Effects of High-Grain Diet on Performance, Ruminal Fermentation, and Rumen Microbial Flora of Lactating Holstein Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Management
2.2. Sample Collection and Analyses
2.3. DNA Extraction and 16S rRNA Gene Sequencing
2.4. Bioinformatics Analysis of the Sequence Data
2.5. Statistical Analysis
3. Results
3.1. Feed Intake and Lactation Performance
3.2. Ruminal Fermentation Characteristics
3.3. Sequencing and Diversity Measures
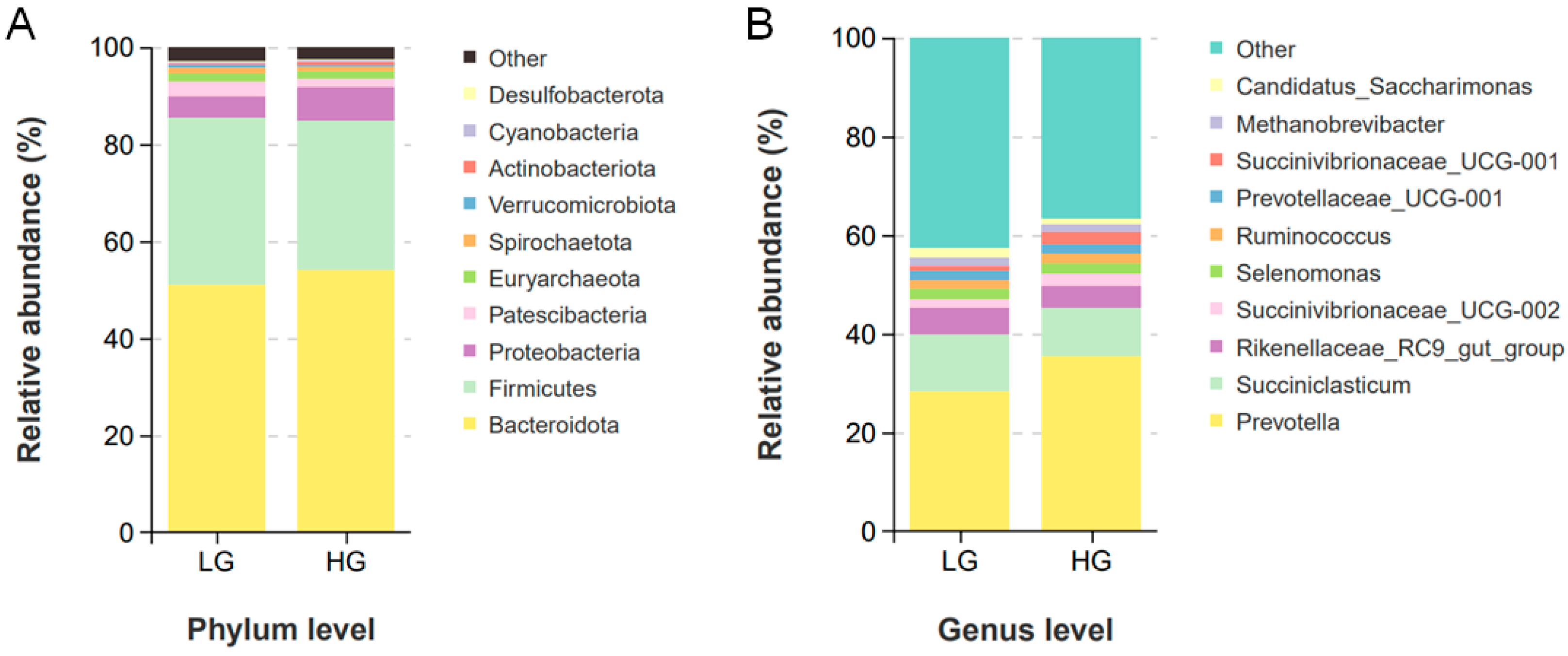
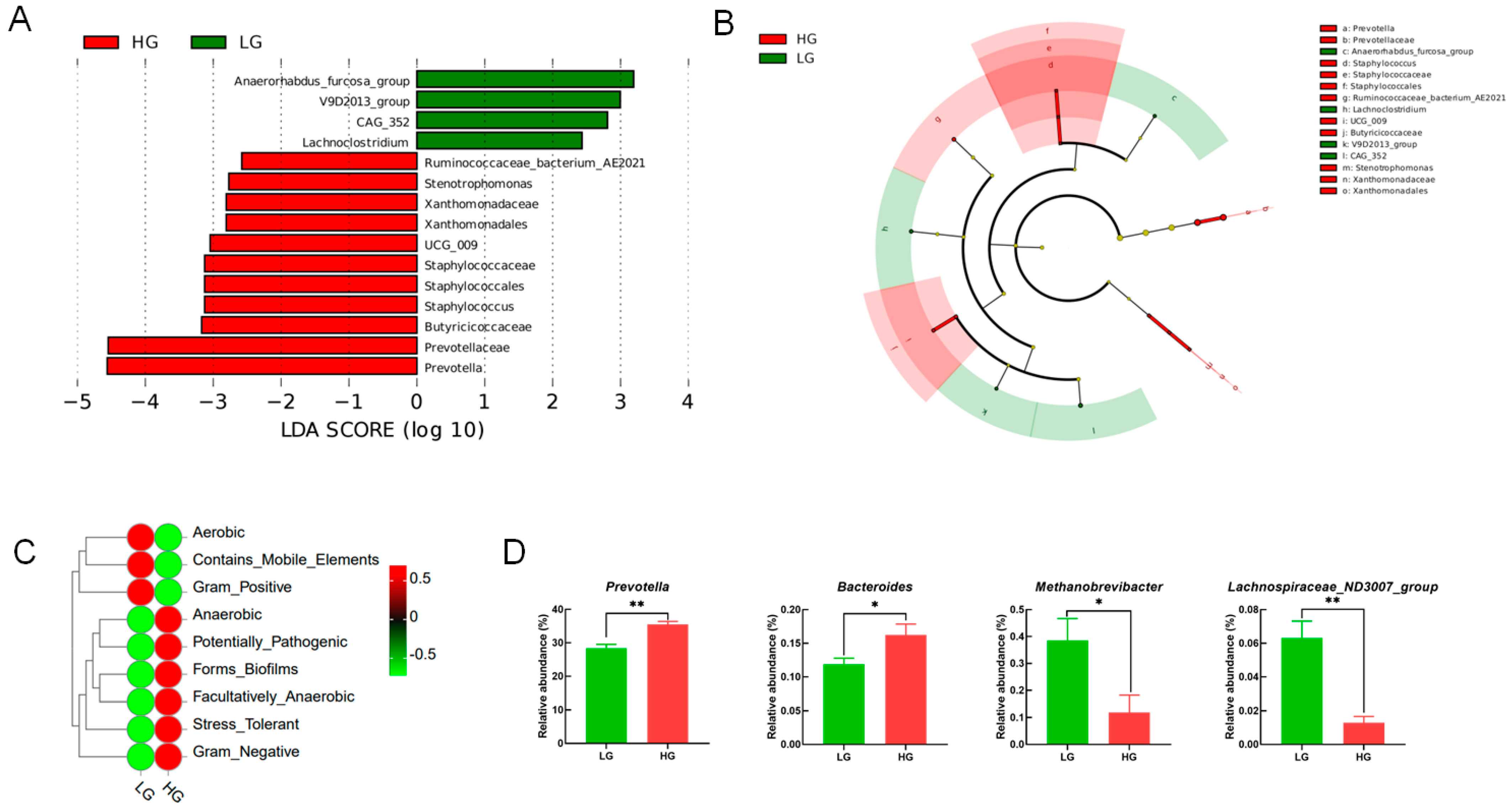
3.4. Ruminal Microbiota Composition
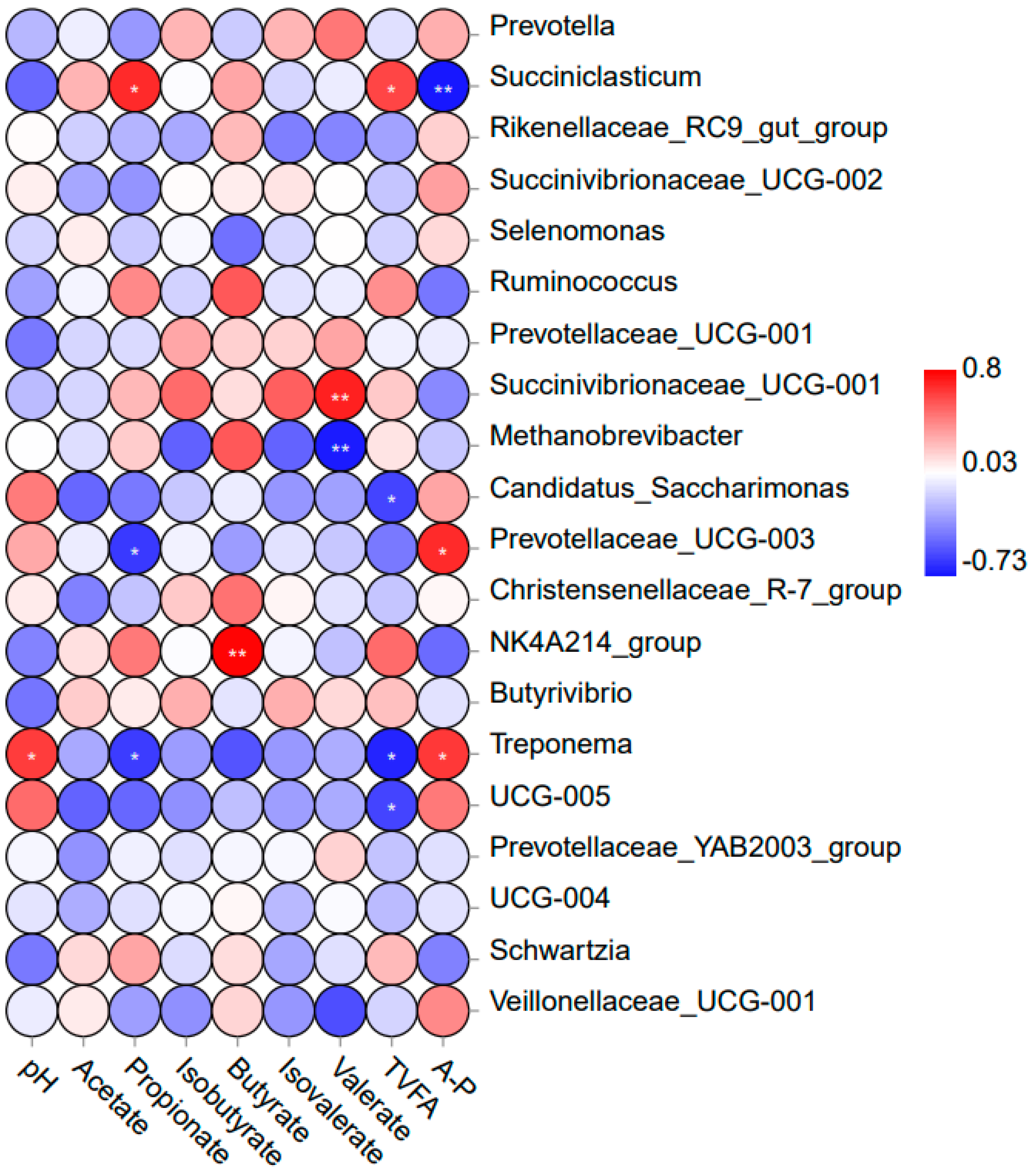
3.5. Correlation Analysis of Rumen Fermentation Parameters and Major Bacteria
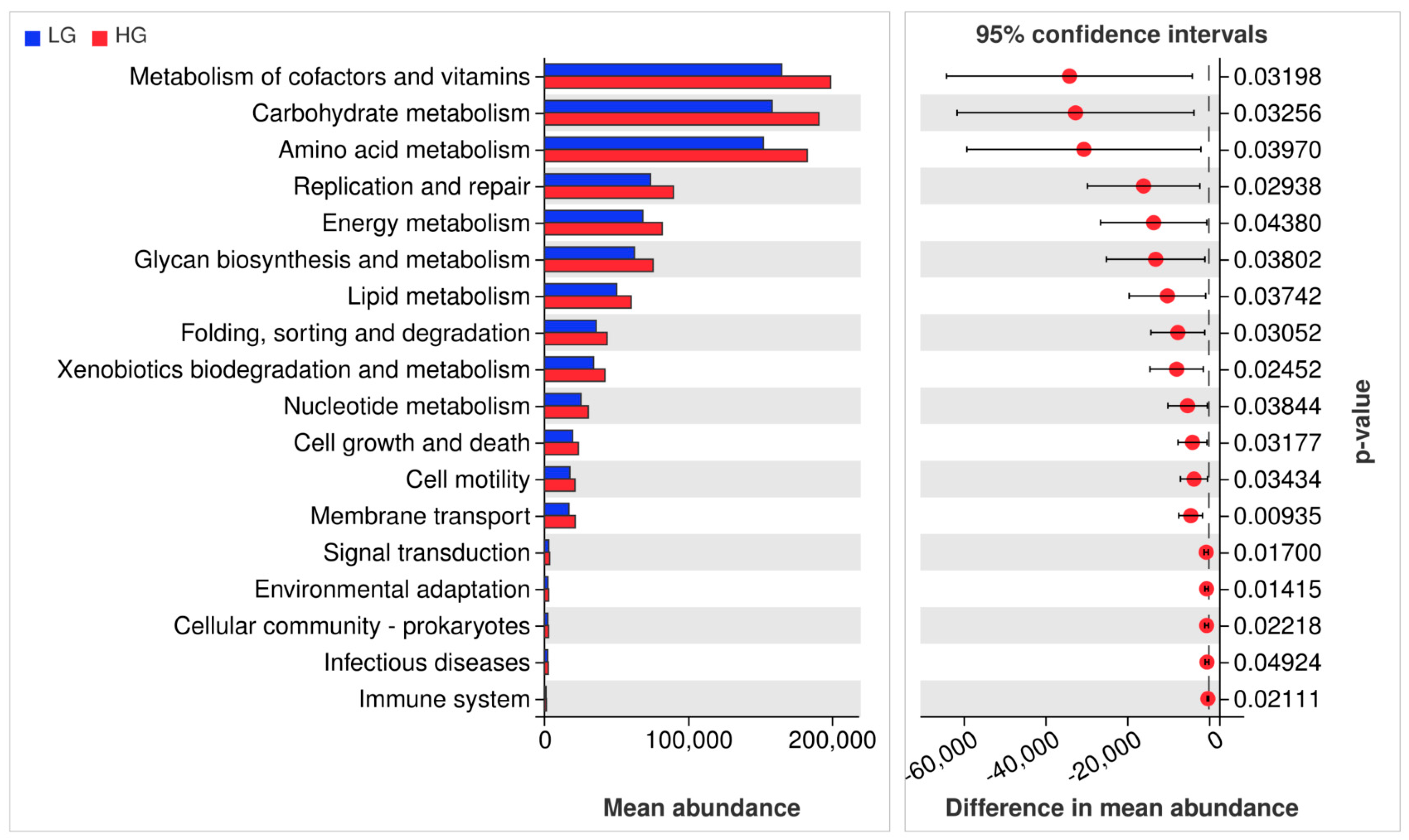
3.6. Functional Prediction of the Microbial Community Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, D.; Gao, Y.; Lu, Y.; Qu, M.; Xiong, X.; Xu, L.; Zhao, X.; Pan, K.; Ouyang, K. Niacin alters the ruminal microbial composition of cattle under high-concentrate condition. Anim. Nutr. 2017, 3, 180–185. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Ma, N.; Chang, G.; Aabdin, Z.U.; Shen, X. Long-term high-concentrate diet feeding induces apoptosis of rumen epithelial cells and inflammation of rumen epithelium in dairy cows. Anim. Biotechnol. 2022, 33, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ye, H.; Liu, J.; Mao, S. High-grain diets altered rumen fermentation and epithelial bacterial community and resulted in rumen epithelial injuries of goats. Appl. Microbiol. Biotechnol. 2017, 101, 6981–6992. [Google Scholar] [CrossRef]
- Gomez, D.E.; Arroyo, L.G.; Costa, M.C.; Viel, L.; Weese, J.S. Characterization of the Fecal Bacterial Microbiota of Healthy and Diarrheic Dairy Calves. J. Vet. Intern. Med. 2017, 31, 928–939. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Elolimy, A.A.; Loor, J.J. Rumen Microbiome, Probiotics, and Fermentation Additives. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, N.; Ren, L.; Wang, M.; Hu, L.; Shen, Y.; Cao, Y.; Li, Q.; Li, J.; Gao, Y. Microbiome-Metabolome Responses in Ruminal Content and Feces of Lactating Dairy Cows With N-Carbamylglutamate Supplementation Under Heat Stress. Front. Vet. Sci. 2022, 9, 902001. [Google Scholar] [CrossRef]
- Steele, M.A.; Penner, G.B.; Chaucheyras-Durand, F.; Guan, L.L. Development and physiology of the rumen and the lower gut: Targets for improving gut health. J. Dairy Sci. 2016, 99, 4955–4966. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2021; p. 502. [Google Scholar]
- Jiang, M.C.; Datsomor, O.; Cheng, Z.Q.; Meng, Z.T.; Zhan, K.; Yang, T.Y.; Huang, Y.H.; Yan, Q.; Zhao, G.Q. Partial Substitution of Alfalfa Hay by Stevia (Stevia rebaudiana) Hay Can Improve Lactation Performance, Rumen Fermentation, and Nitrogen Utilization of Dairy Cows. Front. Vet. Sci. 2022, 9, 899148. [Google Scholar] [CrossRef]
- Jiang, M.C.; Zhang, X.L.; Wang, K.X.; Datsomor, O.; Li, X.; Lin, M.; Feng, C.Y.; Zhao, G.Q.; Zhan, K. Effect of Slow-Release Urea Partial Replacement of Soybean Meal on Lactation Performance, Heat Shock Signal Molecules, and Rumen Fermentation in Heat-Stressed Mid-Lactation Dairy Cows. Animals 2023, 13, 2771. [Google Scholar] [CrossRef]
- Wolff, S.M.; Ellison, M.J.; Hao, Y.; Cockrum, R.R.; Austin, K.J.; Baraboo, M.; Burch, K.; Lee, H.J.; Maurer, T.; Patil, R.; et al. Diet shifts provoke complex and variable changes in the metabolic networks of the ruminal microbiome. Microbiome 2017, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Z.; Peng, W.C.; Liu, J.X.; Xu, G.Z.; Wang, D.M. Effect of chromium methionine supplementation on lactation performance, hepatic respiratory rate and anti-oxidative capacity in early-lactating dairy cows. Anim. Int. J. Anim. Biosci. 2021, 15, 100326. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glockner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef]
- Cao, T.; Li, Q.; Huang, Y.; Li, A. plotnineSeqSuite: A Python package for visualizing sequence data using ggplot2 style. BMC Genom. 2023, 24, 585. [Google Scholar] [CrossRef]
- Spindel, J.; Begum, H.; Akdemir, D.; Virk, P.; Collard, B.; Redona, E.; Atlin, G.; Jannink, J.L.; McCouch, S.R. Genomic selection and association mapping in rice (Oryza sativa): Effect of trait genetic architecture, training population composition, marker number and statistical model on accuracy of rice genomic selection in elite, tropical rice breeding lines. PLoS Genet. 2015, 11, e1004982. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Zhuang, H.; Yang, Z.; Jiang, G.; Liu, Z. Promoting intestinal IgA production in mice by oral administration with anthocyanins. Front. Immunol. 2022, 13, 826597. [Google Scholar] [CrossRef]
- Lei, Z.; Wu, H.; Yang, Y.; Hu, Q.; Lei, Y.; Liu, W.; Nie, Y.; Yang, L.; Zhang, X.; Yang, C.; et al. Ovariectomy Impaired Hepatic Glucose and Lipid Homeostasis and Altered the Gut Microbiota in Mice with Different Diets. Front. Endocrinol. 2021, 12, 708838. [Google Scholar] [CrossRef]
- Di Giorgio, E.; Clocchiatti, A.; Piccinin, S.; Sgorbissa, A.; Viviani, G.; Peruzzo, P.; Romeo, S.; Rossi, S.; Dei Tos, A.P.; Maestro, R.; et al. MEF2 is a converging hub for histone deacetylase 4 and phosphatidylinositol 3-kinase/Akt-induced transformation. Mol. Cell. Biol. 2013, 33, 4473–4491. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Giallongo, F.; Hristov, A.N.; Oh, J.; Frederick, T.; Weeks, H.; Werner, J.; Lapierre, H.; Patton, R.A.; Gehman, A.; Parys, C. Effects of slow-release urea and rumen-protected methionine and histidine on performance of dairy cows. J. Dairy Sci. 2015, 98, 3292–3308. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, C.; Yao, Z.; Cai, L.; Ni, Y.; Mao, S.; Zhao, R. Whole transcriptome analysis of RNA expression profiles reveals the potential regulating action of long noncoding RNA in lactating cows fed a high concentrate diet. Anim. Nutr. 2021, 7, 1315–1328. [Google Scholar] [CrossRef]
- Kim, Y.H.; Nagata, R.; Ohtani, N.; Ichijo, T.; Ikuta, K.; Sato, S. Effects of Dietary Forage and Calf Starter Diet on Ruminal pH and Bacteria in Holstein Calves during Weaning Transition. Front. Microbiol. 2016, 7, 1575. [Google Scholar] [CrossRef]
- Auffret, M.D.; Dewhurst, R.J.; Duthie, C.A.; Rooke, J.A.; John Wallace, R.; Freeman, T.C.; Stewart, R.; Watson, M.; Roehe, R. The rumen microbiome as a reservoir of antimicrobial resistance and pathogenicity genes is directly affected by diet in beef cattle. Microbiome 2017, 5, 159. [Google Scholar] [CrossRef] [PubMed]
- Harthan, L.B.; Cherney, D.J.R. Okara as a protein supplement affects feed intake and milk composition of ewes and growth performance of lambs. Anim. Nutr. 2017, 3, 171–174. [Google Scholar] [CrossRef]
- Ben Meir, Y.A.; Nikbachat, M.; Portnik, Y.; Jacoby, S.; Adin, G.; Moallem, U.; Halachmi, I.; Miron, J.; Mabjeesh, S.J. Effect of forage-to-concentrate ratio on production efficiency of low-efficient high-yielding lactating cows. Anim. Int. J. Anim. Biosci. 2021, 15, 100012. [Google Scholar] [CrossRef]
- Cui, Q.; Lin, L.; Lai, Z.; Mao, S. Effects of high-grain diet feeding on fatty acid profiles in milk, blood, muscle, and adipose tissue, and transcriptional expression of lipid-related genes in muscle and adipose tissue of dairy cows. J. Anim. Sci. Biotechnol. 2023, 14, 41. [Google Scholar] [CrossRef]
- Yang, Y.; Ferreira, G.; Teets, C.L.; Corl, B.A.; Thomason, W.E.; Griffey, C.A. Effects of feeding hulled and hull-less barley with low-and high-forage diets on lactation performance, nutrient digestibility, and milk fatty acid composition of lactating dairy cows. J. Dairy Sci. 2018, 101, 3036–3043. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Vázquez-Fresno, R.; Serra-Cayuela, A.; Dong, E.; Mandal, R.; Hennessy, D.; McAuliffe, S.; Dillon, P.; Wishart, D.S.; Stanton, C.; et al. Pasture Feeding Changes the Bovine Rumen and Milk Metabolome. Metabolites 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, S.; Mu, R.; Guo, J.; Zhao, C.; Cao, Y.; Zhang, N.; Fu, Y. The Rumen Microbiota Contributes to the Development of Mastitis in Dairy Cows. Microbiol. Spectr. 2022, 10, e0251221. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Baudon, M.; Elcoso, G.; Viejo, J.; Courillon, A. Effects on rumen pH and feed intake of a dietary concentrate challenge in cows fed rations containing pH modulators with different neutralizing capacity. J. Dairy Sci. 2023, 106, 4580–4598. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Wang, H.; Nan, X.; Guo, Y.; Xiong, B. Calcium Propionate Supplementation Has Minor Effects on Major Ruminal Bacterial Community Composition of Early Lactation Dairy Cows. Front. Microbiol. 2022, 13, 847488. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of High Forage/Concentrate Diet on Volatile Fatty Acid Production and the Microorganisms Involved in VFA Production in Cow Rumen. Anim. Open Access J. 2020, 10, 223. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Penner, G.B.; Stumpff, F.; Gabel, G. Ruminant nutrition symposium: Role of fermentation acid absorption in the regulation of ruminal pH. J. Anim. Sci. 2011, 89, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, M.; Adamowicz, E.; Basarab, J.A.; Guan, L.L. Characterization of bovine ruminal epithelial bacterial communities using 16S rRNA sequencing, PCR-DGGE, and qRT-PCR analysis. Vet. Microbiol. 2012, 155, 72–80. [Google Scholar] [CrossRef]
- Steele, M.A.; Vandervoort, G.; AlZahal, O.; Hook, S.E.; Matthews, J.C.; McBride, B.W. Rumen epithelial adaptation to high-grain diets involves the coordinated regulation of genes involved in cholesterol homeostasis. Physiol. Genom. 2011, 43, 308–316. [Google Scholar] [CrossRef]
- Zhou, M.; Hernandez-Sanabria, E.; Guan, L.L. Characterization of variation in rumen methanogenic communities under different dietary and host feed efficiency conditions, as determined by PCR-denaturing gradient gel electrophoresis analysis. Appl. Environ. Microbiol. 2010, 76, 3776–3786. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Mulligan, F.J.; Neville, E.W.; Guan, L.L.; Steele, M.A.; Penner, G.B. Invited review: Effect of subacute ruminal acidosis on gut health of dairy cows. J. Dairy Sci. 2022, 105, 7141–7160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiao, L.; Jin, L.; Yan, S.; Niu, D.; Yang, W. Effect of commercial slow-release urea product on in vitro rumen fermentation and ruminal microbial community using RUSITEC technique. J. Anim. Sci. Biotechnol. 2022, 13, 56. [Google Scholar] [CrossRef]
- Li, F.; Feng, Y.; Liu, H.; Kong, D.; Hsueh, C.Y.; Shi, X.; Wu, Q.; Li, W.; Wang, J.; Zhang, Y.; et al. Gut Microbiome and Metabolome Changes in Mice with Acute Vestibular Deficit. Front. Cell. Infect. Microbiol. 2022, 12, 821780. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Qin, J.; Hao, Y.; Fu, L. Association of gut microbiota composition and function with an aged rat model of senile osteoporosis using 16S rRNA and metagenomic sequencing analysis. Aging 2020, 12, 10795–10808. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, K.; Nan, X.; Cai, M.; Yang, L.; Xiong, B.; Zhao, Y. Synergistic Effects of 3-Nitrooxypropanol with Fumarate in the Regulation of Propionate Formation and Methanogenesis in Dairy Cows In Vitro. Appl. Environ. Microbiol. 2022, 88, e0190821. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, H.; Yang, Q.; Yang, D.; Liu, S.; Cui, Z. Evaluating Starter Feeding on Ruminal Function in Yak Calves: Combined 16S rRNA Sequencing and Metabolomics. Front. Microbiol. 2022, 13, 821613. [Google Scholar] [CrossRef]
- Lamendella, R.; Domingo, J.W.; Ghosh, S.; Martinson, J.; Oerther, D.B. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol. 2011, 11, 103. [Google Scholar] [CrossRef]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef]
- Christensen, L.; Vuholm, S.; Roager, H.M.; Nielsen, D.S.; Krych, L.; Kristensen, M.; Astrup, A.; Hjorth, M.F. Prevotella Abundance Predicts Weight Loss Success in Healthy, Overweight Adults Consuming a Whole-Grain Diet Ad Libitum: A Post Hoc Analysis of a 6-Wk Randomized Controlled Trial. J. Nutr. 2019, 149, 2174–2181. [Google Scholar] [CrossRef]
- Pal, D.; Naskar, M.; Bera, A.; Mukhopadhyay, B. Chemical synthesis of the pentasaccharide repeating unit of the O-specific polysaccharide from Ruminococcus gnavus. Carbohydr. Res. 2021, 507, 108384. [Google Scholar] [CrossRef]

| Items | Low-Grain Diets (LG) | High-Grain Diets (HG) |
|---|---|---|
| Ingredient, % of DM | ||
| Alfalfa hay | 24 | 17 |
| Oaten hay | 24 | 17 |
| Corn silage | 12 | 6 |
| Corn grain | 16.6 | 31.5 |
| Soybean meal | 6.84 | 6.84 |
| DDGS 1 | 3.7 | 4 |
| Oatmeal | 4 | 4 |
| Rootlet | 1.6 | 3.4 |
| Spray corn husk | 2 | 4 |
| Corn germ meal | 3 | 4 |
| Premix 2 | 2.26 | 2.26 |
| Total | 100 | 100 |
| Nutrient composition | ||
| DM 3, % | 50.74 | 51.03 |
| Ash, % of DM | 6.79 | 5.67 |
| Crude protein, % of DM | 15.43 | 15.54 |
| Crude fat, % of DM | 2.97 | 3.03 |
| NDF 4, % of DM | 38.02 | 31.08 |
| ADF 5, % of DM | 23.42 | 17.95 |
| Ca, % of DM | 0.84 | 0.85 |
| P, % of DM | 0.35 | 0.38 |
| Starch, % of DM | 17.36 | 29.72 |
| NFC 6 | 36.79 | 44.68 |
| NFC/NDF | 0.97 | 1.44 |
| NEL 7, Mcal/kg of DM | 1.54 | 1.63 |
| Item | Treatment 1 | SEM 2 | p-Value | |
|---|---|---|---|---|
| LG | HG | |||
| DMI 3, kg/d | 21.83 | 21.23 | 0.71 | 0.69 |
| Milk yield, kg/d | 16.79 | 17.85 | 0.75 | 0.50 |
| ECM 4, kg/d | 17.05 | 17.68 | 0.57 | 0.63 |
| Milk composition | ||||
| Fat, % | 4.02 | 3.75 | 0.03 | 0.04 |
| Protein, % | 3.48 | 3.59 | 0.06 | 0.36 |
| Lactose, % | 4.80 | 4.84 | 0.03 | 0.56 |
| Total solids, % | 16.51 | 16.25 | 0.20 | 0.53 |
| SCC 5, ×103/mL | 221.10 | 245.83 | 17.91 | 0.52 |
| MUN 6, mg/dL | 15.73 | 16.61 | 0.26 | 0.09 |
| Item | Treatment 1 | SEM 2 | p-Value | |
|---|---|---|---|---|
| LG | HG | |||
| Rumen pH | 6.03 | 5.56 | 0.09 | <0.01 |
| NH3-N (mg/dL) | 12.34 | 13.12 | 0.88 | 0.46 |
| TVFA 3 (mM) | 108.91 | 143.08 | 4.08 | <0.01 |
| Acetate (mM) | 65.18 | 81.14 | 1.92 | <0.01 |
| Propionate (mM) | 24.28 | 38.47 | 2.45 | <0.01 |
| Butyrate (mM) | 14.24 | 17.52 | 0.78 | 0.03 |
| Isobutyrate (mM) | 1.47 | 1.26 | 0.29 | 0.75 |
| Valerate (mM) | 2.07 | 2.66 | 0.20 | 0.16 |
| Isovalerate (mM) | 1.62 | 2.02 | 0.22 | 0.39 |
| VFA profile (mol/100 mol) | ||||
| Acetate | 59.88 | 56.71 | 0.78 | 0.03 |
| Propionate | 22.30 | 26.89 | 1.07 | 0.02 |
| Butyrate | 13.08 | 12.25 | 0.57 | 0.49 |
| Isobutyrate | 1.35 | 0.88 | 0.27 | 0.42 |
| Valerate | 1.90 | 1.86 | 0.16 | 0.91 |
| Isovalerate | 1.49 | 1.41 | 0.19 | 0.85 |
| Acetate/Propionate | 2.69 | 2.14 | 0.16 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Song, D.; Zhang, X.; Datsomor, O.; Jiang, M.; Zhao, G. Effects of High-Grain Diet on Performance, Ruminal Fermentation, and Rumen Microbial Flora of Lactating Holstein Dairy Cows. Animals 2024, 14, 2522. https://doi.org/10.3390/ani14172522
Wang K, Song D, Zhang X, Datsomor O, Jiang M, Zhao G. Effects of High-Grain Diet on Performance, Ruminal Fermentation, and Rumen Microbial Flora of Lactating Holstein Dairy Cows. Animals. 2024; 14(17):2522. https://doi.org/10.3390/ani14172522
Chicago/Turabian StyleWang, Kexin, Damin Song, Xuelei Zhang, Osmond Datsomor, Maocheng Jiang, and Guoqi Zhao. 2024. "Effects of High-Grain Diet on Performance, Ruminal Fermentation, and Rumen Microbial Flora of Lactating Holstein Dairy Cows" Animals 14, no. 17: 2522. https://doi.org/10.3390/ani14172522
APA StyleWang, K., Song, D., Zhang, X., Datsomor, O., Jiang, M., & Zhao, G. (2024). Effects of High-Grain Diet on Performance, Ruminal Fermentation, and Rumen Microbial Flora of Lactating Holstein Dairy Cows. Animals, 14(17), 2522. https://doi.org/10.3390/ani14172522






