SPRN) in Prion Disease-Resistant Animal, Chickens. Front. Vet. Sci. 2022, 9, 904305. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Hawkins, S.A.; Austin, A.R.; Konold, T.; Green, R.B.; Blamire, I.W.; Dexter, I.; Stack, M.J.; Chaplin, M.J.; Langeveld, J.P.; et al. Studies of the transmissibility of the agent of bovine spongiform encephalopathy to the domestic chicken. BMC Res. Notes 2011, 4, 501. [Google Scholar] [CrossRef]
- Chang, H. Essentials of Animal Genetic Resources Scienc; Agricultural Publishing House of China: Beijing, China, 1995; Volume 123, p. 129. [Google Scholar]
- Kayang, B.B.; Fillon, V.; Inoue-Murayama, M.; Miwa, M.; Leroux, S.; Fève, K.; Monvoisin, J.L.; Pitel, F.; Vignoles, M.; Mouilhayrat, C.; et al. Integrated maps in quail (Coturnix japonica) confirm the high degree of synteny conservation with chicken (Gallus gallus) despite 35 million years of divergence. BMC Genom. 2006, 7, 101. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.C.; Jeong, B.H. Novel Single Nucleotide Polymorphisms (SNPs) and Genetic Features of the Prion Protein Gene (PRNP) in Quail (Coturnix japonica). Front. Vet. Sci. 2022, 9, 870735. [Google Scholar] [CrossRef] [PubMed]
- Pejaver, V.; Urresti, J.; Lugo-Martinez, J.; Pagel, K.A.; Lin, G.N.; Nam, H.J.; Mort, M.; Cooper, D.N.; Sebat, J.; Iakoucheva, L.M.; et al. Inferring the molecular and phenotypic impact of amino acid variants with MutPred2. Nat. Commun. 2020, 11, 5918. [Google Scholar] [CrossRef]
- Ng, P.C.; Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Randall, A.; Baldi, P. Prediction of protein stability changes for single-site mutations using support vector machines. Proteins 2006, 62, 1125–1132. [Google Scholar] [CrossRef]
- Iglesias, V.; Conchillo-Sole, O.; Batlle, C.; Ventura, S. AMYCO: Evaluation of mutational impact on prion-like proteins aggregation propensity. BMC Bioinform. 2019, 20, 24. [Google Scholar] [CrossRef]
- Paladin, L.; Piovesan, D.; Tosatto, S.C.E. SODA: Prediction of protein solubility from disorder and aggregation propensity. Nucleic Acids Res. 2017, 45, W236–W240. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30 (Suppl. S1), S162–S173. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Rohlfs, R.V.; Weir, B.S. Distributions of Hardy-Weinberg equilibrium test statistics. Genetics 2008, 180, 1609–1616. [Google Scholar] [CrossRef]
- Daude, N.; Wohlgemuth, S.; Rogaeva, E.; Farid, A.H.; Heaton, M.; Westaway, D. Frequent missense and insertion/deletion polymorphisms in the ovine Shadoo gene parallel species-specific variation in PrP. PLoS ONE 2009, 4, e6538. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.; Shen, C.; Zhao, D.; Goldmann, W. Genetic analysis of the SPRN gene in ruminants reveals polymorphisms in the alanine-rich segment of shadoo protein. J. Gen. Virol. 2009, 90, 2575–2580. [Google Scholar] [CrossRef]
- Gurgul, A.; Polak, M.P.; Larska, M.; Słota, E. PRNP and SPRN genes polymorphism in atypical bovine spongiform encephalopathy cases diagnosed in Polish cattle. J. Appl. Genet. 2012, 53, 337–342. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.-L.; Du, S.-H.; Wang, S.-Q.; Zhang, Y.-P. Comparative analysis of the Shadoo gene between cattle and buffalo reveals significant differences. PLoS ONE 2012, 7, e46601. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Kim, S.-K.; Won, S.-Y.; Jeong, B.-H. Polymorphisms of shadow of prion protein gene (SPRN) in Korean native cattle (Hanwoo) and Holstein cattle. Sci. Rep. 2020, 10, 15272. [Google Scholar] [CrossRef]
- Peletto, S.; Bertolini, S.; Maniaci, M.G.; Colussi, S.; Modesto, P.; Biolatti, C.; Bertuzzi, S.; Caramelli, M.; Maurella, C.; Acutis, P.L. Association of an indel polymorphism in the 3′UTR of the caprine SPRN gene with scrapie positivity in the central nervous system. J. Gen. Virol. 2012, 93, 1620–1623. [Google Scholar] [CrossRef]
- Lampo, E.; Duchateau, L.; Schepens, B.; Van Poucke, M.; Saelens, X.; Erkens, T.; Van Zeveren, A.; Peelman, L. Identification of polymorphisms in the ovine Shadow of prion protein (SPRN) gene and assessment of their effect on promoter activity and susceptibility for classical scrapie. Anim. Genet. 2010, 41, 169–178. [Google Scholar] [CrossRef]
- Memon, S.; Wang, Z.; Zou, W.-Q.; Kim, Y.-C.; Jeong, B.-H. First Report of Single Nucleotide Polymorphisms (SNPs) of the Leporine Shadow of Prion Protein Gene (SPRN) and Absence of Nonsynonymous SNPs in the Open Reading Frame (ORF) in Rabbits. Animals 2024, 14, 1807. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-D.; Zayed, M.; Kim, Y.-C.; Jeong, B.-H. The First Genetic Characterization of the SPRN Gene in Pekin Ducks (Anas platyrhynchos domesticus). Animals 2024, 14, 1588. [Google Scholar] [CrossRef]
- Choi, D.-I.; Zayed, M.; Kim, Y.-C.; Jeong, B.-H. Novel Polymorphisms and Genetic Studies of the Shadow of Prion Protein gene (SPRN) in Pheasants. Front. Vet. Sci. 2024, 11, 1399548. [Google Scholar] [CrossRef]
- Dickinson, M.E.; Flenniken, A.M.; Ji, X.; Teboul, L.; Wong, M.D.; White, J.K.; Meehan, T.F.; Weninger, W.J.; Westerberg, H.; Adissu, H.; et al. High-throughput discovery of novel developmental phenotypes. Nature 2016, 537, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Young, R.; Passet, B.; Vilotte, M.; Cribiu, E.P.; Béringue, V.; Le Provost, F.; Laude, H.; Vilotte, J.L. The prion or the related Shadoo protein is required for early mouse embryogenesis. FEBS Lett. 2009, 583, 3296–3300. [Google Scholar] [CrossRef]
- Funahashi, J.; Sugita, Y.; Kitao, A.; Yutani, K. How can free energy component analysis explain the difference in protein stability caused by amino acid substitutions? Effect of three hydrophobic mutations at the 56th residue on the stability of human lysozyme. Protein Eng. 2003, 16, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.; Wyatt, K.; Wistow, G.J.; Bateman, O.A.; Wallace, B.A.; Slingsby, C. The P23T cataract mutation causes loss of solubility of folded gammaD-crystallin. J. Mol. Biol. 2004, 343, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.; Annunziata, O.; Asherie, N.; Ogun, O.; Benedek, G.B.; Pande, J. Decrease in protein solubility and cataract formation caused by the Pro23 to Thr mutation in human gamma D-crystallin. Biochemistry 2005, 44, 2491–2500. [Google Scholar] [CrossRef]
- Kim, W.; Hecht, M.H. Generic hydrophobic residues are sufficient to promote aggregation of the Alzheimer’s Abeta42 peptide. Proc. Natl. Acad. Sci. USA 2006, 103, 15824–15829. [Google Scholar] [CrossRef]
- Kaytor, M.D.; Warren, S.T. Aberrant protein deposition and neurological disease. J. Biol. Chem. 1999, 274, 37507–37510. [Google Scholar] [CrossRef]
- Harding, C.F. Learning from bird brains: How the study of songbird brains revolutionized neuroscience. Lab Anim. 2004, 33, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Alm, H.; Mattsson, A.; Nilsson, A.; Kultima, K.; Savitski, M.M.; Fälth, M.; Sköld, K.; Brunström, B.; Andren, P.E.; et al. Neuropeptidomic analysis of the embryonic Japanese quail diencephalon. BMC Dev. Biol. 2010, 10, 30. [Google Scholar] [CrossRef] [PubMed]

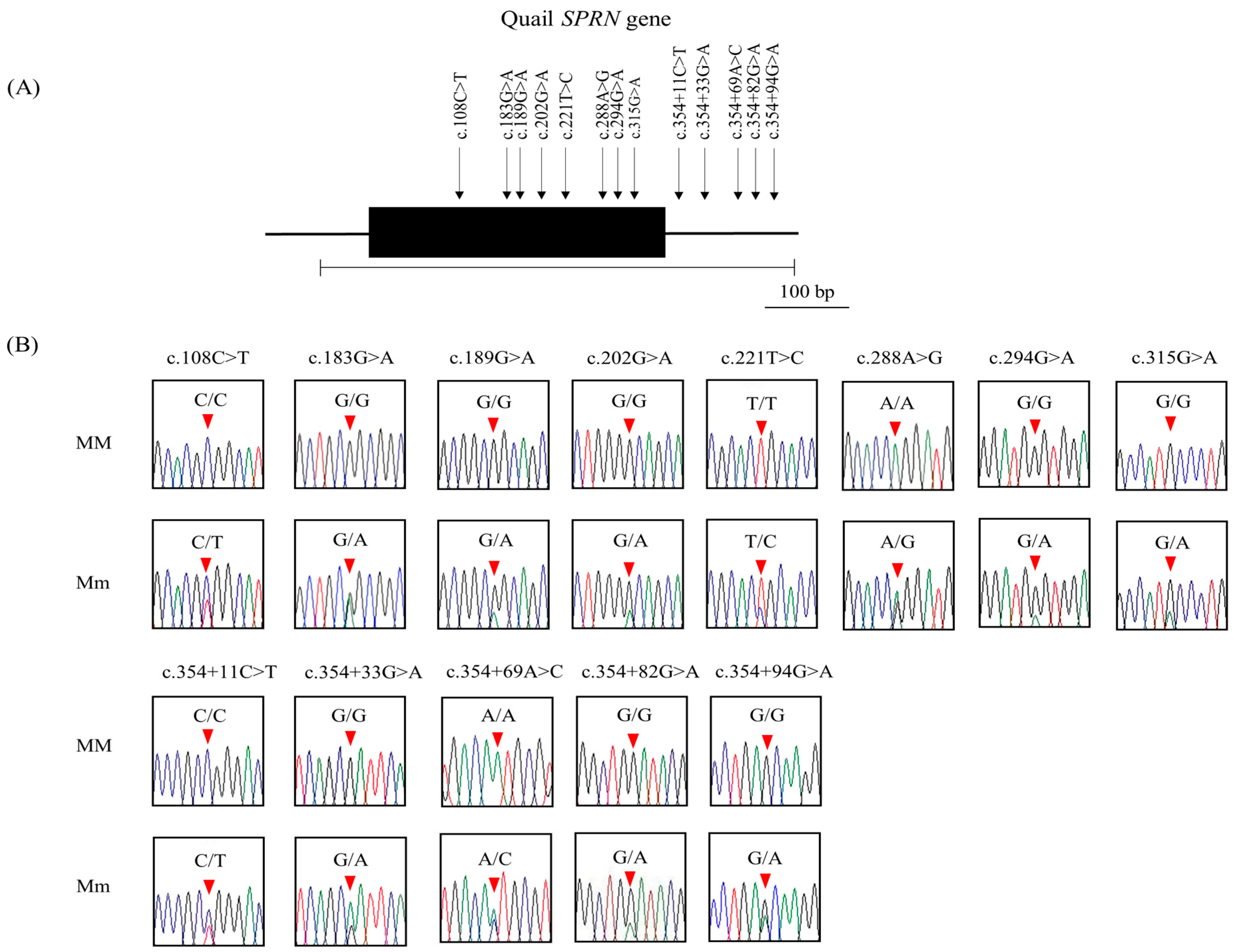
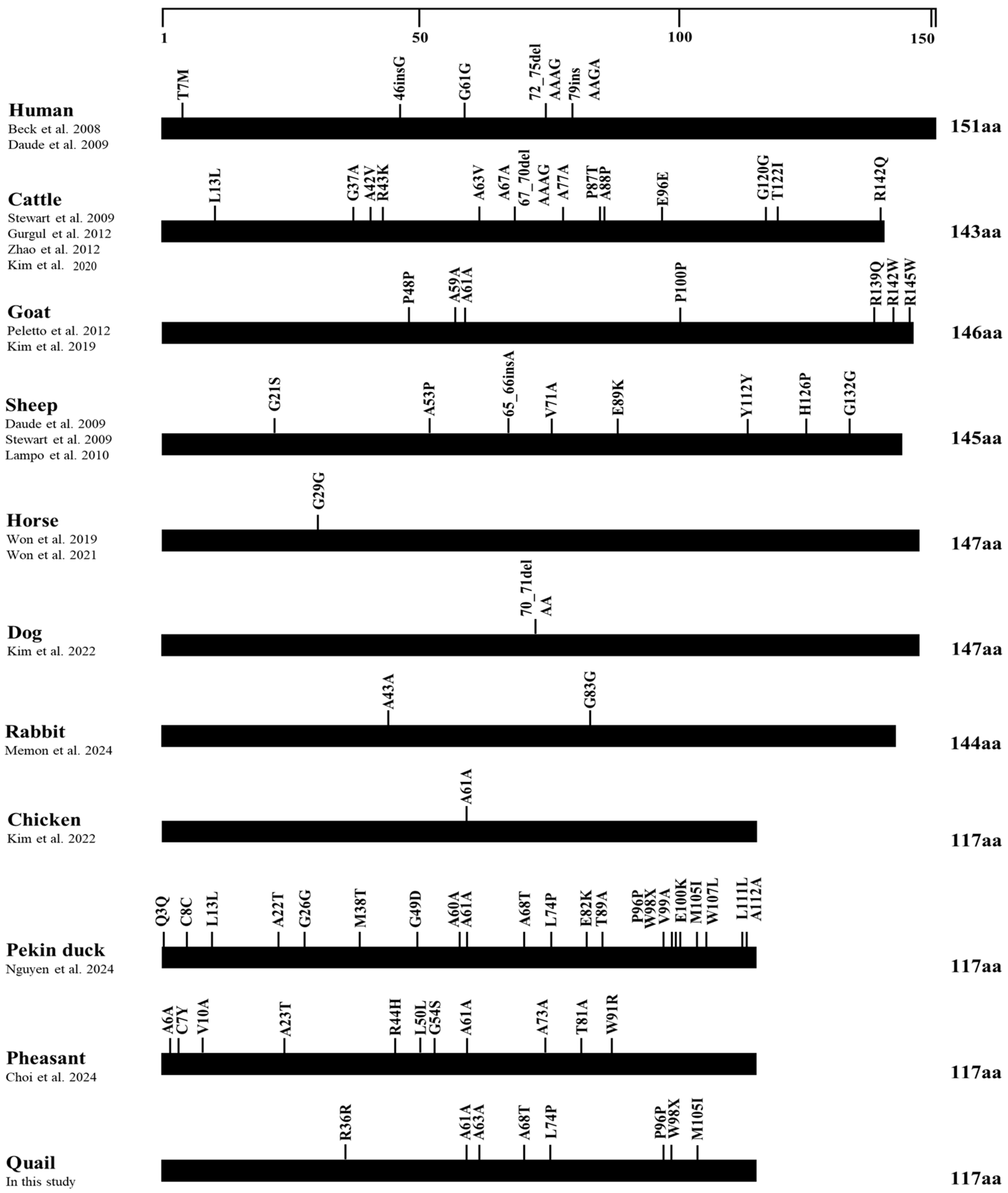
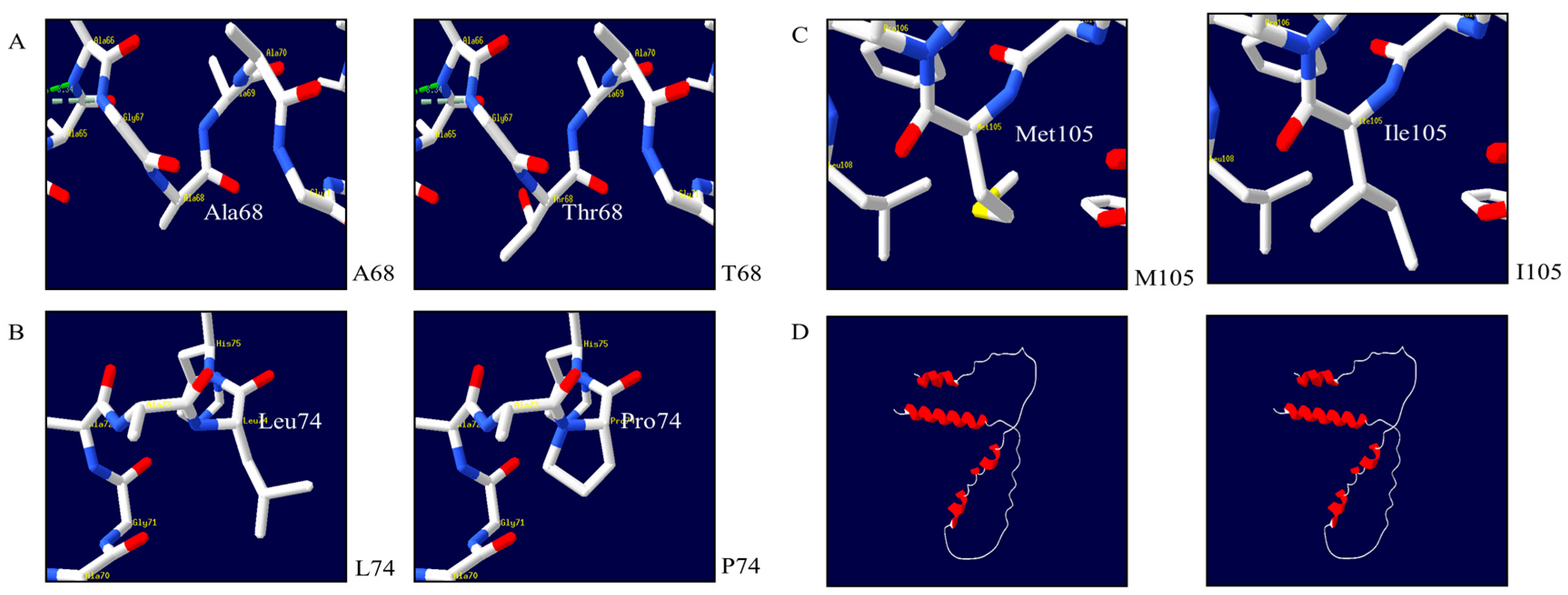
| Polymorphisms | Genotype Frequencies, n (%) | Allele Frequencies, n (%) | HWE | |||
|---|---|---|---|---|---|---|
| MM | Mm | mm | M | m | ||
| c.108C>T | 105 (99.1) | 1 (0.9) | 0 (0) | 211 (99.5) | 1 (0.5) | 0.9610 |
| c.183G>A | 2 (1.9) | 104 (98.1) | 0 (0) | 108 (50.9) | 104 (49.1) | <0.0001 |
| c.189G>A | 78 (73.6) | 28 (26.4) | 0 (0) | 184 (86.8) | 28 (13.2) | 0.1171 |
| c.202G>A (A68T) | 80 (75.5) | 26 (24.5) | 0 (0) | 186 (87.7) | 26 (12.3) | 0.1501 |
| c.221T>C (L74P) | 78 (73.6) | 28 (26.4) | 0 (0) | 184 (86.8) | 28 (13.2) | 0.1171 |
| c.288A>G | 63 (59.4) | 43 (40.6) | 0 (0) | 169 (79.7) | 43 (20.3) | 0.0088 |
| c.294G>A (W98X) | 78 (73.6) | 28 (26.4) | 0 (0) | 184 (86.8) | 28 (13.2) | 0.1171 |
| c.315G>A (M105I) | 105 (99.1) | 1 (0.9) | 0 (0) | 211 (99.5) | 1 (0.5) | 0.9610 |
| c.354+11C>T | 105 (99.1) | 1 (0.9) | 0 (0) | 211 (99.5) | 1 (0.5) | 0.9610 |
| c.354+33G>A | 2 (1.9) | 104 (98.1) | 0 (0) | 108 (50.9) | 104 (49.1) | <0.0001 |
| c.354+69A>C | 20 (18.9) | 86 (81.1) | 0 (0) | 126 (59.4) | 86 (40.6) | <0.0001 |
| c.354+82G>A | 60 (56.6) | 46 (43.4) | 0 (0) | 166 (78.3) | 46 (21.7) | 0.0043 |
| c.354+94G>A | 52 (49.1) | 54 (50.9) | 0 (0) | 158 (74.5) | 54 (25.5) | 0.0004 |
| c.108C>T | c.183G>A | c.189G>A | c.202G>A | c.221T>C | c.288A>G | c.294G>A | c.315G>A | c.354+11C>T | c.354+33G>A | c.354+69A>C | c.354+82G>A | c.354+94G>A | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.108C>T | - | ||||||||||||
| c.183G>A | 0.005 | - | |||||||||||
| c.189G>A | 0.001 | 0.158 | - | ||||||||||
| c.202G>A | 0.001 | 0.145 | 0.058 | - | |||||||||
| c.221T>C | 0.001 | 0.158 | 0.043 | 0.415 | - | ||||||||
| c.288A>G | 0.001 | 0.264 | 0.008 | 0.036 | 0.039 | - | |||||||
| c.294G>A | 0.001 | 0.158 | 0.009 | 0.543 | 0.370 | 0.039 | - | ||||||
| c.315G>A | 0.0 | 0.005 | 0.001 | 0.001 | 0.001 | 0.019 | 0.001 | - | |||||
| c.354+11C>T | 1.0 | 0.005 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.0 | - | ||||
| c.354+33G>A | 0.005 | 1.0 | 0.158 | 0.145 | 0.158 | 0.264 | 0.158 | 0.005 | 0.005 | - | |||
| c.354+69A>C | 0.007 | 0.657 | 0.104 | 0.155 | 0.082 | 0.174 | 0.174 | 0.007 | 0.007 | 0.657 | - | ||
| c.354+82G>A | 0.001 | 0.288 | 0.0 | 0.039 | 0.042 | 0.021 | 0.023 | 0.017 | 0.001 | 0.288 | 0.189 | - | |
| c.354+94G>A | 0.002 | 0.329 | 0.051 | 0.079 | 0.140 | 0.087 | 0.140 | 0.002 | 0.002 | 0.329 | 0.233 | 0.014 | - |
| c.108C>T | c.183G>A | c.189G>A | c.202G>A | c.221T>C | c.288A>G | c.294G>A | c.315G>A | c.354+11C>T | c.354+33G>A | c.354+69A>C | c.354+82G>A | c.354+94G>A | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.56C>T (T19I) | 0.001 | 0.003 | 0.002 | 0.011 | 0.011 | 0.026 | 0.012 | 0.001 | 0.001 | 0.003 | 0.0 | 0.001 | 0.002 |
| c.61G>A (V21I) | 0.001 | 0.0 | 0.016 | 0.001 | 0.001 | 0.025 | 0.002 | 0.001 | 0.001 | 0.0 | 0.006 | 0.0 | 0.004 |
| c.64G>T (A22S) | 0.001 | 0.003 | 0.001 | 0.011 | 0.011 | 0.017 | 0.011 | 0.001 | 0.001 | 0.003 | 0.0 | 0.003 | 0.001 |
| Haplotype | Frequency, n (%) |
|---|---|
| CGGGTAGGCGAGG | 106 (0.500) |
| CAGGTAGGCACGG | 9 (0.042) |
| CAGGTGGGCACGG | 7 (0.033) |
| CAGGTGGGCACAG | 6 (0.028) |
| CAGACAAGCACGA | 6 (0.028) |
| CAGGTAGGCACAG | 5 (0.024) |
| CAGGTGGGCACGA | 5 (0.024) |
| CAAGTAGGCAAAA | 4 (0.019) |
| CAGGTGGGCACAA | 4 (0.019) |
| CAAGTGGGCACGG | 3 (0.014) |
| CAGGTAGGCAAGA | 3 (0.014) |
| CGGGTAGGCGCGA | 2 (0.010) |
| CAGGTAGGCAAAG | 2 (0.010) |
| Others * | 50 (0.235) |
| Total | 212 (1.0) |
| Species | Polymorphisms | Total | References |
|---|---|---|---|
| Chicken | Synonymous c.183G>A Non-synonymous | 1 | Kim et al., 2022 [22]. |
| Pekin duck | Synonymous c.9G>A, c.24C>T, c.39G>T, c.78C>T, c.180T>C, c.183G>A, c.288A>G, c.333C>T, c.336T>C. Non-synonymous c.64G>A (A22T), c.113T>C (M38T), c.146G>A (G49D), c.202G>A (A68T), c.221T>C (L74P), c.244G>A (E82K), c.265A>G (T89A), c.294G>A (W98X), c.296T>C (V99A), c.298G>A (E100K), c.315G>A (M105I), and c.320G>T (W107L). | 21 | Nguyen et al., 2024 [43]. |
| Pheasant | Synonymous c.18C>T, c.148C>T, c.183A>G, c.219A>G Non-synonymous c.20G>A (C7Y), c.29T>C (V10A), c.67G>A (A23T), c.131G>A (R44H), c.160G>A (G54S), c.241A>G (T81A), c.271T>C (W91R). | 11 | Choi et al., 2024 [44]. |
| Quail | Synonymous c.108C>T, c.183G>A, c.189G>A, c.288A>G Non-synonymous c.202G>A (A68T), c.221T>C (L74P), c.294G>A (W98X), c.315G>A (M105I) | 8 | This study |
| Polymorphism | Method | Score | Prediction |
|---|---|---|---|
| c.202G>A (A68T) | MutPred2 | 0.049 | Benign |
| SIFT | 0.0 | Deleterious | |
| MUpro | −0.672 | Decrease | |
| AMYCO | 0.0 | Neutral | |
| SODA | −125.746 | Less soluble | |
| c.221T>C (L74P) | MutPred2 | 0.112 | Benign |
| SIFT | 0.0 | Deleterious | |
| MUpro | −1.964 | Decrease | |
| AMYCO | 0.0 | Neutral | |
| SODA | 148.129 | Soluble | |
| c.315G>A (M105I) | MutPred2 | 0.030 | Benign |
| SIFT | 0.0 | Deleterious | |
| MUpro | −0.468 | Decrease | |
| AMYCO | 0.0 | Neutral | |
| SODA | −2.04302 | Less soluble |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.-I.; Zayed, M.; Jeong, B.-H. Novel Single-Nucleotide Polymorphisms (SNPs) and Genetic Studies of the Shadow of Prion Protein (SPRN) in Quails. Animals 2024, 14, 2481. https://doi.org/10.3390/ani14172481
Choi D-I, Zayed M, Jeong B-H. Novel Single-Nucleotide Polymorphisms (SNPs) and Genetic Studies of the Shadow of Prion Protein (SPRN) in Quails. Animals. 2024; 14(17):2481. https://doi.org/10.3390/ani14172481
Chicago/Turabian StyleChoi, Da-In, Mohammed Zayed, and Byung-Hoon Jeong. 2024. "Novel Single-Nucleotide Polymorphisms (SNPs) and Genetic Studies of the Shadow of Prion Protein (SPRN) in Quails" Animals 14, no. 17: 2481. https://doi.org/10.3390/ani14172481






